Blood Melatonin in Breast Milk-Fed Preterm Infants: Longitudinal Biomonitoring to 38 Weeks’ Postmenstrual Age (ProMote Study)
Abstract
1. Introduction
- (i)
- (ii)
- Quantifying and bounding differences in melatonin of the umbilical cord blood by time of birth (day vs. night).
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Blood Melatonin Collection Procedure
2.4. Data Analysis
2.5. Sensitivity Analysis
3. Results
3.1. Baseline Characteristics
3.2. Day Versus Night Births
3.3. Melatonin Analysis
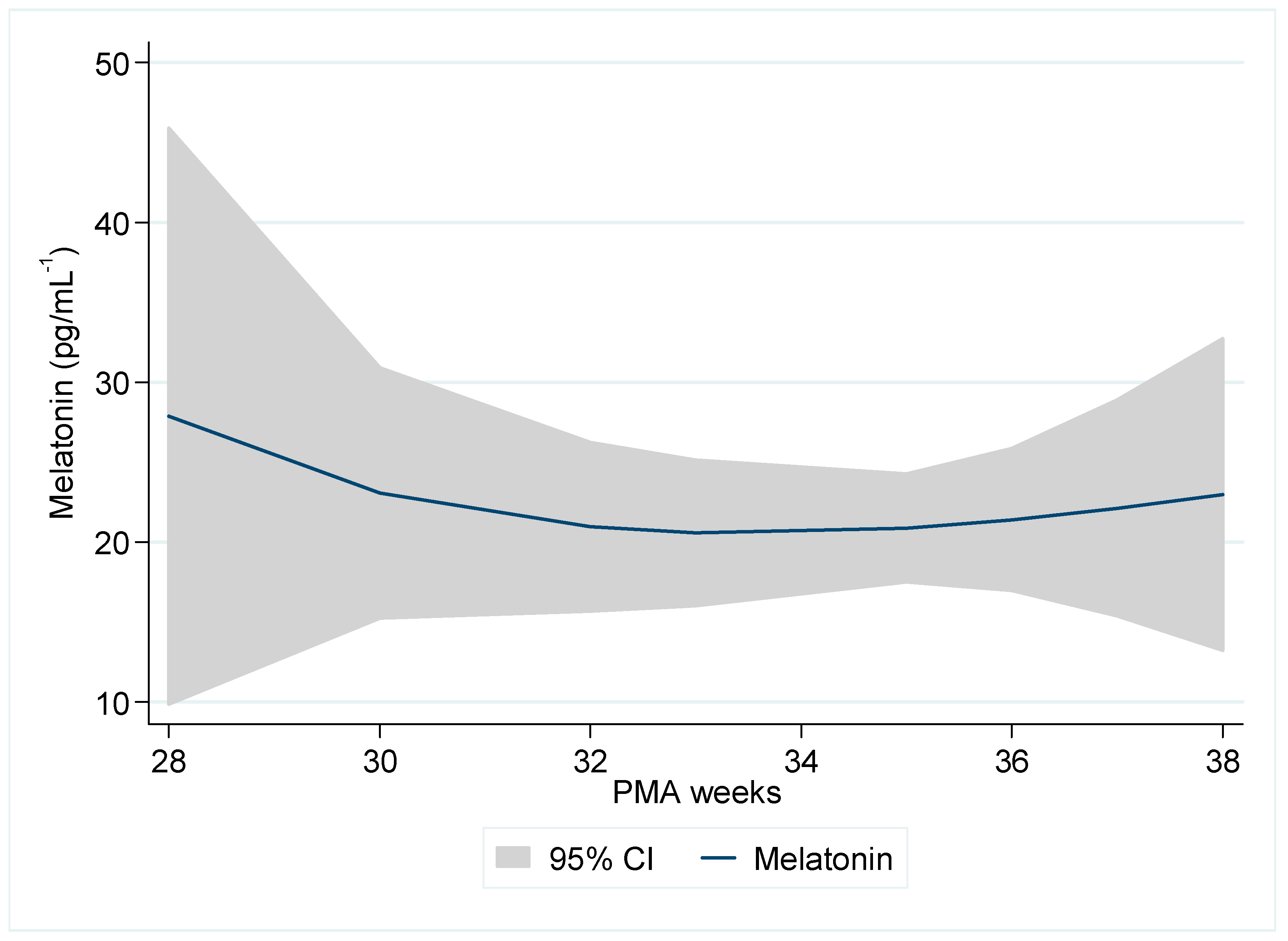
Initial Profile of Morning Melatonin by PMA
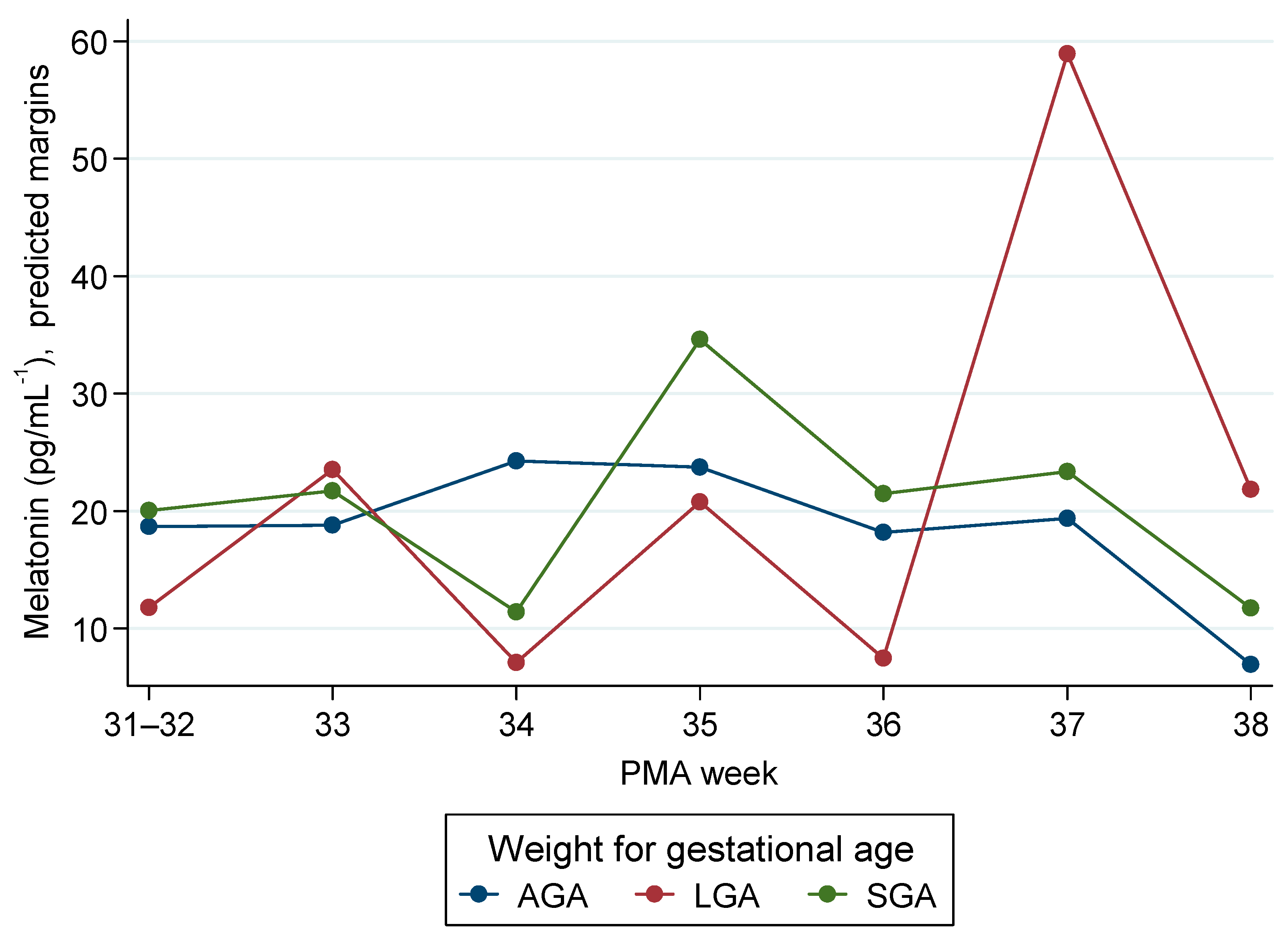
3.4. Mixed-Models Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLT | Melatonin |
| PMA | Postmenstrual age (gestational age at birth + time since birth) |
| GA | Gestational age |
| GW | Gestational weeks |
| WfGA | Weight-for-gestational-age |
| AGA | Appropriate for gestational age |
| SGA | Small for gestational age |
| LGA | Large for gestational age |
| NICU | Neonatal Intensive Care Unit |
| DOL | Day of life |
| ELISA | Enzyme-linked immunosorbent assay |
| SPE | Solid-phase extraction |
| PNPP | p-Nitrophenyl phosphate |
| SCN | Suprachiasmatic nucleus |
| aMT6s | 6-Sulfatoxymelatonin |
| 6-OHMS | 6-Hydroxymelatonin sulfate |
| IQR | Interquartile range |
| SD | Standard deviation |
| CI | Confidence interval |
| SE | Standard error |
| ML | Maximum likelihood |
| REML | Restricted maximum likelihood |
| L-BFGS | Limited-memory Broyden–Fletcher–Goldfarb–Shanno (optimizer) |
References
- Joseph, T.; Schuch, V.; Hossack, D.; Chakraborty, R.; Johnson, E. Melatonin: The placental antioxidant and anti-inflammatory. Front. Immunol. 2024, 15, 1339304. [Google Scholar] [CrossRef]
- Katzer, D.; Pauli, L.; Mueller, A.; Reutter, H.; Reinsberg, J.; Fimmers, R.; Bartmann, P.; Bagci, S. Melatonin Concentrations and Antioxidative Capacity of Human Breast Milk According to Gestational Age and the Time of Day. J. Hum. Lact. 2016, 32, NP105–NP110. [Google Scholar] [CrossRef]
- Qin, Y.; Shi, W.; Zhuang, J.; Liu, Y.; Tang, L.; Bu, J.; Sun, J.; Bei, F. Variations in melatonin levels in preterm and term human breast milk during the first month after delivery. Sci. Rep. 2019, 9, 17984. [Google Scholar] [CrossRef] [PubMed]
