Effect of Slow Versus Rapid Advancement of Enteral Feeding on Intestinal Oxygenation in Preterm Infants
Highlights
- Intermittent bolus feeding increased intestinal oxygenation, with a more pronounced percentage change observed during and after feeding in the rapid advancement group.
- No significant difference in gastrointestinal adverse outcomes, such as feeding intolerance or necrotizing enterocolitis, was found between the slow and rapid advancement groups.
- The use of abdominal near-infrared spectroscopy allowed continuous, real-time assessment of mesenteric oxygenation, demonstrating its potential value as a non-invasive monitoring tool in preterm infants.
- Rapid advancement of enteral feeding, when closely monitored clinically and/or with abdominal near-infrared spectroscopy, may be safely implemented without increasing the risk of gastrointestinal complications.
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Randomization
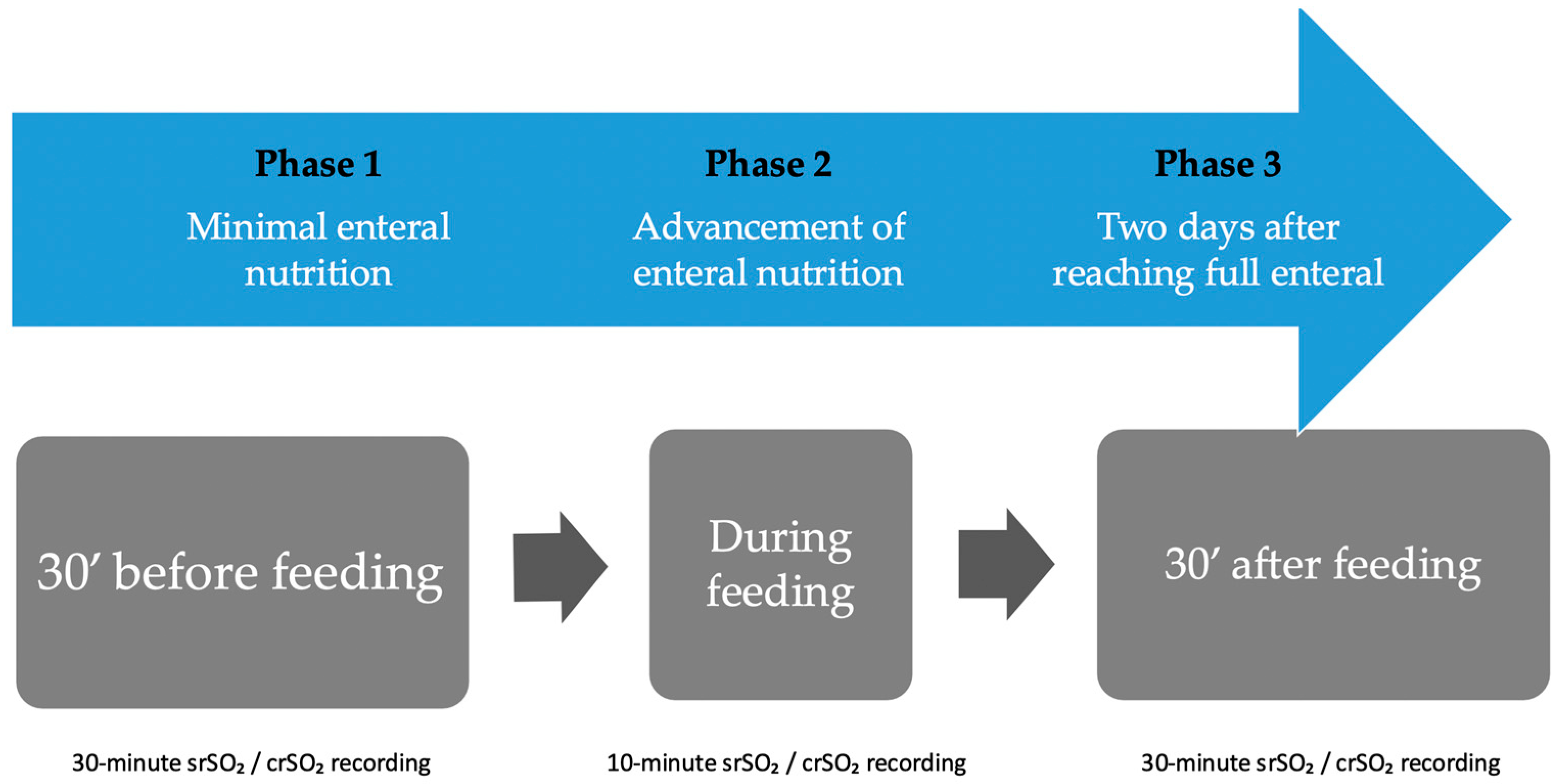
2.3. Intervention
2.4. Sample Size
2.5. Statistical Analysis
3. Results
3.1. Evaluation of srSO2 and SCOR Alterations in Group 1 and Group 2
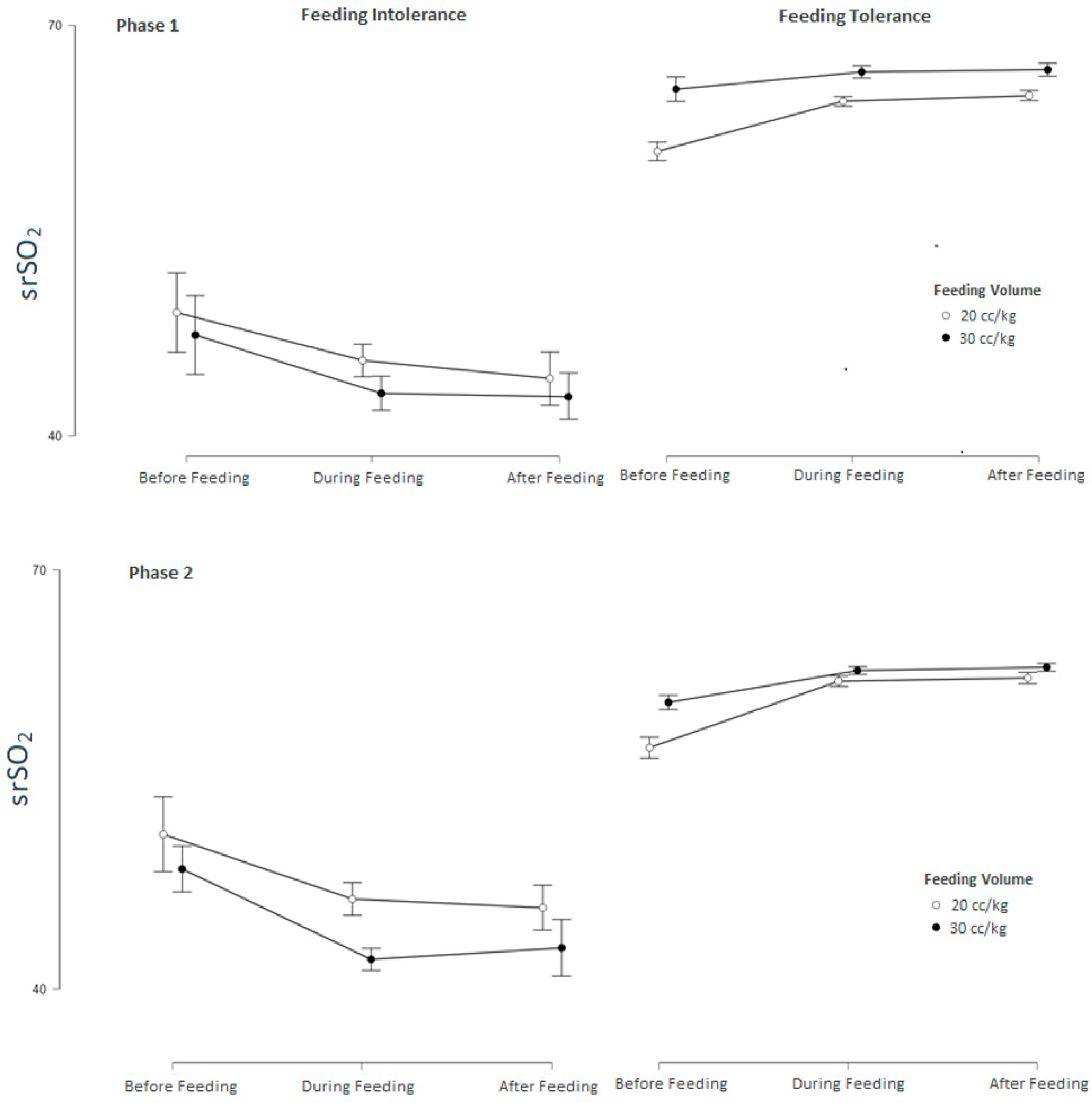
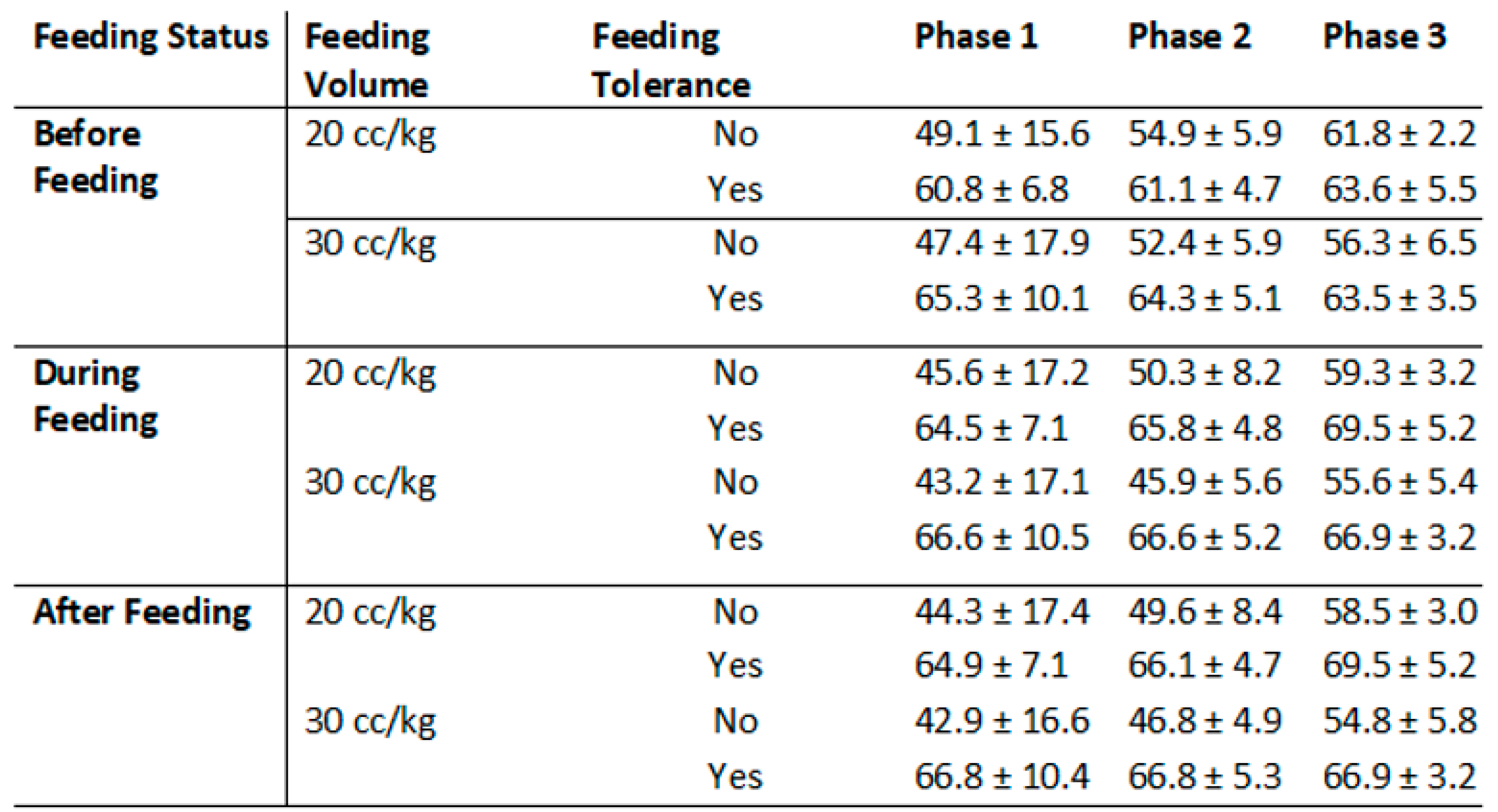
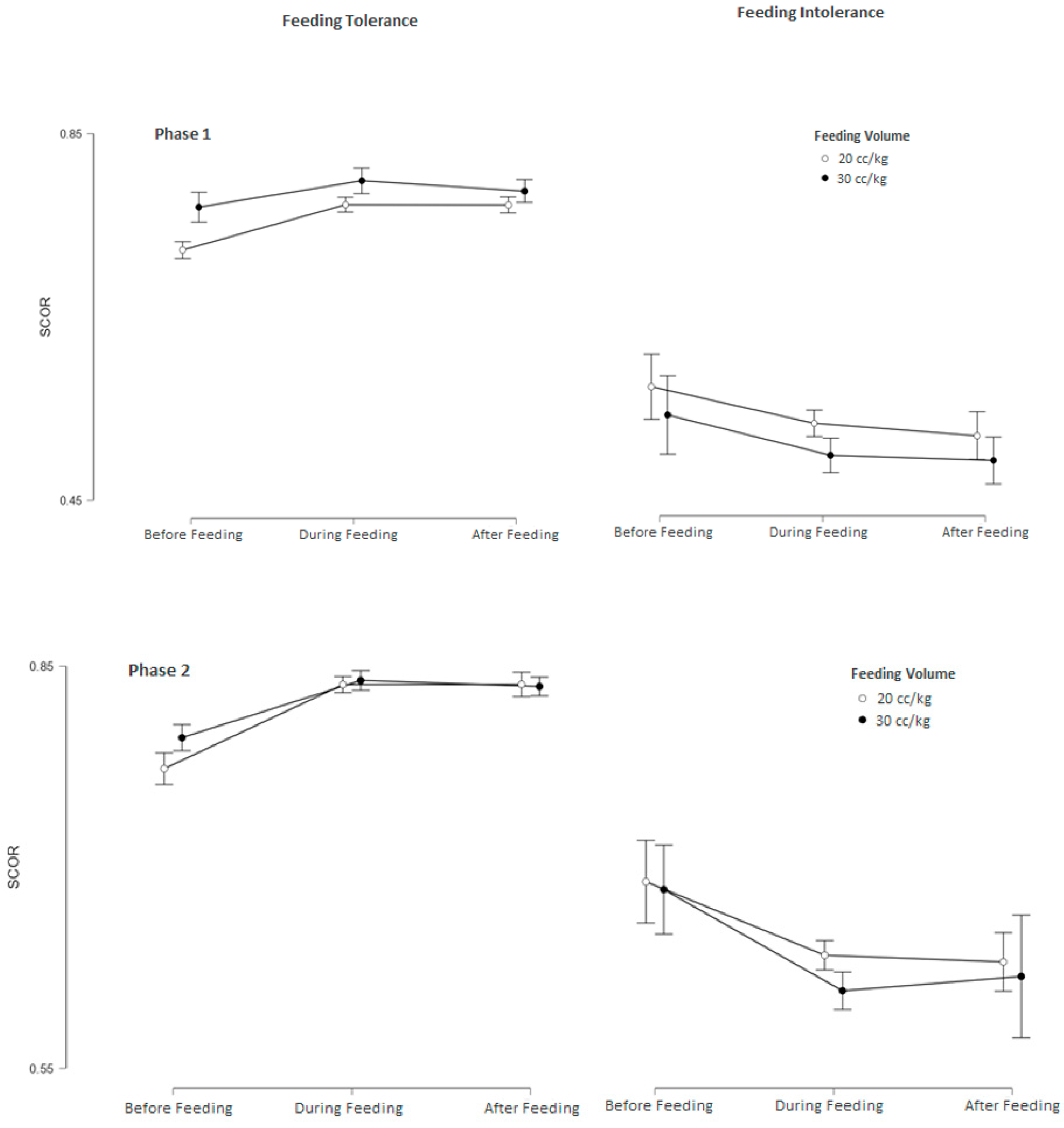
3.2. Subgroup Analysis of srSO2 and SCOR According to Feeding Tolerance vs. Feeding Intolerance in Groups 1 and 2
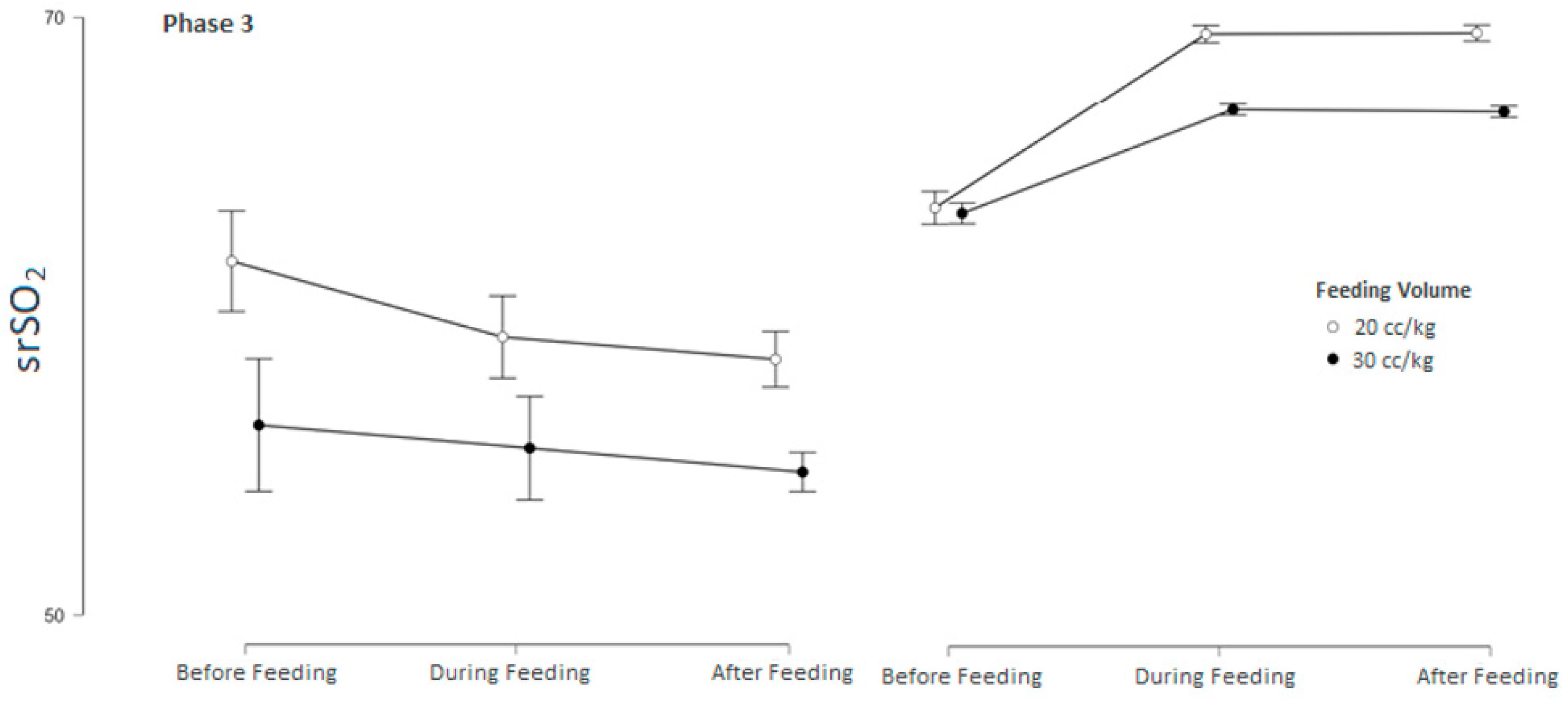
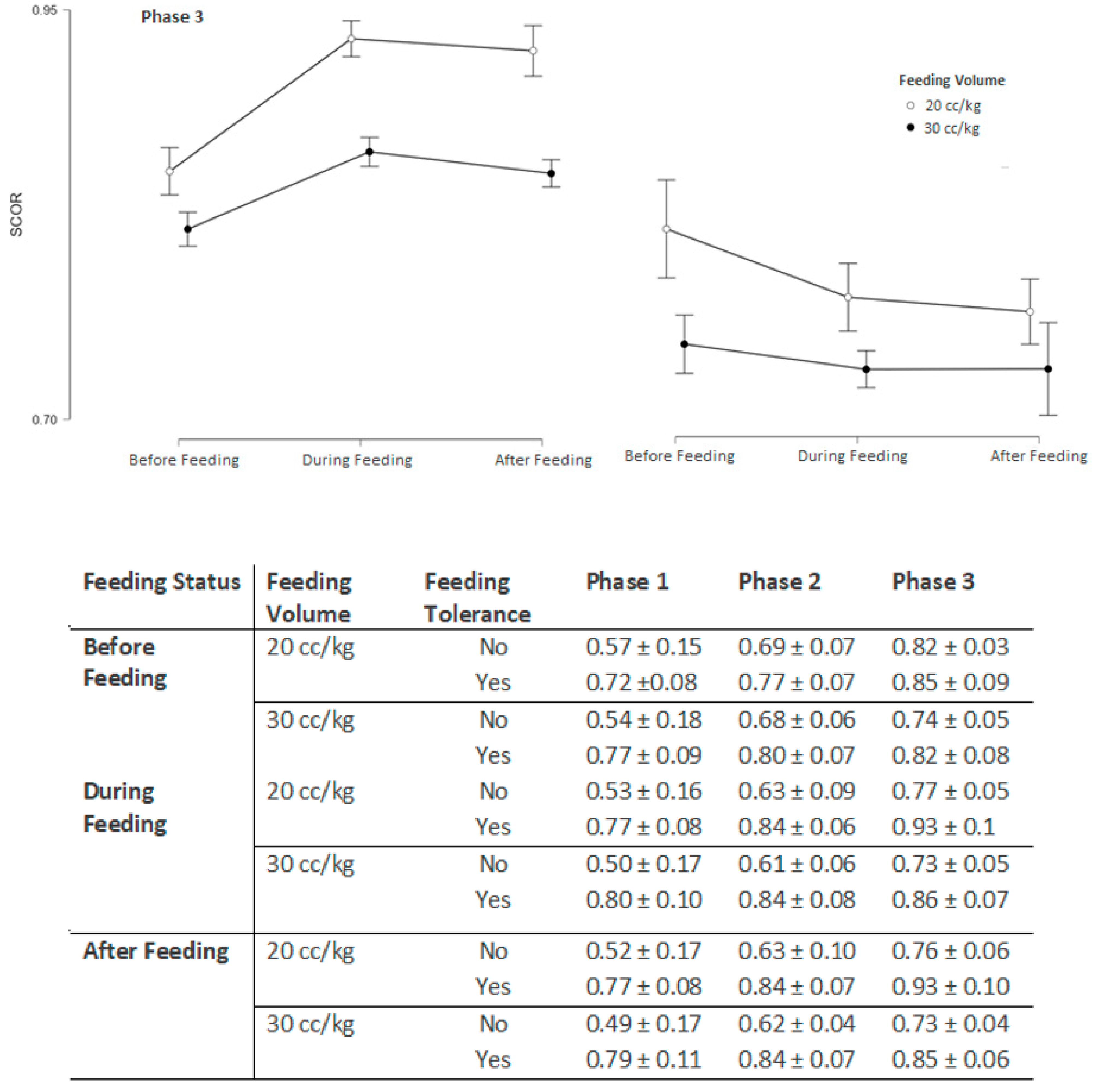
3.3. srSO2 and SCOR Changes Across Feeding Phases in Groups 1 and 2 Based on Feeding Tolerance vs. Feeding Intolerance
3.4. Estimating Feeding Tolerance Using a Composite ROC-Based Chart
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIRS | Near-infrared spectroscopy |
| SMA | Superior mesenteric artery |
| NEC | Necrotizing enterocolitis |
| srSO2 | Splanchnic oxygenation |
References
- Oddie, S.J.; Young, L.; McGuire, W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2017, 8, CD001241. [Google Scholar] [CrossRef]
- Oddie, S.J.; Young, L.; McGuire, W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2021, 8, CD001241. [Google Scholar] [CrossRef]
