Analysis of Clinical Characteristics of Refractory Food Protein-Induced Allergic Proctocolitis
Highlights
- Refractory food protein-induced allergic proctocolitis (FPIAP) is associated with prolonged symptoms, growth retardation, and dietary intolerance.
- Non-early-onset FPIAP cases showed significantly more growth issues, lower hemoglobin levels, and higher corticosteroid use than early-onset cases.
- Early identification of FPIAP onset may help reduce the risk of growth delays and the need for corticosteroids.
- Individualized treatment strategies are needed to improve long-term outcomes and food tolerance in children with refractory FPIAP.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patients’ Selection
3.2. Demographic Data
3.3. Clinical Data
| Indicator | Value | Indicator | Value |
|---|---|---|---|
| Gender [No. (%)] Male 0–3 months 4–6 months 7–9 months Female | 25 (71.4%) 14 (70.0%) 5 (62.5%) 6 (85.7%) 10 (28.6%) | Select Amino acid formula proportion Breastfeeding Mixed feeding Formula feeding | 23/24 (95.8%) 8/9 (88.9%) 2/2 (100%) |
| Age distribution of symptom onset [No. (%)] 0–3 months 4–6 months 7–9 months | 20 (57.1%) 8 (22.9%) 7 (20%) | Symptoms occurrence [No. (%)] Before complementary food introduction After complementary food introduction | 30 (85.7%) 5 (14.3%) |
| Age of symptom onset [M (P25, P75), months] | 3 (1, 6) | Family history of allergic diseases [No. (%)] | 11 (31.4%) |
| Hospitalization age [M (P25, P75), months] | 12 (9, 22) | Complementary food introduction time [M (P25, P75), months] | 6 (6, 8) |
3.4. Laboratory Tests
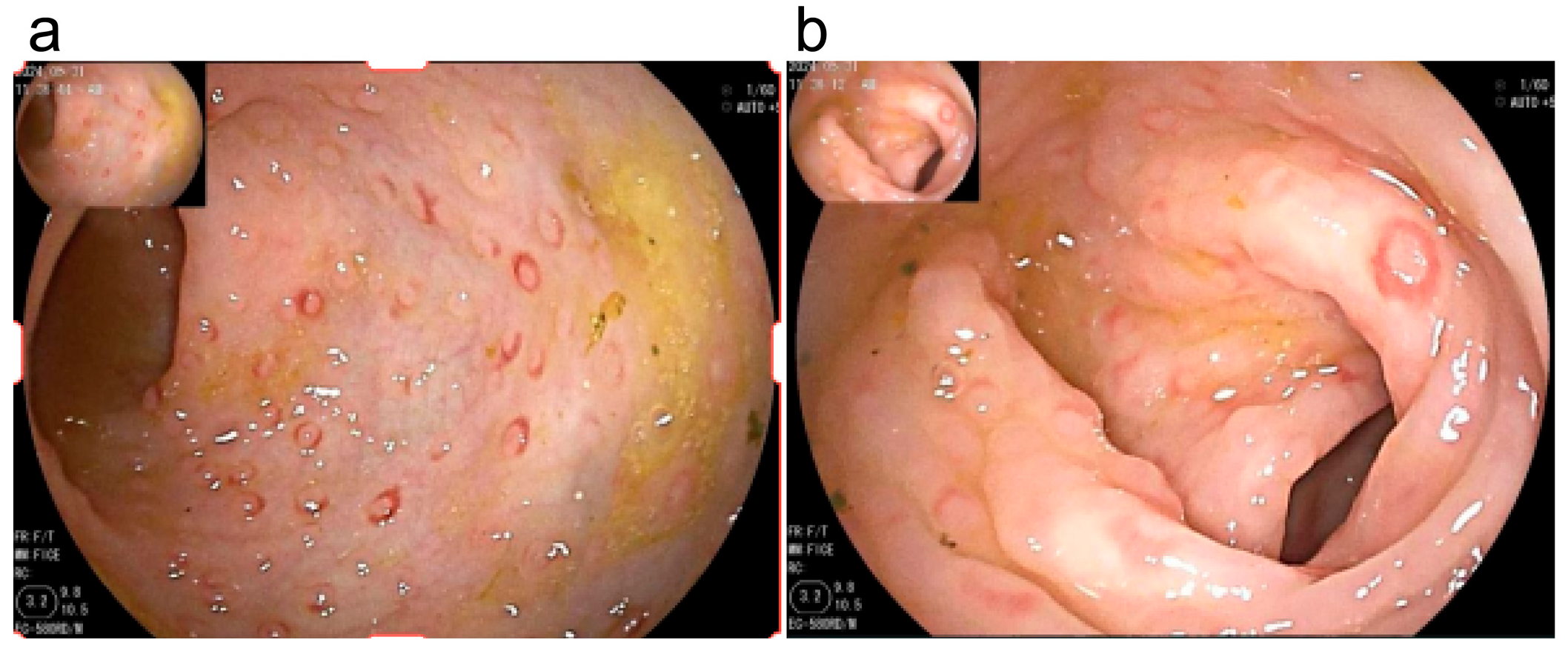
3.5. Endoscopic Examination
3.6. Treatment and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMV | Cytomegalovirus |
| DNA | Deoxyribonucleic Acid |
| EBV | Epstein–Barr Virus |
| EOG | Early-Onset Group |
| ESPGHAN | European Society for Paediatric Gastroenterology, Hepatology and Nutrition |
| FPIAP | Food Protein-Induced Allergic Proctocolitis |
| IgE | Immunoglobulin E |
| LNH | Lymphonodular Hyperplasia |
| nEOG | Non-Early-Onset Group |
| SPSS | Statistical Package for the Social Sciences |
| TNF-α | Tumor Necrosis Factor Alpha |
| T-SPOT | Tuberculin Infection T-cell Test |
| VEO-IBD | Very Early-Onset Inflammatory Bowel Disease |
References
- Vandenplas, Y.; Broekaert, I.; Domellöf, M.; Indrio, F.; Lapillonne, A.; Pienar, C.; Ribes-Koninckx, C.; Shamir, R.; Szajewska, H.; Thapar, N.; et al. An ESPGHAN position paper on the diagnosis, management, and prevention of Cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 386–413. [Google Scholar] [CrossRef]