- Gombert, M.; Codoñer-Franch, P. Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System. Int. J. Mol. Sci. 2021, 22, 6809. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tain, Y.-L.; Sheen, J.-M.; Huang, L.-T. Melatonin utility in neonates and children. J. Formos. Med. Assoc. 2012, 111, 57–66. [Google Scholar] [CrossRef]
- Verteramo, R.; Pierdomenico, M.; Greco, P.; Milano, C. The Role of Melatonin in Pregnancy and the Health Benefits for the Newborn. Biomedicines 2022, 10, 3252. [Google Scholar] [CrossRef]
- McCarthy, R.; Jungheim, E.S.; Fay, J.C.; Bates, K.; Herzog, E.D.; England, S.K. Riding the Rhythm of Melatonin Through Pregnancy to Deliver on Time. Front. Endocrinol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Häusler, S.; Lanzinger, E.; Sams, E.; Fazelnia, C.; Allmer, K.; Binder, C.; Reiter, R.J.; Felder, T.K. Melatonin in Human Breast Milk and Its Potential Role in Circadian Entrainment: A Nod towards Chrononutrition? Nutrients 2024, 16, 1422. [Google Scholar] [CrossRef]
- D’Angelo, G.; Chimenz, R.; Reiter, R.J.; Gitto, E. Use of Melatonin in Oxidative Stress Related Neonatal Diseases. Antioxidants 2020, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, A.; Rager, K.; Gupta, D. Ontogeny of Circadian Rhythmicity for Melatonin, Serotonin, and N-Acetylserotonin in Humans. J. Pineal Res. 1986, 3, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Stamp, G.E.; Goble, F.C. Development of melatonin production in infants and the impact of prematurity. J. Clin. Endocrinol. Metab. 1992, 75, 367–369. [Google Scholar] [CrossRef]
- Illnerová, H.; Buresová, M.; Presl, J. Melatonin rhythm in human milk. J. Clin. Endocrinol. Metab. 1993, 77, 838–841. [Google Scholar] [CrossRef]
- Biran, V.; Decobert, F.; Bednarek, N.; Boizeau, P.; Benoist, J.-F.; Claustrat, B.; Barré, J.; Colella, M.; Frérot, A.; Garnotel, R. Melatonin levels in preterm and term infants and their mothers. Int. J. Mol. Sci. 2019, 20, 2077. [Google Scholar] [CrossRef]
- Commentz, J.C.; Henke, A.; Dammann, O.; Hellwege, H.H.; Willig, R.P. Decreasing melatonin and 6-hydroxymelatonin sulfate excretion with advancing gestational age in preterm and term newborn male infants. Eur. J. Endocrinol. 