Neonatal Pyruvate Kinase Deficiency Presenting with Severe Hemolytic Anemia and Liver Failure
Abstract
1. Introduction
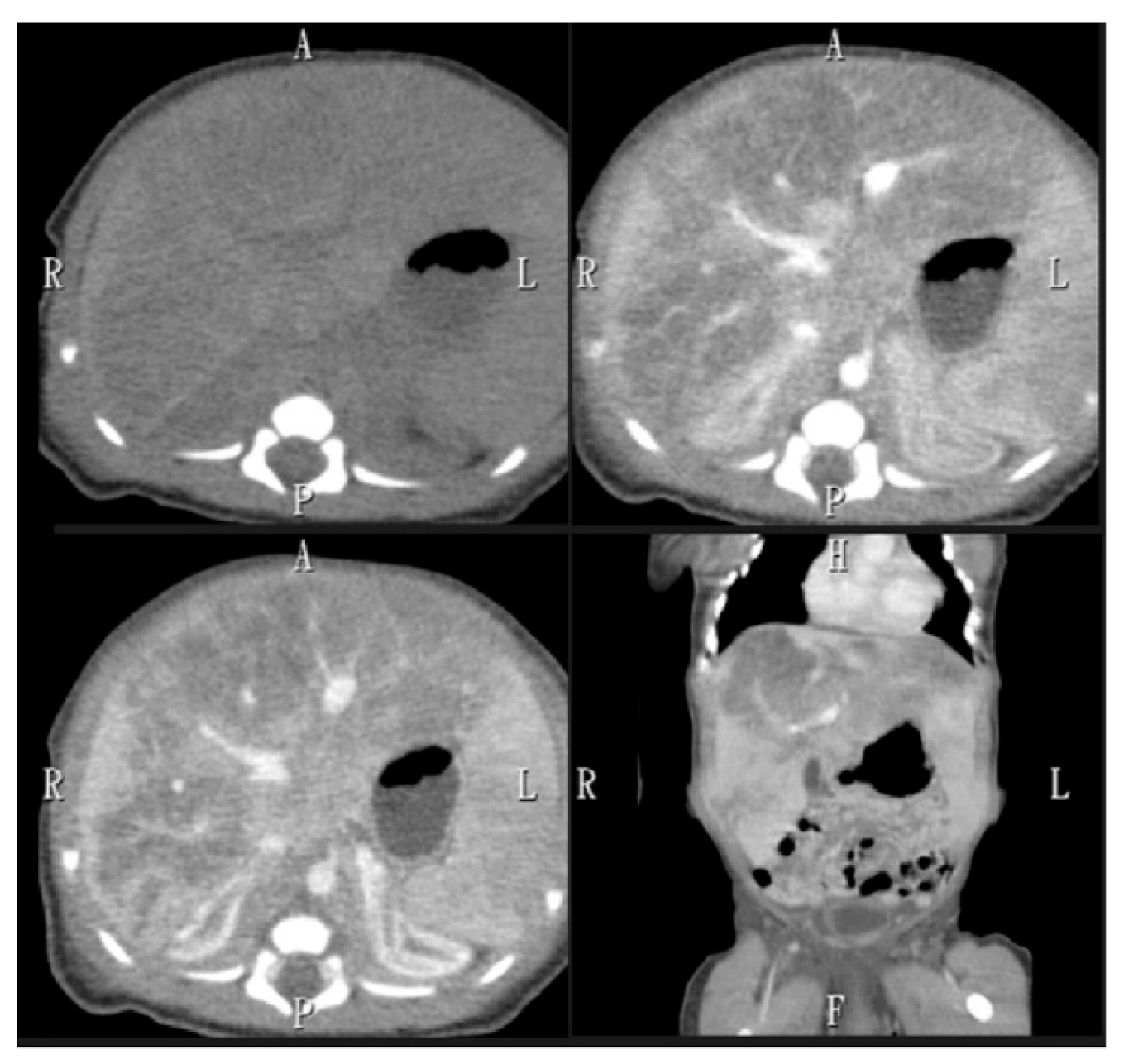
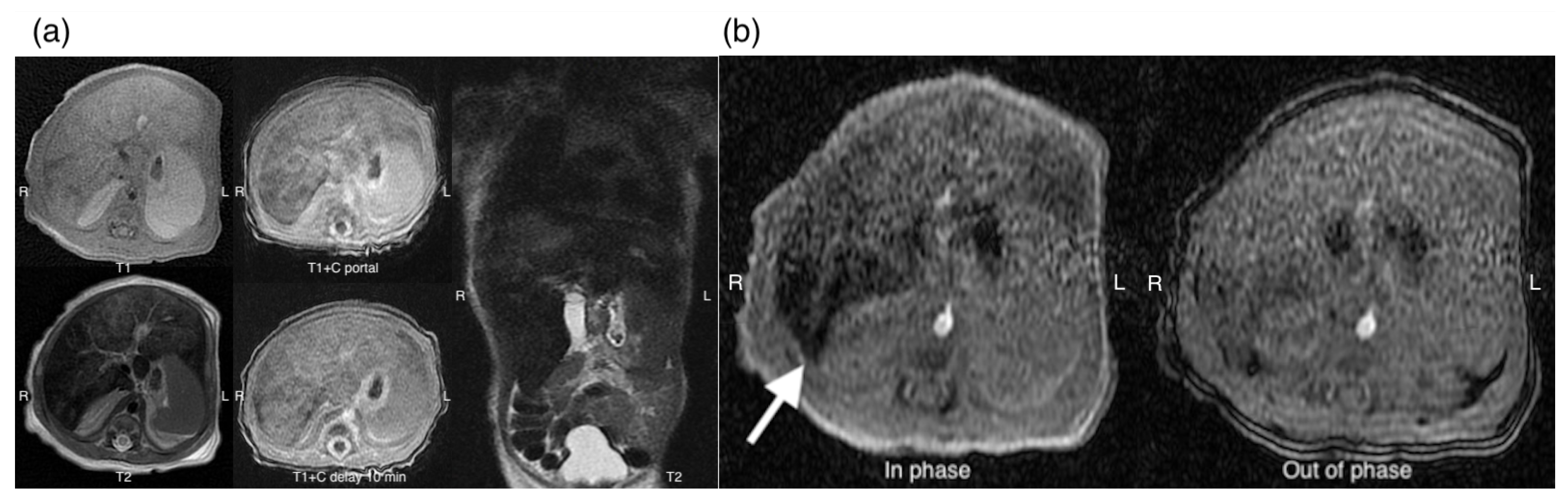
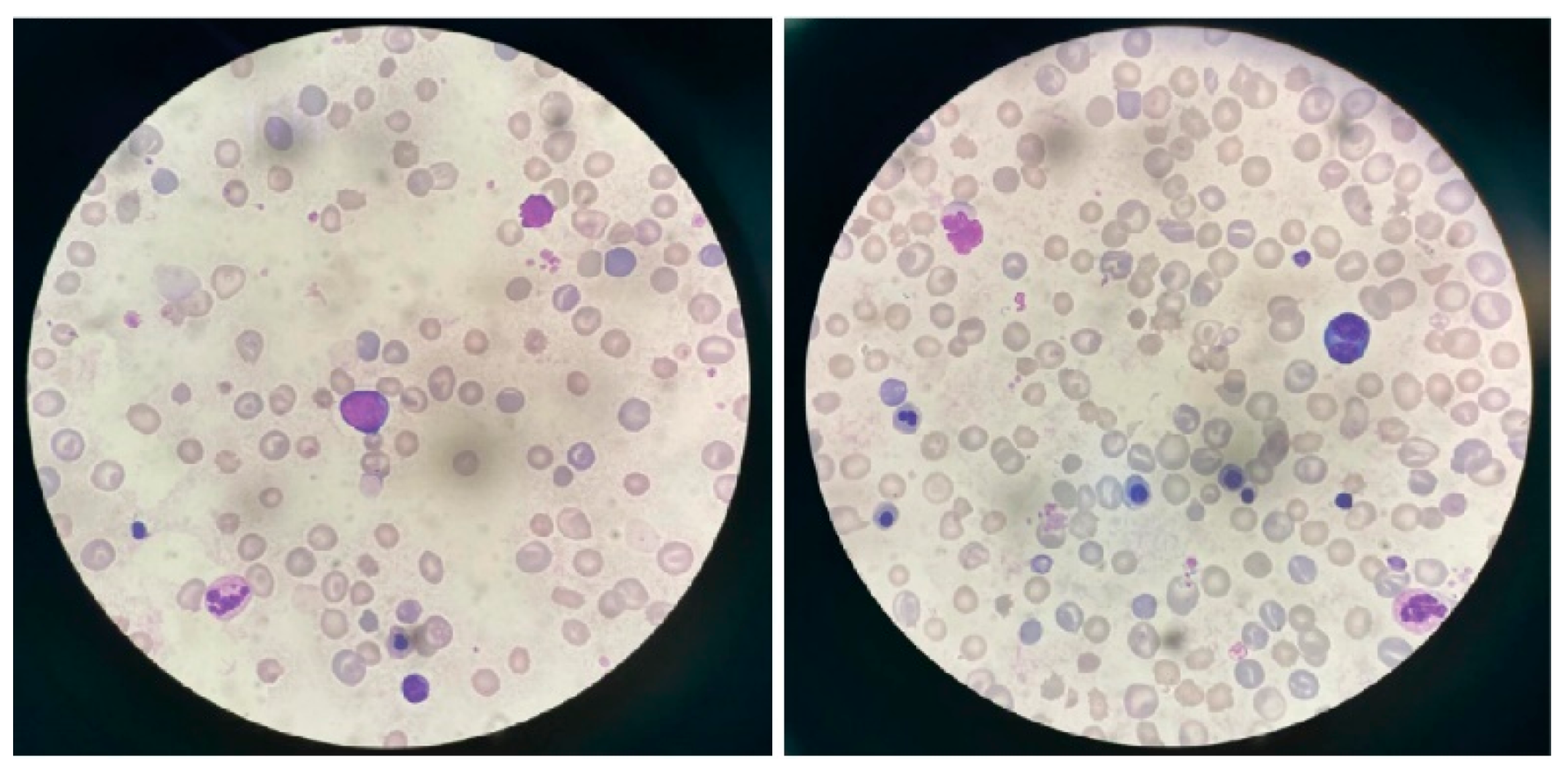
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PKD | Pyruvate Kinase Deficiency |
| GA | Gestational Age |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| RBC/HPF | Red Blood Cell Per High-Power Field |
| PT | Prothrombin Time |
| INR | International Normalized Ratio |
| LDH | Lactate Dehydrogenase |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| HHA | Hereditary Hemolytic Anemia |
| PK activity | Pyruvate Kinase Activity |
References
- Canu, G.; De Bonis, M.; Minucci, A.; Capoluongo, E. Red blood cell PK deficiency: An update of PK-LR gene mutation database. Blood Cells Mol. Dis. 2016, 57, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Rets, A.; Clayton, A.L.; Christensen, R.D.; Agarwal, A.M. Molecular diagnostic update in hereditary hemolytic anemia and neonatal hyperbilirubinemia. Int. J. Lab. Hematol. 2019, 41 (Suppl. 1), 95–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Shehata, N.; Lang-Robertson, K.; Bianchi, P.; Glenthøj, A.; Sheth, S.; Neufeld, E.J.; Rees, D.C.; Chonat, S.; Kuo, K.H.M.; et al. Diagnosis and management of pyruvate kinase deficiency: International expert guidelines. Lancet Haematol. 2024, 11, e228–e239. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.Y.; Yeh, T.C.; Huang, T.H.; Lin, P.C.; Liu, H.C.; Chang, J.G. A novel mutation of PKLR gene in a Taiwanese neonate initially presented with severe hemolytic anemia. Pediatr. Neonatol. 