Food Pattern, Food Selectivity and Sensory Profile in Autism Spectrum Disorder: An Exploratory Analysis in Chilean Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.4. Measure Variables
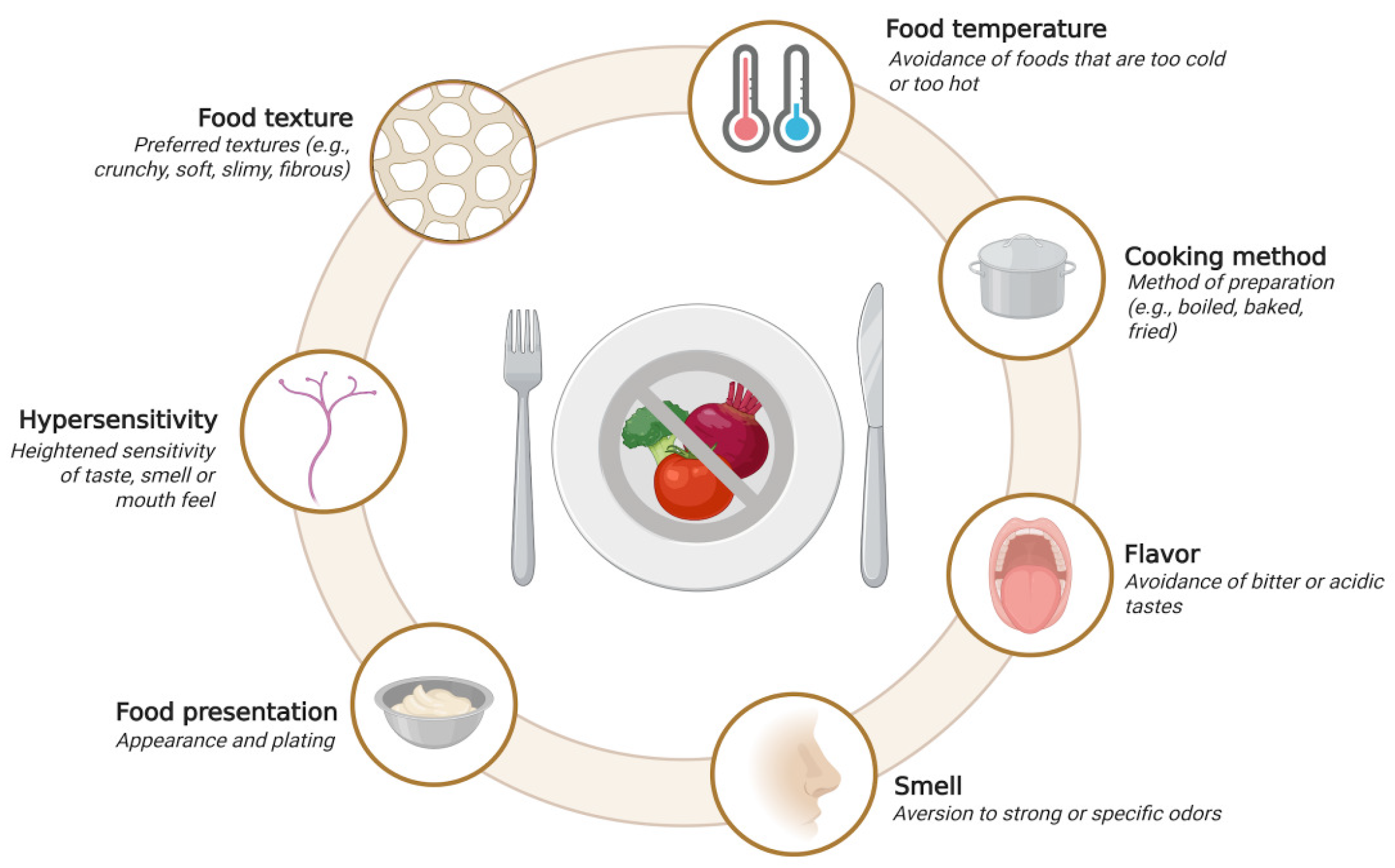
2.4.1. Sensory Processing and Hypersensitivity
2.4.2. Food Selectivity and Food Pattern
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| ASI | Ayres Sensory Integration |
| BAMBI | Brief Autism Mealtime Behaviour Inventory |
| DSM-V | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| FFQ | Food Frequency Questionnaire |
| RTEBC | Ready-To-Eat Breakfast Cereal |
| SOS | Sequential Oral Sensory Approach |
References
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Almandil, N.B.; Alkuroud, D.N.; AbdulAzeez, S.; AlSulaiman, A.; Elaissari, A.; Borgio, J.F. Environmental and Genetic Factors in Autism Spectrum Disorders: Special Emphasis on Data from Arabian Studies. Int. J. Environ. Res. Public Health 2019, 16, 658. [Google Scholar] [CrossRef]
- Talantseva, O.I.; Romanova, R.S.; Shurdova, E.M.; Dolgorukova, T.A.; Sologub, P.S.; Titova, O.S.; Kleeva, D.F.; Grigorenko, E.L. The global prevalence of autism spectrum disorder: A three-level meta-analysis. Front. Psychiatry 2023, 14, 1071181. [Google Scholar] [CrossRef]
- López-Espejo, M. Tendencias en la prevalencia y carga del trastorno del espectro autista en Chile desde 1990 a 2021. Andes Pediatr. 2025, 96, 191–199. [Google Scholar] [CrossRef]
- Martelli, M.E.; Gigliotti, F.; Giovannone, F.; Lentini, G.; Manti, F.; Sogos, C. Developmental Patterns in Autism and Other Neurodevelopmental Disorders in Preschool Children. Children 2025, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Tafolla, M.; Lord, C. Longitudinal Analyses of Mental Health in Autistic Individuals: A Systematic Review. Brain Sci. 2024, 14, 1033. [Google Scholar] [CrossRef]
- Rouphael, M.; Hojeij, B.; Ezzedine, D.; Mortada, H.; Sacre, Y.; Bitar, T.; Naim, E.; Hleihel, W.; Hoteit, M. Assessment of Feeding Behaviors and Parents’ Frustrations of Children with Autism Spectrum Disorder in Lebanon: A Case-Control Study. Children 2023, 10, 117. [Google Scholar] [CrossRef]