1996, 135, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Hoyos, A.; Bonillo-Perales, A.; Avila-Villegas, R.; González-Ripoll, M.; Uberos, J.; Florido-Navío, J.; Molina-Carballo, A. Melatonin levels during the first week of life and their relation with the antioxidant response in the perinatal period. Neonatology 2007, 92, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.; Roger, M.; Lemaitre, B.; Massias, J.; Chaussain, J. Plasma and urinary melatonin in male infants during the first 12 months of life. Clin. Chim. Acta 1982, 121, 37–42. [Google Scholar] [CrossRef]
- Muñoz-Hoyos, A.; Jaldo-Alba, F.; Molina-Carballo, A.; Rodríguez-Cabezas, T.; Molina-Font, J.; Acuña-Castroviejo, D. Absence of plasma melatonin circadian rhythm during the first 72 hours of life in human infants. J. Clin. Endocrinol. Metab. 1993, 77, 699–703. [Google Scholar]
- Kakatsaki, I.; Papanikolaou, S.; Roumeliotaki, T.; Anagnostatou, N.H.; Lygerou, I.; Hatzidaki, E. The prevalence of small for gestational age and extrauterine growth restriction among extremely and very preterm neonates, using different growth curves, and its association with clinical and nutritional factors. Nutrients 2023, 15, 3290. [Google Scholar] [CrossRef]
- Kakatsaki, I.; Anagnostatou, N.H.; Roumeliotaki, T.; Panteris, E.; Liapikos, T.; Papanikolaou, S.; Hatzidaki, E. Evaluating Prevalence of Preterm Postnatal Growth Faltering Using Fenton 2013 and INTERGROWTH-21st Growth Charts with Logistic and Machine Learning Models. Nutrients 2025, 17, 1726. [Google Scholar] [CrossRef]
- Kokkinaki, T.; Anagnostatou, N.; Markodimitraki, M.; Roumeliotaki, T.; Tzatzarakis, M.; Vakonaki, E.; Giannakakis, G.; Tsatsakis, A.; Hatzidaki, E. The development of preterm infants from low socio-economic status families: The combined effects of melatonin, autonomic nervous system maturation and psychosocial factors (ProMote): A study protocol. PLoS ONE 2025, 20, e0316520. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.A.; Lucke, A.M.; Cummings, J.J.; Committee on Fetus and Newborn. Postnatal Cord Blood Sampling: Clinical Report. Pediatrics 2025, 155, e2025071811. [Google Scholar] [CrossRef]
- Prakash, N.; Decristofaro, J.; Maduekwe, E.T. One Less Painful Procedure: Using Umbilical Cord Blood as Alternative Source to Admission Complete Blood Count. Am. J. Perinatol. 2017, 34, 1178–1184. [Google Scholar] [CrossRef]
- Carroll, P.D.; Nankervis, C.A.; Iams, J.; Kelleher, K. Umbilical cord blood as a replacement source for admission complete blood count in premature infants. J. Perinatol. 2012, 32, 97–102. [Google Scholar] [CrossRef]