- Dorling, J.; Hewer, O.; Hurd, M.; Bari, V.; Bosiak, B.; Bowler, U.; King, A.; Linsell, L.; Murray, D.; Omar, O.; et al. Two speeds of increasing milk feeds for very preterm or very low-birthweight infants: The SIFT RCT. Health Technol. Assess. 2020, 24, 1–94. [Google Scholar]
- Dong, Y.; Speer, C.P. Late-onset neonatal sepsis: Recent developments. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F257–F263. [Google Scholar]
- Ramaswamy, V.V.; Bandyopadhyay, T.; Ahmed, J.; Bandiya, P.; Zivanovic, S.; Roehr, C.C. Enteral Feeding Strategies in Preterm Neonates ≤32 weeks Gestational Age: A Systematic Review and Network Meta-Analysis. Ann. Nutr. Metab. 2021, 77, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.; Brown, J.V.E.; Copperthwaite, B.R.; Oddie, S.J.; McGuire, W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 12, CD013542. [Google Scholar] [CrossRef] [PubMed]
- Berseth, C.L.; Bisquera, J.A.; Paje, V.U. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2003, 111, 529–534. [Google Scholar] [CrossRef] [PubMed]
- McKeown, R.E.; Marsh, T.D.; Amarnath, U.; Garrison, C.Z.; Addy, C.L.; Thompson, S.J.; Austin, J.L. Role of delayed feeding and of feeding increments in necrotizing enterocolitis. J. Pediatr. 1992, 121, 764–770. [Google Scholar] [CrossRef]
- Modi, M.; Ramji, S.; Jain, A.; Kumar, P.; Gupta, N. Early Aggressive Enteral Feeding in Neonates Weighing 750–1250 Grams: A Randomized Controlled Trial. Indian. Pediatr. 2019, 56, 294–298. [Google Scholar] [CrossRef]
- Brown, E.G.; Sweet, A.Y. Preventing necrotizing enterocolitis in neonates. JAMA 1978, 240, 2452–2454. [Google Scholar] [CrossRef]
- Henderson, G.; Craig, S.; Brocklehurst, P.; McGuire, W. Enteral feeding regimens and necrotising enterocolitis in preterm infants: A multicentre case-control study. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F120–F123. [Google Scholar]
- Uauy, R.D.; Fanaroff, A.A.; Korones, S.B.; Phillips, E.A.; Phillips, J.B.; Wright, L.L. Necrotizing enterocolitis in very low birth weight infants: Biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr. 1991, 119, 630–638. [Google Scholar]