- Bandini, L.G.; Curtin, C.; Phillips, S.; Anderson, S.E.; Maslin, M.; Must, A. Changes in Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Alibrandi, A.; Zirilli, A.; Loschiavo, F.; Gangemi, M.C.; Sindoni, A.; Tribulato, G.; Giudice, R.L.; Famà, F. Food Selectivity in Children with Autism Spectrum Disorder: A Statistical Analysis in Southern Italy. Children 2023, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.; Krall, J.R.; Baranova, A.; Slavin, M. Nutritional Intake and Sensory Processing in School-Aged Children with Autism Spectrum Disorder. Nutrients 2025, 17, 604. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.V.; Gomes, D.L. Eating Behavior and Nutritional Profile of Children with Autism Spectrum Disorder in a Reference Center in the Amazon. Nutrients 2024, 16, 452. [Google Scholar] [CrossRef]
- Chistol, L.T.; Bandini, L.G.; Must, A.; Phillips, S.; Cermak, S.A.; Curtin, C. Sensory Sensitivity and Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 583–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nimbley, E.; Golds, L.; Sharpe, H.; Gillespie-Smith, K.; Duffy, F. Sensory processing and eating behaviours in autism: A systematic review. Eur. Eat. Disord. Rev. 2022, 30, 538–559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Westby, C.; Roman, R. Developing Knowledge of Autism in Majority World Countries: Examples of Bolivia and Paraguay. Neuropsychiatr. Dis. Treat. 2024, 20, 1583–1595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivera-Figueroa, K.; Marfo, N.Y.A.; Eigsti, I.M. Parental Perceptions of Autism Spectrum Disorder in Latinx and Black Sociocultural Contexts: A Systematic Review. Am. J. Intellect. Dev. Disabil. 2022, 127, 42–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Concha-Cisternas, Y.; Petermann-Rocha, F.; Castro-Piñero, J.; Parra, S.; Albala, C.; Wyngard, V.V.; Vásquez, J.; Cigarroa, I.; Celis-Morales, C. Handgrip strength as a predictor of adverse health outcomes. Rev. Medica Chile 2022, 150, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W. Sensory Profile 2. Available online: https://www.pearsonassessments.com/en-us/Store/Professional-Assessments/Motor-Sensory/Sensory-Profile-2/p/100000822?srsltid=AfmBOorcE4ovQOwxHVQJC517iOUsp24UzuPQQ_KHLFU3SkGaowtkc9ja (accessed on 13 November 2025).