- Barni, S.; Pessina, B.; Fioretti, L.; Scarallo, L.; Di Siena, A.; Bramuzzo, M.; Liccioli, G.; Sarti, L.; Tomei, L.; Giovannini, M.; et al. Food Protein-Induced Allergic Proctocolitis: Real-World Experience from an Italian Cohort. Nutrients 2025, 17, 98. [Google Scholar] [CrossRef]
- Uncuoğlu, A.; Aydoğan, M.; Şimşek, I.E.; Çöğürlü, M.T.; Uçak, K.; Acar, H.C. A prospective assessment of clinical characteristics and responses to dietary elimination in food protein-induced allergic proctocolitis. J. Allergy Clin. Immunol. Pract. 2022, 10, 206–214.e1. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Meyer, R.; Hoffman, I.; Alliet, P.; De Greef, E.; Hauser, B.; Huysentruyt, K. Discrepancies in the Diagnosis, Treatment and Prevention of Cow Milk Allergy Among Paediatricians. Acta Paediatr. 2025, 114, 3000–3012. [Google Scholar] [CrossRef]
- Mennini, M.; Fiocchi, A.G.; Cafarotti, A.; Montesano, M.; Mauro, A.; Villa, M.P.; Di Nardo, G. Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organ. J. 2020, 13, 100471. [Google Scholar] [CrossRef]
- Cetinkaya, P.G.; Kahveci, M.; Karaatmaca, B.; Esenboga, S.; Sahiner, U.M.; Sekerel, B.E.; Soyer, O. Predictors for late tolerance development in food protein-induced allergic proctocolitis. Allergy Asthma Proc. 2020, 41, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Folegatti, A.; Ferrigno, C.; Pensabene, L.; Agosti, M.; D’Auria, E. To diet or not to diet: This is the question in food-protein-induced allergic proctocolitis (FPIAP)-a comprehensive review of current recommendations. Nutrients 2024, 16, 589. [Google Scholar] [CrossRef] [PubMed]
- Mennini, M.; Felici, E.; Di Nardo, G. The need for standardization in the diagnosis and management of food protein-induced allergic proctocolitis (FPIAP): The time has come to act. Minerva Pediatr. 2025, 77, 7–9. [Google Scholar] [CrossRef]
- Li, Z.; Gong, S. The Subspecialty Group of Gastroenterology, the Society of Pediatrics, Chinese Medical Association. Expert consensus of food allergic gastrointestinal disease. Zhonghua Er Ke Za Zhi 2017, 55, 487–492. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Amil-Dias, J.; Auth, M.K.H.; Chehade, M.; Collins, M.H.; Gupta, S.K.; Gutiérrez-Junquera, C.; Orel, R.; Vieira, M.C.; Zevit, N.; et al. Joint ESPGHAN/NASPGHAN guidelines on childhood eosinophilic gastrointestinal disorders beyond eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 122–152. [Google Scholar] [CrossRef]