- Patole, S.K.; de Klerk, N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: A systematic review and meta-analysis of observational studies. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F147–F151. [Google Scholar] [CrossRef]
- Ramani, M.; Ambalavanan, N. Feeding practices and necrotizing enterocolitis. Clin. Perinatol. 2013, 40, 1–10. [Google Scholar] [CrossRef]
- Senterre, T. Practice of enteral nutrition in very low birth weight and extremely low birth weight infants. World Rev. Nutr. Diet. 2014, 110, 201–214. [Google Scholar]
- Kuik, S.J.; van Zoonen, A.G.J.F.; Bos, A.F.; Van Braeckel, K.N.J.A.; Hulscher, J.B.F.; Kooi, E.M.W. The effect of enteral bolus feeding on regional intestinal oxygen saturation in preterm infants is age-dependent: A longitudinal observational study. BMC Pediatr. 2019, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.J.; Coombs, R.C.; Evans, D.H.; Levin, R.J. Effect of feed interval and feed type on splanchnic haemodynamics. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F49–F53. [Google Scholar] [PubMed]
- Hsu, C.H.; Lee, H.C.; Huang, F.Y. Duplex ultrasonographic assessment of gut blood flow velocity: Effect of meal composition in normal full-term newborns after first feed. J. Ultrasound Med. 1994, 13, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Gillam-Krakauer, M.; Cochran, C.M.; Slaughter, J.C.; Polavarapu, S.; McElroy, S.J.; Hernanz-Schulman, M.; Engelhardt, B. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J. Perinatol. 2013, 33, 609–612. [Google Scholar] [CrossRef]
- Marin, T.; Moore, J. Understanding near-infrared spectroscopy. Adv. Neonatal Care 2011, 11, 382–388. [Google Scholar] [CrossRef]
- Naulaers, G.; Meyns, B.; Miserez, M.; Leunens, V.; Van Huffel, S.; Casaer, P.; Weindling, M.; Devlieger, H. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology 2007, 92, 120–126. [Google Scholar]
- Cortez, J.; Gupta, M.; Amaram, A.; Pizzino, J.; Sawhney, M.; Sood, B.G. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J. Matern. Fetal Neonatal Med. 2011, 24, 574–582. [Google Scholar]