- Romero-Sanchez, J. Sensory processing differences between preterm and term infants: The role of the occupational therapist. Rev. Chil. De Ter. Ocup. 2016, 1, 47–56. [Google Scholar] [CrossRef]
- Lamboglia, A.; Romano, R.; Valente, D.; Berardi, A.; Cavalli, G.; Giovannone, F.; Sogos, C.; Tofani, M.; Galeoto, G. Brief Autism Mealtime Behavior Inventory (BAMBI): Italian Translation and Validation. Children 2023, 10, 1201. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. Validity of the food frequency questionnaire for adults in nutritional epidemiological studies: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 1670–1688. [Google Scholar] [CrossRef]
- Dehghan, M.; Martinez, S.; Zhang, X.; Seron, P.; Lanas, F.; Islam, S.; Merchant, A.T. Relative validity of an FFQ to estimate daily food and nutrient intakes for Chilean adults. Public Health Nutr. 2013, 16, 1782–1788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yañez-Flores, K.; Castillo-Tapia, J.; Alegría-Villablanca, M.; López-Espinoza, M.Á. Eating behavior of children and adolescentswith autism spectrum disorder who attend a group in a commune in the region of Ñuble, Chile. Salud Cienc. Y Tecnol. Ser. Conf. 2025, 4, 1456. [Google Scholar] [CrossRef]
- Díaz Vargas, D.; Leonario Rodríguez, M. Effectiveness of nutritional interventions on behavioral symptomatology of autism spectrum disorder: A systematic review. Nutr. Hosp. 2022, 39, 1378–1388. [Google Scholar]
- Correale, C.; Borgi, M.; Cirulli, F.; Laghi, F.; Trimarco, B.; Ferraro, M.; Venerosi, A. The Impact of Health and Social Services on the Quality of Life in Families of Adults with Autism Spectrum Disorder (ASD): A Focus Group Study. Brain Sci. 2022, 12, 177. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef]
- Byrska, A.; Błażejczyk, I.; Faruga, A.; Potaczek, M.; Wilczyński, K.M.; Janas-Kozik, M. Patterns of Food Selectivity among Children with Autism Spectrum Disorder. J. Clin. Med. 2023, 12, 5469. [Google Scholar] [CrossRef] [PubMed]
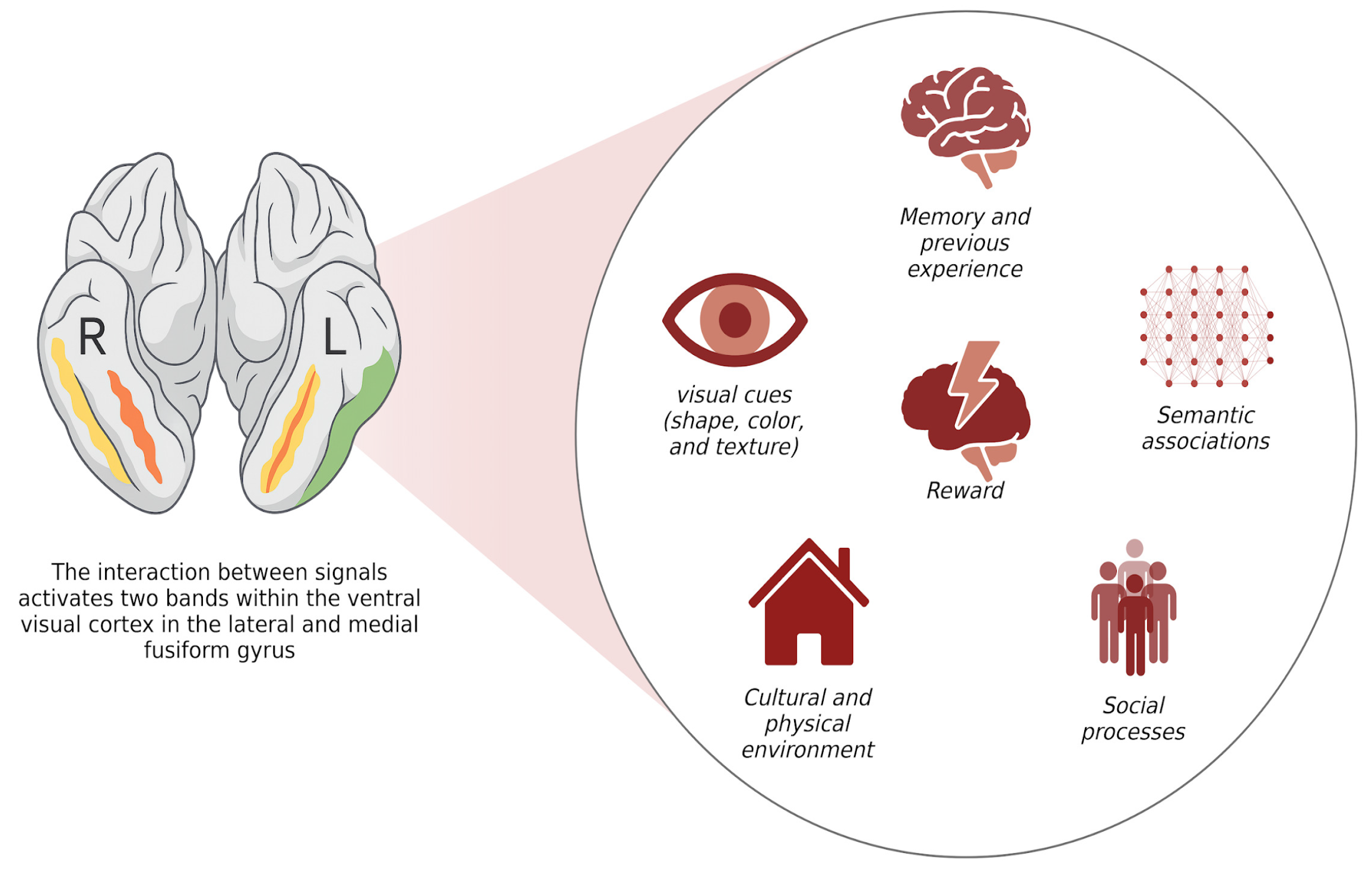
- Jain, N.; Wang, A.; Henderson, M.M.; Lin, R.; Prince, J.S.; Tarr, M.J.; Wehbe, L. Selectivity for food in human ventral visual cortex. Commun. Biol. 2023, 6, 175. [Google Scholar] [CrossRef]