- Vicente, P.; García, A.; Alvarez, E.; Clemente, S.; Blázquez, E. Presence of Melatonin in the Umbilical Cord Blood of Full-Term Human Newborns. J. Pineal Res. 1989, 6, 135–140. [Google Scholar] [CrossRef]
- Kivelä, A.; Kauppila, A.; Leppäluoto, J.; Vakkuri, O. Melatonin in infants and mothers at delivery and in infants during the first week of life. Clin. Endocrinol. 1990, 32, 593–598. [Google Scholar] [CrossRef]
- Paditz, E. Postnatal Development of the Circadian Rhythmicity of Human Pineal Melatonin Synthesis and Secretion (Systematic Review). Children 2024, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Goble, F.C.; Stamp, G.E. Factors influencing the development of melatonin rhythmicity in humans. J. Clin. Endocrinol. Metab. 1996, 81, 1525–1532. [Google Scholar] [PubMed]
- Sivan, Y.; Laudon, M.; Tauman, R.; Zisapel, N. Melatonin Production in Healthy Infants: Evidence for Seasonal Variations. Pediatr. Res. 2001, 49, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar] [CrossRef]
- Bagci, S.; Berner, A.L.; Reinsberg, J.; Gast, A.-S.; Zur, B.; Welzing, L.; Bartmann, P.; Mueller, A. Melatonin concentration in umbilical cord blood depends on mode of delivery. Early Hum. Dev. 2012, 88, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Kok, E.Y.; Kaur, S.; Mohd Shukri, N.H.; Abdul Razak, N.; Takahashi, M.; Teoh, S.C.; Tay, J.E.F.; Shibata, S. The role of light exposure in infant circadian rhythm establishment: A scoping review perspective. Eur. J. Pediatr. 2024, 184, 112. [Google Scholar] [CrossRef] [PubMed]
- Booker, L.A.; Lenz, K.E.; Spong, J.; Deacon-Crouch, M.; Wilson, D.L.; Nguyen, T.H.; Skinner, T.C. High-Temperature Pasteurization Used at Donor Breast Milk Banks Reduces Melatonin Levels in Breast Milk. Breastfeed. Med. 2023, 18, 549–552. [Google Scholar] [CrossRef]
- Chrustek, A.; Sinkiewicz-Darol, E.; Lampka, M.; Olszewska-Słonina, D.; Sperkowska, B.; Linowiecka, K. Effect of pasteurization on melatonin concentration in human breast milk. Postępy Hig. I Med. Doświadczalnej 2022, 76, 220–227. [Google Scholar] [CrossRef]
- Morag, I.; Ohlsson, A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst. Rev. 2013, 8, CD006982. [Google Scholar] [CrossRef]

| Parameter | N (%) or Median (IQR) |
|---|---|