- Kültürsay, N.; Bilgen, H.; Türkyılmaz, C. Turkish Neonatal Society guideline on enteral feeding of the preterm infant. Turk. Pediatri Ars. 2018, 53 (Suppl. 1), S109–S118. [Google Scholar]
- Dave, V.; Brion, L.P.; Campbell, D.E.; Scheiner, M.; Raab, C.; Nafday, S.M. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J. Perinatol. 2009, 29, 213–218. [Google Scholar] [PubMed]
- Fang, S.; Kempley, S.T.; Gamsu, H.R. Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch. Dis. Child. Fetal Neonatal Ed. 2001, 85, F42–F45. [Google Scholar] [PubMed]
- Dix, L.M.; van Bel, F.; Lemmers, P.M. Monitoring Cerebral Oxygenation in Neonates: An Update. Front. Pediatr. 2017, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- da Costa, C.S.; Greisen, G.; Austin, T. Is near-infrared spectroscopy clinically useful in the preterm infant? Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F558–F561. [Google Scholar]
- Yavanoglu Atay, F.; Bozkurt, O.; Sahin, S.; Bidev, D.; Sari, F.N.; Uras, N. A Comparison of Slow Infusion Intermittent Feeding versus Gravity Feeding in Preterm Infants: A Randomized Controlled Trial. Children 2023, 10, 1389. [Google Scholar] [CrossRef]
- Lucchini, R.; Bizzarri, B.; Giampietro, S.; De Curtis, M. Feeding intolerance in preterm infants. How to understand the warning signs. J. Matern. Fetal Neonatal Med. 2011, 24 (Suppl. 1), 72–74. [Google Scholar] [CrossRef]
- Mihatsch, W.A.; von Schoenaich, P.; Fahnenstich, H.; Dehne, N.; Ebbecke, H.; Plath, C.; von Stockhausen, H.B.; Muche, R.; Franz, A.; Pohlandt, F. The significance of gastric residuals in the early enteral feeding advancement of extremely low birth weight infants. Pediatrics 2002, 109, 457–459. [Google Scholar] [CrossRef]
- Yang, W.C.; Fogel, A.; Lauria, M.E.; Ferguson, K.; Smith, E.R. Fast Feed Advancement for Preterm and Low Birth Weight Infants: A Systematic Review and Meta-analysis. Pediatrics 2022, 150 (Suppl. 1), e2022057092G. [Google Scholar] [CrossRef]