- Esposito, M.; Mirizzi, P.; Fadda, R.; Pirollo, C.; Ricciardi, O.; Mazza, M.; Valenti, M. Food Selectivity in Children with Autism: Guidelines for Assessment and Clinical Interventions. Int. J. Environ. Res. Public Health 2023, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; Ikeda, Y. Dealing with food selectivity and mealtime behaviour in school-children with autism: A qualitative study of special education teachers in Japan. Int. J. Dev. Disabil. 2023, 69, 860–868. [Google Scholar] [CrossRef] [PubMed]
- de Paula Ivnuk, L.; Ferreira, M.C.; de Farias, O.F.; de Brito Bello, S.R.; Pazello, C.T.; dos Santos Rodrigues, S.G.; Silva, R.W. Seletividade alimentar infantil: Uma revisão integrativa. Res. Soc. Dev. 2023, 12, e130121244099. [Google Scholar] [CrossRef]
- Breda, C.; Santero, S.; Conti, M.V.; Cena, H. Programmes to manage food selectivity in individuals with autism spectrum disorder. Nutr. Res. Rev. 2025, 38, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Zetler, N.K.; Cermak, S.A.; Engel-Yeger, B.; Baranek, G.; Gal, E. Association Between Sensory Features and High-Order Repetitive and Restricted Behaviors and Interests Among Children With Autism Spectrum Disorder. Am. J. Occup. Ther. 2022, 76, 7603205010. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, P.; Burnette, C.B. Challenges and opportunities for conceptualizing intuitive eating in autistic people. Int. J. Eat. Disord. 2023, 56, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.V.; Morgan, L.; Hilton, C. Autism and Mental Health: The Role of Occupational Therapy. Am. J. Occup. Ther. 2023, 77, 7702170010. [Google Scholar] [CrossRef]
- Willman, R.; Hobbs, M.; Thomas, J.J. The potential role of occupational therapy in the treatment of avoidant/restrictive food intake disorder. Int. J. Eat. Disord. 2024, 57, 1985–1990. [Google Scholar] [CrossRef]
- Reche-Olmedo, L.; Torres-Collado, L.; Compañ-Gabucio, L.M.; Garcia-de-la-Hera, M. The Role of Occupational Therapy in Managing Food Selectivity of Children with Autism Spectrum Disorder: A Scoping Review. Children 2021, 8, 1024. [Google Scholar] [CrossRef]
- Riccio, M.P.; Marino, M.; Garotti, R.; Tassiello, A.; Maffettone, V.; Pezone, M.; Bravaccio, C. Food selectivity in Autism Spectrum Disorder: Implications of eating, sensory and behavioural profile. Front. Psychiatry 2025, 16, 1587454. [Google Scholar] [CrossRef]