- Senocak, N.; Ertugrul, A.; Ozmen, S.; Bostanci, I. Clinical features and clinical course of food protein-induced allergic proctocolitis: 10-year experience of a tertiary medical center. J. Allergy Clin. Immunol. Pract. 2022, 10, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Xanthakos, S.A.; Schwimmer, J.B.; Melin-Aldana, H.; Rothenberg, M.E.; Witte, D.P.; Cohen, M.B. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: A prospective cohort study. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 16–22. [Google Scholar] [CrossRef]
- Arvola, T.; Ruuska, T.; Keränen, J.; Hyöty, H.; Salminen, S.; Isolauri, E. Rectal bleeding in infancy: Clinical, allergological, and microbiological examination. Pediatrics 2006, 117, e760–e768. [Google Scholar] [CrossRef] [PubMed]
- Elizur, A.; Cohen, M.; Goldberg, M.R.; Rajuan, N.; Cohen, A.; Leshno, M.; Katz, Y. Cow’s milk associated rectal bleeding: A population based prospective study. Pediatr. Allergy Immunol. 2012, 23, 766–770. [Google Scholar] [CrossRef]
- Martin, V.M.; Virkud, Y.V.; Seay, H.; Hickey, A.; Ndahayo, R.; Rosow, R.; Southwick, C.; Elkort, M.; Gupta, B.; Kramer, E.; et al. Prospective assessment of pediatrician-diagnosed food protein-induced allergic proctocolitis by gross or occult blood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1692–1699.e1. [Google Scholar] [CrossRef]
- Buyuktiryaki, B.; Kulhas Celik, I.; Erdem, S.B.; Capanoglu, M.; Civelek, E.; Guc, B.U.; Guvenir, H.; Cakir, M.; Dibek Misirlioglu, E.; Akcal, O.; et al. Risk factors influencing tolerance and clinical features of food protein-induced allergic proctocolitis. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 574–579. [Google Scholar] [CrossRef]
- Koksal, B.T.; Barış, Z.; Ozcay, F.; Yilmaz Ozbek, O. Single and multiple food allergies in infants with proctocolitis. Allergol. Immunopathol. 2018, 46, 3–8. [Google Scholar] [CrossRef]
- Celik, P.; Yilmaz, D.; Yuksel, F.; Akpinar, F.; Karabag, K.; Uzun, A.K.; Dibek Misirlioglu, E. Behavioral feeding problems and parenting stress in toddlers with food protein-induced allergic proctocolitis. Ann. Allergy Asthma Immunol. 2025, 134, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Concha, S.; Cabalín, C.; Iturriaga, C.; Pérez-Mateluna, G.; Gomez, C.; Cifuentes, L.; Harris, P.R.; Gana, J.C.; Borzutzky, A. Diagnostic validity of fecal occult blood test in infants with food protein-induced allergic proctocolitis. Rev. Chil. Pediatr. 2018, 89, 630–637. [Google Scholar] [CrossRef]
- Czaja-Bulsa, G.; Bulsa, K.; Łokieć, M.; Drozd, A. Can faecal zonulin and calprotectin levels be used in the diagnosis and follow-up in infants with milk protein-induced allergic proctocolitis? Nutrients 2024, 16, 2949. [Google Scholar] [CrossRef]
- Lake, A.M. Food-induced eosinophilic proctocolitis. J. Pediatr. Gastroenterol. Nutr. 2000, 30, S58–S60. [Google Scholar] [CrossRef]