- Dani, C.; Pratesi, S.; Barp, J.; Bertini, G.; Gozzini, E.; Mele, L.; Parrini, L. Near-infrared spectroscopy measurements of splanchnic tissue oxygenation during continuous versus intermittent feeding method in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 652–656. [Google Scholar] [PubMed]
- Corvaglia, L.; Martini, S.; Battistini, B.; Rucci, P.; Aceti, A.; Faldella, G. Bolus vs. continuous feeding: Effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatr. Res. 2014, 76, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Lazar, D.A.; Burrin, D.G.; Smith, E.O.; Magliaro, T.J.; Stark, A.R.; Brandt, M.L.; Zamora, I.J.; Sheikh, F.; Akinkuotu, A.C.; et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr. Crit. Care Med. 2014, 15, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Palleri, E.; Wackernagel, D.; Wester, T.; Bartocci, M. Low Splanchnic Oxygenation and Risk for Necrotizing Enterocolitis in Extremely Preterm Newborns. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 401–406. [Google Scholar] [CrossRef]

| Groups 1 (n: 30) | Groups 2 (n: 30) | p | |
|---|---|---|---|
| Gestational age (weeks), mean ± SD | 29.8 ± 1.3 | 29.6 ± 0.2 | 0.555 |
| Birth weight (g), median (min–max) | 1193 (1100–1426) | 1405 (1199–1458) | 0.190 |
| Gender, (M) n (%) | 16 (53) | 14 (47) | 1 |
| Antenatal steroid, n (%) | 17 (57) | 18 (60) | 1 |
| APGAR at 5 min, median (min–max) | 8 (7–9) | 8 (7–8) | 0.780 |
| SNAPPE II, median (min–max) | 0 (0–4.3) | 0 (0–3.9) | 0.553 |
| Prolonged membrane rupture, n (%) | 3 (10.0) | 4 (13.3) | 0.127 |
| Chorioamnionitis, n (%) | 0 | 1 (3.3) | 1 |
| Gestational hypertension, n (%) | 7 (23.3) | 4 (13.3) | 0.313 |
| Preeclampsia, n (%) | 10 (33.3) | 6 (20.0) | 0.237 |
| Caffeine, n (%) | 21 (70.0) | 16 (53.3) | 0.158 |
| Surfactant, n (%) | 19 (63.3) | 16 (56.6) | 0.132 |
| Invasive mechanical ventilation duration (days), median (min–max) | 2 (0–5.3) | 1 (0–3.3) | 0.15 |
| Non-invasive mechanical ventilation duration (days), median (min–max) | 5 (3–13) | 6 (2.8–12.5) | 0.889 |
| Stage ≥ 2 NEC, n (%) | 0 | 0 | N/A * |
| Late onset sepsis, n (%) | 5 (16.7) | 6 (20.0) | 0.217 |
| HsPDA, n (%) | 5 (16.7) | 4 (13.3) | 0.716 |