2020, 61, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Raphaël, M.F.; Van Wijk, R.; Schweizer, J.J.; Schouten-van Meeteren, N.A.; Kindermann, A.; van Solinge, W.W.; Smiers, F.J. Pyruvate kinase deficiency associated with severe liver dysfunction in the newborn. Am. J. Hematol. 2007, 82, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Yaish, H.M.; Christensen, R.D.; Lemons, R.S. Neonatal nonimmune hemolytic anemia. Curr. Opin. Pediatr. 2017, 29, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, V.; Manzoni, F.; Raffaeli, G.; Cavallaro, G.; Fattizzo, B.; Amelio, G.S.; Gulden, S.; Amodeo, I.; Giannotta, J.A.; Mosca, F.; et al. Severe presentation of congenital hemolytic anemias in the neonatal age: Diagnostic and therapeutic issues. Diagnostics 2021, 11, 1549. [Google Scholar] [CrossRef] [PubMed]
- Grace, R.F.; Barcellini, W. Management of pyruvate kinase deficiency in children and adults. Blood 2020, 136, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, X.; Liu, Y.; Liu, Y.; Sun, L.; Chen, F. PKM2, function and expression and regulation. Cell Biosci. 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, P.; Fermo, E.; Lezon-Geyda, K.; van Beers, E.J.; Morton, H.D.; Barcellini, W.; Glader, B.; Chonat, S.; Ravindranath, Y.; Newburger, P.E.; et al. Genotype-phenotype correlation and molecular heterogeneity in pyruvate kinase deficiency. Am. J. Hematol. 2020, 95, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Chartier, M.-E.; Hart, L.; Paganelli, M.; Ahmed, N.; Bilodeau, M.; Alvarez, F. Successful liver transplants for liver failure associated with pyruvate kinase deficiency. Pediatrics 2018, 141 (Suppl. 5), S385–S389. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.E.; Kyle, R.A.; Schoenfield, L.J. Increased serum conjugated bilirubin in hemolytic anemia. Postgrad. Med. 1974, 55, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Elkin, B.; Allende, D.S.; SenGupta, S. Acute-on-chronic liver failure and successful liver transplantation in pyruvate kinase deficiency. ACG Case Rep. J. 2023, 10, e01143. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, A.N.; Yan, Y.; Hedgeman, E.; van Beers, E.J.; Al-Samkari, H.; Barcellini, W.; Eber, S.W.; Glader, B.; Yaish, H.M.; Chonat, S.; et al. Comorbidities and complications in adults with pyruvate kinase deficiency. Eur. J. Haematol. 2021, 106, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hua, X.; Li, J.; Li, Y. Novel compound heterozygous PKLR mutation induced pyruvate kinase deficiency with persistent pulmonary hypertension in a neonate: A case report. Front. Cardiovasc. Med. 2022, 9, 872172. [Google Scholar] [CrossRef] [PubMed]
- Dulmovits, B.M.; Wild, K.T.; Flibotte, J.; Lambert, M.P.; Kwiatkowski, J.; Thom, C.S. Neonatal thrombocytopenia as a presenting finding in de novo pyruvate kinase deficiency. Neonatology 2023, 120, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Olivier, F.; Wieckowska, A.; Piedboeuf, B.; Alvarez, F. Cholestasis and hepatic failure in a neonate: A case report of severe pyruvate kinase deficiency. Pediatrics 2015, 136, e1366–e1368. [Google Scholar] [CrossRef] [PubMed]
| Gene | Physical Position | Transcript | Exon | Nucleotide (AA Change) | Zygosity | MAF | CADD Phred Score | ClinVar | ACMG Interpretation | Inheritance |
|---|---|---|---|---|---|---|---|---|---|---|