- Lamont, R.F.; Møller Luef, B.; Stener Jørgensen, J. Childhood inflammatory and metabolic disease following exposure to antibiotics in pregnancy, antenatally, intrapartum and neonatally. F1000Research 2020, 9, 144. [Google Scholar] [CrossRef]
- Huang, K.Y.; Liang, B.S.; Zhang, X.Y.; Chen, H.; Ma, N.; Lan, J.L.; Li, D.Y.; Zhou, Z.W.; Yang, M. Molecular characterization of Clostridium perfringens isolates from a tertiary Children’s Hospital in Guangzhou, China, establishing an association between bacterial colonization and food allergies in infants. Gut Pathog. 2023, 15, 47. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A. Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy Asthma Proc. 2015, 36, 172–184. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non-IgE-mediated gastrointestinal food allergy. J. Allergy Clin. Immunol. 2015, 135, 1114–1124. [Google Scholar] [CrossRef]
- Rojas Gallegos, M.B.; Crissinger, K.D. Outcomes of Infants with Severe Refractory Food Protein-Induced Allergic Proctocolitis Treated with Mesalamine. JPGN Rep. 2020, 2, e024. [Google Scholar] [CrossRef]
- Chinese Medical Association of Allergy and Immunology. Expert consensus on mechanisms and targeted therapy of Type 2 inflammatory diseases. Chin. Med. J. 2022, 102, 3349–3373. [Google Scholar]
- Liu, W.; Ma, X.; Zhou, W. Adverse events of Benralizumab in moderate to severe eosinophilic asthma: A meta-analysis. Medicine 2019, 98, e15868. [Google Scholar] [CrossRef]
- Chung, H.L.; Hwang, J.B.; Park, J.J.; Kim, S.G. Expression of transforming growth factor beta1, transforming growth factor type I and II receptors, and TNF-alpha in the mucosa of the small intestine in infants with food protein-induced enterocolitis syndrome. J. Allergy Clin. Immunol. 2002, 109, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Nomura, I.; Orihara, K.; Yoshida, K.; Akasawa, A.; Tachimoto, H.; Ohtsuka, Y.; Namai, Y.; Futamura, M.; Shoda, T.; et al. Antigen-specific T-cell responses in patients with non-IgE-mediated gastrointestinal food allergy are predominantly skewed to T(H)2. J. Allergy Clin. Immunol. 2013, 131, 590–592.e1. [Google Scholar] [CrossRef]
- Erdem, S.B.; Nacaroglu, H.T.; Karaman, S.; Erdur, C.B.; Karkıner, C.U.; Can, D. Tolerance development in food protein-induced allergic proctocolitis: Single centre experience. Allergol. Immunopathol. 2017, 45, 212–219. [Google Scholar] [CrossRef] [PubMed]
| Symptom | EOG (n = 20) | nEOG (n = 15) | χ2 Value/Z | p-Value |
|---|---|---|---|---|