| Stage 3 ≥ ROP, n (%) | 0 | 0 | N/A |
| Stage 2 ≥ IVH, n (%) | 0 | 0 | N/A |
| Exclusive breastfeeding, n (%) | 21 (70.0) | 23 (76.6) | 0.771 |
| Minimal enteral feeding duration (days), mean ± SD | 2.8 ± 1.7 | 2.3 ± 0.9 | 0.184 |
| Feeding intolerance, n (%) | 8 (26.6) | 6 (20.0) | 0.192 |
| Full enteral feeding transition duration (days), median (min–max) | 9 (7.8–11.1) | 7 (6.3–9.2) | 0.005 |
| Hospital stay (days), mean ± SD | 41.2 ± 18.5 | 40.9 ± 19.9 | 0.955 |
| Mortality, n (%) | 0 | 0 | N/A |
| Groups 1 | Groups 2 | p | |
|---|---|---|---|
| Phase 1 | |||
| Pre-feeding (baseline) srSO2 | 57.51 ± 11.23 | 59.81 ± 12.82 | 0.13 |
| During-feeding srSO2 | 59.14 ± 13.72 | 60.35 ± 14.06 | 0.26 |
| After-feeding srSO2 | 59.08 ± 14.35 | 60.42 ± 14.07 | 0.25 |
| Percentage Change (baseline to during-after feeding) | 2.78 ± 0.86 | 2.54 ± 0.92 | 0.73 |
| Pre-feeding (baseline) SCOR | 0.71 ± 0.13 | 0.72 ± 0.12 | 0.12 |
| During-feeding SCOR | 0.75 ± 0.25 | 0.76 ± 0.27 | 0.23 |
| After-feeding SCOR | 0.76 ± 0.28 | 0.77 ± 0.24 | 0.29 |
| Percentage Change (baseline to during-after feeding) | 2.92 ± 1.34 | 3.53 ± 1.42 | 0.9 |
| Phase 2 | |||
| Pre-feeding (baseline) srSO2 | 59.34 ± 5.74 | 60.61 ± 6.62 | 0.42 |
| During-feeding srSO2 | 62.45 ± 9.23 | 66.75 ± 9.07 | 0.07 |
| After-feeding srSO2 | 62.83 ± 9.53 | 66.92 ± 8.85 | 0.08 |
| Percentage Change (baseline to during-after feeding) | 5.25 ± 2.16 | 10.14 ± 3.24 | 0.03 |
| Pre-feeding (baseline) SCOR | 0.74 ± 0.13 | 0.73 ± 0.12 | 0.96 |
| During-feeding SCOR | 0.81 ± 0.11 | 0.85 ± 0.11 | 0.06 |
| After-feeding SCOR | 0.81 ± 0.12 | 0.87 ± 0.12 | 0.06 |
| Percentage Change (baseline to during-after feeding) | 3.93 ± 2.34 | 11.97 ± 3.26 | 0.02 |
| Phase 3 | |||
| Pre-feeding (baseline) srSO2 | 62.26 ± 4.93 | 62.74 ± 4.46 | 0.71 |
| During-feeding srSO2 | 69.94 ± 6.63 | 72.75 ± 5.07 | 0.06 |
| After-feeding srSO2 | 69.75 ± 6.87 | 72.84 ± 5.23 | 0.06 |
| Percentage Change (baseline to during-after feeding) | 12.42 ± 3.45 | 15.91 ± 4.85 | 0.02 |
| Pre-feeding (baseline) SCOR | 0.70 ± 0.08 | 0.74 ± 0.07 | 0.77 |