| Melatonin (pg/mL−1) | |
| t0-Birth | 10.4 (5.6–32.4) |
| t1—4th–7th day of life | 10.6 (5.2–26.3) |
| t2—10th–14th day of life | 9.4 (6.5–26.8) |
| t3—35th–36th week PMA | 8.3 (2.9–15.4) |
| Time of birth | |
| 05:00–16:59 | 93 (76.9) |
| 17:00–04:59 | 28 (23.1) |
| Delivery type | |
| Vaginal | 7 (5.7) |
| Caesarean section | 115 (94.3) |
| Infant sex | |
| Male | 63 (51.6) |
| Female | 59 (48.4) |
| Oxygen at birth | |
| No | 38 (31.2) |
| Yes | 84 (68.9) |
| Intubated | |
| No | 112 (91.8) |
| Yes | 10 (8.2) |
| Length (cm) | 45.8 (43.0–47.0) |
| Head circumference (cm) | 32.0 (30.5–32.5) |
| Weight (g) | 2155 (1890–2530) |
| Gestational age (weeks) | 34.0 (33.0–35.0) |
| Preterm category | |
| Extremely preterm | 2 (1.6) |
| Very preterm | 15 (12.3) |
| Moderate preterm | 35 (28.7) |
| Late preterm | 70 (57.4) |
| Weight for GA | |
| Small for GA (SGA) | 11 (9.0) |
| Appropriate for GA (AGA) | 103 (84.4) |
| Large for GA (LGA) | 8 (6.6) |
| Twins | |
| No | 84 (68.9) |
| Yes | 38 (31.2) |
| Maternal age (years) | 34.3 (29.8–39.2) |
| Maternal nationality: Greek | |
| No | 11 (9.1) |
| Yes | 110 (90.9) |
| Maternal education | |
| Compulsory | 7 (5.8) |
| Secondary | 43 (35.5) |
| Tertiary | 71 (58.7) |
| Melatonin in Colostrum (pg/mL−1) | 16.3 (7.3–30.6) |
| Variable | Day (05:00–16:59) Median (IQR) | Night (17:00–04:59) Median (IQR) | p-Value |
|---|---|---|---|
| GA (weeks) | 34 (33–35) | 33 (32–34) | 0.005 |
| Length (cm) | 46 (44–48) | 43 (42–46) | 0.006 |
| Head circumference (cm) | 32 (31–33) | 31 (30–32) | 0.046 |
| Weight (g) | 2260 (1930–2600) | 1985 (1780–2285) | 0.031 |
| Maternal age (years) | 34.1 (29.7–38.6) | 34.3 (29.1–39.7) | 0.503 |
| PMA | N | Median | IQR |
|---|---|---|---|
| 26–28 | 6 | 7.8 | (2.3–31.2) |
| 29–30 | 14 | 15.2 | (8.1–31.7) |
| 31–32 | 27 | 8.2 | (5.5–14.6) |
| 33 | 34 | 8.7 | (5.2–20.9) |
| 34 | 46 | 9.0 | (5.7–23.8) |
| 35 | 81 | 11.6 | (5.5–26.1) |
| 36 | 50 | 10.1 | (5.6–30.8) |
| 37 | 14 | 17.8 | (3.7–45.4) |
| 38 | 6 | 9.2 | (4.3–19.1) |
| Total | 278 | 10.1 | (5.4–25.3) |
| Birth Time Group * | t0 (At Birth or 24 h) | t1 (4th–7th day) | t2/t (10th–14th day) | p-Value (Pairs) | p-Value (Pairs) |
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | (t1 vs. t0) | (t2/t3 vs. t1) | |
| Day (N = 93) (05:00–16:59) | 10.71 (5.49–31.01) | 13.11 (5.46–31.66) | 6.73 (2.90–15.77) | 0.563 | 0.027 |