- Zulkifli, M.N.; Kadar, M.; Fenech, M.; Hamzaid, N.H. Interrelation of food selectivity, oral sensory sensitivity, and nutrient intake in children with autism spectrum disorder: A scoping review. Res. Autism Spectr. Disord. 2022, 93, 101928. [Google Scholar] [CrossRef]
- Montiel-Nava, C.; Montenegro, M.C.; Ramirez, A.C.; Valdez, D.; Rosoli, A.; Garcia, R.; Garrido, G.; Cukier, S.; Rattazzi, A.; Paula, C.S. Age of autism diagnosis in Latin American and Caribbean countries. Autism 2024, 28, 58–72. [Google Scholar] [CrossRef]
- Adams, D.; Dargue, N.; Paynter, J. Longitudinal studies of challenging behaviours in autistic children and adults: A systematic review and meta-analysis. Clin. Psychol. Rev. 2023, 104, 102320. [Google Scholar] [CrossRef]


| n (%) | n (%) | n (%) | |||
|---|---|---|---|---|---|
| Sex | Physician | Nutritionist | |||
| Male | 33 (57.9) | Yes | 5 (8.8) | Yes | 0 (0.0) |
| Female | 24 (42.1) | No | 52 (91.2) | No | 57 (100) |
| Age | Occupational Therapy | Texture | |||
| 6 to 9 | 42 (76.7) | Yes | 21 (38.8) | Yes | 32 (56.1) |
| 10 to 12 | 15 (26.3) | No | 36 (63.2) | No | 25 (43.9) |
| ASD | Speech Therapist | Color | |||
| Yes | 32 (56.1) | Yes | 19 (33.3) | Yes | 20 (35.1) |
| No | 25 (43.9) | No | 38 (66.7) | No | 37 (64.9) |
| School Type | Psychologist | Smell | |||
| Traditional | 48 (84.2) | Yes | 20 (36.1) | Yes | 25 (43.9) |
| Special | 9 (15.8) | No | 37 (64.9) | No | 32 (56.1) |
| Teraphy | Physical Therapist | Ritual | |||
| Yes | 34 (59.6) | Yes | 2 (3.50) | Yes | 25 (43.9) |
| No | 23 (40.4) | No | 55 (96.5) | No | 32 (56.1) |
| ASD n (%) | Non ASD n (%) | OR | CI 95% | p Value | |
|---|---|---|---|---|---|
| Texture | |||||
| Yes | 25 (78.1) | 7 (28.0) | 9.18 | 2.78–27.5 | 0.0002 |
| No | 7 (21.9) | 18 (72.0) | 0.11 | 0.03–0.36 | *** |
| Color | |||||
| Yes | 17 (53.1) | 3 (12.0) | 8.31 | 2.07–29.4 | 0.0018 |
| No | 15 (46.9) | 22 (88.0) | 0.12 | 0.03–0.48 | ** |
| Smell | |||||
| Yes | 17 (53.1) | 8 (32.0) | 2.41 | 0.86–6.53 | 0.1783 |
| No | 15 (46.9) | 17 (68.0) | 0.42 | 0.15–1.16 | ns |
| Ritual | |||||
| Yes | 23 (71.9) | 2 (8.00) | 29.39 | 5.47–136.2 | <0.0001 |
| No | 9 (28.1) | 23 (92.0) | 0.03 | 0.01–0.18 | **** |