| During-feeding SCOR | 0.84 ± 0.11 | 0.86 ± 0.08 | 0.07 |
| After-feeding SCOR | 0.83 ± 0.12 | 0.88 ± 0.07 | 0.08 |
| Percentage Change (baseline to during-after feeding) | 13.34 ± 5.21 | 16.04 ± 5.41 | 0.04 |
| Feeding Intolerance (n: 14) | Feeding Tolerance (n: 46) | p | |
|---|---|---|---|
| Phase 1 | |||
| Pre-feeding (baseline) srSO2 | 48.53 ± 15.64 | 63.34 ± 9.04 | 0.008 |
| During-feeding srSO2 | 44.83 ± 16.46 | 65.65 ± 9.12 | 0.001 |
| After-feeding srSO2 | 43.84 ± 16.42 | 65.91 ± 9.06 | <0.001 |
| Pre-feeding (baseline) SCOR | 0.57 ± 0.15 | 0.72 ± 0.08 | <0.001 |
| During-feeding SCOR | 0.54 ± 0.18 | 0.77 ± 0.09 | <0.001 |
| After-feeding SCOR | 0.53 ± 0.165 | 0.77 ± 0.10 | <0.001 |
| Phase 2 | |||
| Pre-feeding (baseline) srSO2 | 54.15 ± 5.73 | 62.91 ± 5.17 | <0.001 |
| During-feeding srSO2 | 48.83 ± 7.52 | 66.34 ± 5.08 | <0.001 |
| After-feeding srSO2 | 48.71 ± 7.35 | 66.52 ± 5.07 | <0.001 |
| Pre-feeding (baseline) SCOR | 0.69 ± 0.07 | 0.77 ± 0.07 | <0.001 |
| During-feeding SCOR | 0.68 ± 0.06 | 0.80 ± 0.07 | <0.001 |
| After-feeding SCOR | 0.63 ± 0.09 | 0.84 ± 0.06 | <0.001 |
| Phase 3 | |||
| Pre-feeding (baseline) srSO2 | 60.25 ± 4.42 | 63.51 ± 4.53 | 0.035 |
| During-feeding srSO2 | 58.23 ± 4.15 | 68.16 ± 4.47 | <0.001 |
| After-feeding srSO2 | 57.43 ± 4.17 | 68.13 ± 4.45 | <0.001 |
| Pre-feeding (baseline) SCOR | 0.82 ± 0.03 | 0.85 ± 0.084 | 0.165 |
| During-feeding SCOR | 0.74 ± 0.05 | 0.82 ± 0.09 | <0.001 |
| After-feeding SCOR | 0.77 ± 0.05 | 0.88 ± 0.09 | <0.001 |
| (a) | |||||||||||
| Cutpoint | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | Metric Score | |||||
| 0 | 100% | 0% | 21.43% | NaN% | 0.989 | 1 | |||||
| 1 | 100% | 54.55% | 37.50% | 100% | 0.989 | 1.55 | |||||
| 2 | 100% | 90.91% | 75% | 100% | 0.989 | 1.91 | |||||
| 3 | 75% | 100% | 100% | 93.62% | 0.989 | 1.75 | |||||
| 4 | 58.33% | 100% | 100% | 89.80% | 0.989 | 1.58 | |||||
| (b) | |||||||||||
| srSO2 Baseline | |||||||||||
| Cutpoint | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | ||||||
| 52 | 100% | 66.67% | 91.67% | 100% | 0.805 | ||||||