| PKLR | 1:155,294,335 | NM_000298.6 | Exon7 | c.1015dupG (p.Asp339GlyfsTer62) | Het | 8.96 × 10−6 | NA | NA | Pathogenic | Mother |
| PKLR | 1:155,295,116 | NM_000298.6 | Exon5 | c.694G>C (p.Gly232Arg) | Het | NA | 35 | Likely Pathogenic | Likely Pathogenic | Father |
| birth | 10 h/o | 24 h/o | 48 h/o | 72 h/o | 7 d/o | 1 m/o | 3 m/o | 1 y/o | 2 y/o | 3 y/o | |
| Hb (g/dL) | 8.5 | 11.6 | 10.3 | 9.2 | 9.9 | 8.2 | 4.7 | 8.4 | 9.3 | 8.0 | |
| Platelet (/µL) | 173,000 | 89,000 | 87,000 | 79,000 | 90,000 | 278,000 | 219,000 | 306,000 | 309,000 | 395,000 | |
| AST (IU/L) | 1462 | 4139 | 3001 | 1040 | 30 | 93 | 929 | 32 | 42 | 47 | |
| ALT (IU/L) | 333 | 506 | 361 | 283 | 54 | 69 | 401 | 24 | 59 | 32 | |
| Total bilirubin (mg/dL) | 15.6 | 16.6 | 16.9 | 13.5 | 12.2 | 11.1 | 45.8 | 1.5 | 1.8 | 2.4 | |
| Direct bilirubin (mg/dL) | 0.8 | 1.2 | 3.7 | 5.8 | 7.5 | 6.4 | 28.8 | 0.4 | 0.4 | 0.5 |
| GA (Weeks) | BBW | Clinical Manifestations | Associated Diagnosis | Thrombo-cytopenia | Hepatic Failure | Cholestasis | Diagnosis Method | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lin et al. [15] | 37 + 6 | 2.4 kg | Severe dyspnea, extreme anemia, skin pallor, jaundice, and hypoxemia | Heart failure, persistent pulmonary hypertension in the neonate (PPHN) | Yes | No | Yes | Whole-exome sequencing | Mechanical ventilator, RBC transfusion, nutrition support | No significant complications |
| Dulmovits et al. [16] | Term | 3.6 kg | Respiratory distress, abdominal distension, jaundice, hepatosplenomegaly, petechiae, anemia, and a diffuse blue macular rash | Incomplete neonatal Kawasaki disease | Yes | No | Yes | Whole-exome sequencing | Platelet transfusions, intensive phototherapy, double volume exchange transfusion, aspirin, IVIG | Stabilized, transfusion- dependent |