| ASD Mean (SD) | Non ASD Mean (SD) | p Value | ASD Mean (SD) | Non ASD Mean (SD) | p Value | ||
|---|---|---|---|---|---|---|---|
| Cereal grains | Legumes | ||||||
| Bread | 23.5 (8.4) | 23.8 (7.0) | 0.639 | Beans | 2.94 (2.9) | 3.04 (2.6) | 0.729 |
| Rice | 6.63 (5.6) | 7.44 (4.1) | 0.365 | Lentils | 3.53 (5.0) | 3.08 (2.0) | 0.802 |
| Potatoes | 8.94 (7.5) | 8.72 (5.3) | 0.618 | Chickpeas | 1.66 (3.3) | 1.00 (1.8) | 0.726 |
| Pasta | 10.8 (7.1) | 9.76 (5.9) | 0.639 | Green peas | 1.03 (2.3) | 2.36 (3.1) | 0.005 |
| Oat | 4.09 (7.5) | 4.08 (7.1) | 0.990 | ||||
| Lipids and Fats | |||||||
| Vegetables | Butter | 7.53 (9.8) | 7.08 (8.6) | 0.930 | |||
| Lettuce | 6.25 (9.5) | 11.6 (7.5) | 0.002 | Margarine | 2.63 (7.2) | 6.24 (9.9) | 0.048 |
| Tomatoes | 6.25 (9.3) | 10.0 (8.1) | 0.016 | Olive oil | 11.8 (12.7) | 8.80 (13.1) | 0.336 |
| Carrots | 9.94 (7.8) | 13.4 (9.7) | 0.182 | Sunflower oil | 13.9 (12.6) | 15.7 (13.6) | 0.714 |
| Cucumber | 4.75 (7.7) | 6.52 (7.8) | 0.132 | Avocado | 5.44 (7.6) | 11.0 (8.3) | 0.007 |
| Nuts | 4.97 (9.3) | 2.76 (4.1) | 0.776 | ||||
| Fruits | Olives | 1.97 (3.6) | 1.48 (2.7) | 0.928 | |||
| Banana | 8.28 (8.8) | 10.6 (7.8) | 0.130 | ||||
| Apple | 7.78 (8.7) | 9.96 (8.7) | 0.220 | Sugars & Snacks | |||
| Pear | 3.00 (4.5) | 2.40 (3.2) | 0.931 | Sacarose | 7.37 (11.5) | 15.4 (13.4) | 0.015 |
| Orange | 7.5 (10.3) | 8.72 (8.0) | 0.097 | Sweet Biscuits | 14.8 (9.4) | 18.5 (8.6) | 0.121 |
| RTEBC | 9.94 (9.1) | 9.84 (9.4) | 0.894 | ||||
| Dairy Products | Flavoured milk additives | 4.78 (10.4) | 7.68 (10.0) | 0.089 | |||
| Milk | 17.5 (13.1) | 18.9 (11.9) | 0.868 | Packaged potato chips | 7.25 (7.8) | 5.80 (4.4) | 0.823 |
| Yogurt | 17.2 (10.3) | 16.3 (8.9) | 0.572 | Savory crackers | 7.00 (8.9) | 5.76 (9.1) | 0.441 |
| Soft fresh cheese | 0.94 (3.8) | 1.68 (2.8) | 0.037 | Savoury snack sticks | 2.78 (4.5) | 2.52 (3.4) | 0.556 |
| Cheese | 9.53 (9.8) | 10.2 (7.8) | 0.481 | ||||
| Beverages | |||||||
| Animal Proteins | Water | 23.1 (10.4) | 27.4 (3.2) | 0.048 | |||
| Eggs | 8.88 (7.8) | 11.9 (8.3) | 0.175 | Carbonated | 4.09 (7.2) | 6.56 (6.2) | 0.005 |
| Chicken | 8.86 (5.1) | 10.4 (4.0) | 0.321 | Sweetened | 14.5 (12.1) | 14.8 (11.1) | 0.721 |
| Meat | 5.50 (5.1) | 9.12 (5.9) | 0.021 | ||||
| Pork | 1.19 (1.8) | 3.00 (2.5) | 0.003 | ||||