| Gender (Male) Hospitalization age [M (P25, P75), months] Feeding method [No. (%)] Breastfeeding Mixed feeding Formula feeding Special formula use time before hospitalization [M (P25, P75), months] Complementary food introduction time [M (P25, P75), months] Family history of allergies | 14 (70.0%) 13.5 (12.0, 24.75) 14 (70.0%) 6 (30.0%) 0 (0%) 8.0 (4.25, 17.75) 6.0 (6.0, 8.0) 9 (45.0%) | 11 (73.3%) 12.0 (9.0, 20.5) 10 (66.7%) 3 (20.0%) 2 (13.3%) 4.5 (1.25, 13.25) 6.5 (6.0, 7.75) 2 (13.3%) | - −1.173 b - −1.804 b −0.075 b - | 1.000 a 0.241 0.314 a 0.071 0.940 0.069 a |
| Antibiotic use | 5 (25.0%) | 10 (52.6%) | - | 0.019 a |
| Refusal to feed | 1 (5.0%) | 2 (13.3%) | - | 0.565 a |
| Reflux | 2 (10.0%) | 1 (6.7%) | - | 1.000 a |
| Eczema | 17 (85.0%) | 11 (73.3%) | - | 0.43 a |
| Diarrhea | 18 (90.0%) | 10 (66.7%) | - | 0.112 a |
| Stools with excessive mucus | 18 (90.0%) | 12(80.0%) | - | 0.631 a |
| Bloody stools | 20 (100%) | 12 (80.0%) | - | 0.07 a |
| Night crying | 2 (10.0%) | 2 (13.3%) | - | 1.000 a |
| Growth retardation | 7 (35.0%) | 11 (73.3%) | - | 0.041 a |
| Malnutrition | 4 (20.0%) | 3 (20.0%) | - | 1.000 a |
| Laboratory Tests | EOG (n = 20) | nEOG (n = 15) | χ2/t/Z Value | p-Value |
|---|---|---|---|---|
| Positive for Clostridium difficile toxin | 3 (15.0%) | 1 (6.7%) | - | 0.619 a |
| Positive for glutamate dehydrogenase antigen | 13 (65.0%) | 7 (46.7%) | - | 0.321 a |
| CMV IgM antibody positivity | 0 (0%) | 2 (13.3%) | - | 0.176 a |
| Hemoglobin (g/L) | 118.95 ± 11.26 | 107.93 ± 14.61 | 2.521 b | 0.017 |
| Absolute eosinophil count [M (P25, P75), ×109/L] | 0.17 (0.060, 0.313) | 0.23 (0.09, 0.33) | −0.634 c | 0.526 |
| Vitamin A (μmol/L) | 0.85 ± 0.34 (n = 12) | 0.71 ± 0.21 (n = 8) | 1.045 c | 0.310 |
| Fecal calprotectin [M (P25, P75), μg/g] | 116.96 (n = 11) (58.0, 263.98) | 116.65 (n = 9) (42.51, 195.83) | −0.418 c | 0.676 |
| Allergen-specific IgE sensitization rate | 6/20 (30.0%) | 7/15 (46.7%) | - | 0.481 a |
| Item | EOG (n = 20) | nEOG (n = 15) | p-Value |
|---|---|---|---|
| Number of colonoscopies 1 time 2 times 3 times | 20 (100%) 0 0 | 11 (73.3%) 2 (13.3%) 2 (13.3%) | 0.026 a |
| Involved intestinal segments Terminal ileum Ileocecal valve Ascending colon Transverse colon Descending colon Sigmoid colon Rectum | 6 (30.0%) 3 (15.0%) 12 (60.0%) 13 (65.0%) 17 (85.0%) 18 (90.0%) 18 (90.0%) | 5 (33.3%) 3 (20.0%) 13 (86.7%) 14 (93.3%) 15 (100%) 15 (100%) 15 (100%) | 1.000 a 1.000 a 0.134 a 0.101 a 0.244 a 0.496 a 0.496 a |
| Lesion characteristics in terminal ileum Uneven mucosa Erythema Erosion | 5 (25.0%) 2 (10.0%) 1 (5.0%) | 4 (26.7%) 3 (20.0%) 1 (6.7%) | 1.000 a 0.631 a 1.000 a |
| Lesion characteristics in ileocecal valve Erythema | 3 (15.0%) | 3 (20.0%) | 1.000 a |