| 52.5 | 97.73% | 66.67% | 91.49% | 88.89% | 0.805 | ||||||
| 55 | 88.64% | 75% | 92.86% | 64.29% | 0.805 | ||||||
| BW | |||||||||||
| Cutpoint | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | ||||||
| 1180 | 84.09% | 91.67% | 97.37% | 61.11% | 0.918 | ||||||
| 1195 | 79.55% | 100% | 100% | 57.14% | 0.918 | ||||||
| 1205 | 77.27% | 100% | 100% | 54.55% | 0.918 | ||||||
| 1245 | 75% | 100% | 100% | 52.17% | 0.918 | ||||||
| GW | |||||||||||
| Cutpoint | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | ||||||
| 30 | 63.64% | 100% | 100% | 42.86% | 0.811 | ||||||
| SCOR Baseline | |||||||||||
| Cutpoint | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | ||||||
| 0.6318 | 0.9773 | 0.7500 | 0.9348 | 0.9000 | 0.8470 | ||||||
| 0.6322 | 0.9545 | 0.7500 | 0.9333 | 0.8182 | 0.8470 | ||||||
| 0.6353 | 0.9318 | 0.7500 | 0.9318 | 0.7500 | 0.8470 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozdemir, H.; Gulcan Kersin, S.; Can Buker, H.S.; Cetinkaya, M.; Kandemir, I.; Memisoglu, A.; Bilgen, H.S. Effect of Slow Versus Rapid Advancement of Enteral Feeding on Intestinal Oxygenation in Preterm Infants. Children 2025, 12, 1527. https://doi.org/10.3390/children12111527
Ozdemir H, Gulcan Kersin S, Can Buker HS, Cetinkaya M, Kandemir I, Memisoglu A, Bilgen HS. Effect of Slow Versus Rapid Advancement of Enteral Feeding on Intestinal Oxygenation in Preterm Infants. Children. 2025; 12(11):1527. https://doi.org/10.3390/children12111527
Chicago/Turabian StyleOzdemir, Hulya, Sinem Gulcan Kersin, Halime Sema Can Buker, Merih Cetinkaya, Ibrahim Kandemir, Asli Memisoglu, and Hulya Selva Bilgen. 2025. "Effect of Slow Versus Rapid Advancement of Enteral Feeding on Intestinal Oxygenation in Preterm Infants" Children 12, no. 11: 1527. https://doi.org/10.3390/children12111527
APA StyleOzdemir, H., Gulcan Kersin, S., Can Buker, H. S., Cetinkaya, M., Kandemir, I., Memisoglu, A., & Bilgen, H. S. (2025). Effect of Slow Versus Rapid Advancement of Enteral Feeding on Intestinal Oxygenation in Preterm Infants. Children, 12(11), 1527. https://doi.org/10.3390/children12111527