| Fish | 3.84 (4.4) | 5.48 (4.9) | 0.154 |
| Domain | r | 95% CI | R2 | p Value |
|---|---|---|---|---|
| Auditory (ASD) | 0.27 | (−0.09, 0.56) | 0.07 | 0.14 |
| Attentional (ASD) | 0.17 | (−0.19, 0.49) | 0.03 | 0.36 |
| Behavioural (ASD) | 0.21 | (−0.15, 0.52) | 0.04 | 0.26 |
| Oral (ASD) | 0.53 | (0.22, 0.74) | 0.28 | 0.002 |
| Socioemotional (ASD) | 0.51 | (0.20, 0.73) | 0.26 | 0.003 |
| Somatic (ASD) | 0.40 | (0.06, 0.66) | 0.16 | 0.025 |
| Tactile (ASD) | 0.33 | (−0.03, 0.61) | 0.11 | 0.069 |
| Visual (ASD) | 0.18 | (−0.19, 0.49) | 0.03 | 0.34 |
| Auditory (non-ASD) | 0.16 | (−0.25, 0.52) | 0.03 | 0.45 |
| Attentional (non-ASD) | 0.09 | (−0.31, 0.47) | 0.01 | 0.66 |
| Behavioural (non-ASD) | 0.24 | (−0.17, 0.58) | 0.06 | 0.24 |
| Oral (non-ASD) | 0.74 | (0.49, 0.88) | 0.55 | <0.001 |
| Socioemotional (non-ASD) | 0.29 | (−0.12, 0.61) | 0.08 | 0.16 |
| Somatic (non-ASD) | −0.27 | (−0.60, 0.15) | 0.07 | 0.20 |
| Tactile (non-ASD) | 0.12 | (−0.29, 0.49) | 0.01 | 0.57 |
| Visual (non-ASD) | 0.09 | (−0.32, 0.47) | 0.01 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, F.; Manzur, M.J.; Morales-Zepeda, D.; Flores, O.; Schwencke, C.; Leonario-Rodriguez, M. Food Pattern, Food Selectivity and Sensory Profile in Autism Spectrum Disorder: An Exploratory Analysis in Chilean Children. Children 2025, 12, 1560. https://doi.org/10.3390/children12111560
Mora F, Manzur MJ, Morales-Zepeda D, Flores O, Schwencke C, Leonario-Rodriguez M. Food Pattern, Food Selectivity and Sensory Profile in Autism Spectrum Disorder: An Exploratory Analysis in Chilean Children. Children. 2025; 12(11):1560. https://doi.org/10.3390/children12111560
Chicago/Turabian StyleMora, Fernanda, María José Manzur, David Morales-Zepeda, Oscar Flores, Constanza Schwencke, and Marcell Leonario-Rodriguez. 2025. "Food Pattern, Food Selectivity and Sensory Profile in Autism Spectrum Disorder: An Exploratory Analysis in Chilean Children" Children 12, no. 11: 1560. https://doi.org/10.3390/children12111560
APA StyleMora, F., Manzur, M. J., Morales-Zepeda, D., Flores, O., Schwencke, C., & Leonario-Rodriguez, M. (2025). Food Pattern, Food Selectivity and Sensory Profile in Autism Spectrum Disorder: An Exploratory Analysis in Chilean Children. Children, 12(11), 1560. https://doi.org/10.3390/children12111560