| Lesion characteristics in ascending colon LNH with erythema Erosion Ulcer | 12 (60.0%) 2 (10.0%) 0 | 13 (86.7%) 3 (20.0%) 2 (13.3%) | 0.134 a 0.631 a 0.176 a |
| Lesion characteristics in the transverse colon LNH with erythema Erosion Ulcer | 13 (65.0%) 4 (20.0%) 1 (5.0%) | 14 (93.3%) 3 (20.0%) 2 (13.3%) | 0.101 a 1.000 a 0.565 a |
| Lesion characteristics in the descending colon LNH with erythema Erosion Ulcer | 17 (85.0%) 4 (20.0%) 0 | 15 (100.0%) 5 (33.3%) 1 (6.7%) | 0.244 a 0.451 a 0.429 a |
| Lesion characteristics in the sigmoid colon LNH with erythema Erosion Ulcer | 17 (85.0%) 5 (25.0%) 0 | 15 (100.0%) 6 (40.0%) 1 (6.7%) | 0.244 a 0.467 a 0.429 a |
| Lesion characteristics in the rectum LNH with erythema Erosion Ulcer | 18 (90.0%) 5 (25.0%) 1 (5.0%) | 15 (100.0%) 5 (33.3%) 2 (13.3%) | 0.496 a 0.712 a 0.565 a |
| Eosinophil presence in pathology | 4 (20.0%) | 7 (46.7%) | 0.144 a |
| Item | EOG (n = 20) | nEOG (n = 15) | p-Value |
|---|---|---|---|
| Number of gastroscopies 0 1 time 2 times 3 times | 5 (25.0%) 15 (75.0%) 0 0 | 1 (6.7%) 11 (73.3%) 1 (6.7%) 2 (13.3%) | 0.085 a |
| Involved sites and lesion characteristics Cardiac ulcer Fundic erythema Corpus erythema Angular incisura erythema Antral erythema Antral erosion Duodenal bulb erosion Erosion of the descending part of the duodenum | 1 (6.7%) 1 (6.7%) 3 (20.0%) 0 (0.0%) 5 (33.3%) 3 (20.0%) 5 (33.3%) 2 (13.3%) | 3 (21.4%) 6 (42.9%) 8 (57.1%) 4 (28.6%) 5 (35.7%) 3 (21.4%) 2 (14.3%) 3 (21.4%) | 0.330 a 0.035 a 0.060 a 0.042 a 1.000 a 1.000 a 0.390 a 0.651 a |
| Eosinophil presence in pathology | 2 (13.3%) | 2 (14.3%) | 1.000 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wu, H.; Li, J.; Liu, X.; Shi, X.; Zhang, H.; Li, Z. Analysis of Clinical Characteristics of Refractory Food Protein-Induced Allergic Proctocolitis. Children 2025, 12, 1494. https://doi.org/10.3390/children12111494
Zhang J, Wu H, Li J, Liu X, Shi X, Zhang H, Li Z. Analysis of Clinical Characteristics of Refractory Food Protein-Induced Allergic Proctocolitis. Children. 2025; 12(11):1494. https://doi.org/10.3390/children12111494
Chicago/Turabian StyleZhang, Juan, Hui Wu, Jun Li, Xun Liu, Xueying Shi, Hua Zhang, and Zailing Li. 2025. "Analysis of Clinical Characteristics of Refractory Food Protein-Induced Allergic Proctocolitis" Children 12, no. 11: 1494. https://doi.org/10.3390/children12111494
APA StyleZhang, J., Wu, H., Li, J., Liu, X., Shi, X., Zhang, H., & Li, Z. (2025). Analysis of Clinical Characteristics of Refractory Food Protein-Induced Allergic Proctocolitis. Children, 12(11), 1494. https://doi.org/10.3390/children12111494