| Hou et al. [4] | NA | NA | Respiratory distress and anemia | NA | No | No | No | Whole-exome sequencing | RBC transfusion | Stabilized, transfusion- dependent |
| Olivier et al. [17] | Term | 3.4 kg | Hypotonic, generalized edema, and anemia | NA | Yes | Yes | Yes | PK enzyme level, genetic study | Blood transfusion, exchange transfusion | Died of sepsis at 3 months old |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-H.; Jiang, C.-B.; Hou, J.-Y.; Jim, W.-T.; Lin, S.-P.; Chang, S.-W.; Tseng, K.-T.; Lee, N.-C. Neonatal Pyruvate Kinase Deficiency Presenting with Severe Hemolytic Anemia and Liver Failure. Children 2025, 12, 1539. https://doi.org/10.3390/children12111539
Hsu Y-H, Jiang C-B, Hou J-Y, Jim W-T, Lin S-P, Chang S-W, Tseng K-T, Lee N-C. Neonatal Pyruvate Kinase Deficiency Presenting with Severe Hemolytic Anemia and Liver Failure. Children. 2025; 12(11):1539. https://doi.org/10.3390/children12111539
Chicago/Turabian StyleHsu, Yung-Han, Chuen-Bin Jiang, Jen-Yin Hou, Wai-Tim Jim, Shuan-Pei Lin, Szu-Wen Chang, Kai-Ti Tseng, and Ni-Chung Lee. 2025. "Neonatal Pyruvate Kinase Deficiency Presenting with Severe Hemolytic Anemia and Liver Failure" Children 12, no. 11: 1539. https://doi.org/10.3390/children12111539
APA StyleHsu, Y.-H., Jiang, C.-B., Hou, J.-Y., Jim, W.-T., Lin, S.-P., Chang, S.-W., Tseng, K.-T., & Lee, N.-C. (2025). Neonatal Pyruvate Kinase Deficiency Presenting with Severe Hemolytic Anemia and Liver Failure. Children, 12(11), 1539. https://doi.org/10.3390/children12111539