| Night (N = 28) (17:00–04:59) | 11.30 (6.24–37.58) | 8.47 (5.09–18.76) | 11.09 (3.39–18.63) | 0.233 | 0.929 |
| Parameters | Effect Estimates | 95% CI | p-Value | |
|---|---|---|---|---|
| TIME GROUP (Ref. Day) | 60.7 | −13.1 | 134.4 | 0.107 |
| PMA (Ref. 26–28 weeks) | ||||
| 29–30 | 26.9 | −11.5 | 65.2 | 0.170 |
| 31–32 | 7.1 | −11.3 | 25.6 | 0.448 |
| 33 | 13.7 | −6.0 | 33.3 | 0.173 |
| 34 | 17.8 | −2.2 | 37.8 | 0.081 |
| 35 | 18.5 | 0.4 | 36.5 | 0.045 |
| 36 | 10.0 | −7.0 | 27.0 | 0.249 |
| 37 | 19.1 | −3.8 | 42.0 | 0.102 |
| 38 | −0.7 | −17.2 | 15.9 | 0.937 |
| TIME GROUP-PMA Interaction | ||||
| 2#29–30 | −79.0 | −154.3 | −3.8 | 0.040 |
| 2#31–32 | −43.2 | −118.2 | 31.9 | 0.260 |
| 2#33 | −63.8 | −138.4 | 10.9 | 0.094 |
| 2#34 | −66.5 | −140.9 | 7.9 | 0.080 |
| 2#35 | −66.6 | −141.5 | 8.3 | 0.081 |
| 2#36 | −58.0 | −134.8 | 18.8 | 0.139 |
| 2#37 | N/A | - | - | - |
| 2#38 | −41.5 | −113.7 | 30.6 | 0.259 |
| Parameters | Effect Estimate | 95% CI | p-Value | |
|---|---|---|---|---|
| Weight for GA (Ref. AGA) | ||||
| LGA | −6.9 | −19.0 | 5.2 | 0.262 |
| SGA | 1.4 | −8.7 | 11.5 | 0.792 |
| PMA (Ref. 31–32 weeks) | ||||
| 33 | 0.1 | −10.9 | 11.2 | 0.983 |
| 34 | 5.6 | −6.5 | 17.6 | 0.363 |
| 35 | 5.0 | −4.3 | 14.4 | 0.293 |
| 36 | −0.5 | −11.2 | 10.2 | 0.928 |
| 37 | 0.7 | −16.0 | 17.4 | 0.934 |
| 38 | −11.8 | −21.3 | −2.2 | 0.016 |
| L/SGA-PMA Interaction | ||||
| LGA#33 | 11.6 | −11.3 | 34.6 | 0.321 |
| LGA#34 | −10.3 | −24.7 | 4.1 | 0.162 |
| LGA#35 | 4.0 | −17.9 | 25.8 | 0.722 |
| LGA#36 | −3.8 | −18.6 | 11.0 | 0.615 |
| LGA#37 | 46.5 | 21.0 | 71.9 | 0.000 |
| LGA#38 | 21.9 | 8.0 | 35.8 | 0.002 |
| SGA#33 | 1.5 | −10.8 | 13.9 | 0.806 |
| SGA#34 | −14.3 | −26.9 | −1.6 | 0.027 |
| SGA#35 | 9.5 | −15.8 | 34.9 | 0.462 |
| SGA#36 | 1.9 | −16.9 | 20.7 | 0.843 |
| SGA#37 | 2.6 | −18.5 | 23.7 | 0.809 |
| SGA#38 | 3.5 | −8.8 | 15.8 | 0.581 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinaki, T.; Tzatzarakis, M.; Vakonaki, E.; Anagnostatou, N.; Roumeliotaki, T.; Panteris, E.; Markodimitraki, M.; Kakatsaki, I.; Kondylakis, H.; Tsatsakis, A.; et al. Blood Melatonin in Breast Milk-Fed Preterm Infants: Longitudinal Biomonitoring to 38 Weeks’ Postmenstrual Age (ProMote Study). Children 2025, 12, 1490. https://doi.org/10.3390/children12111490
Kokkinaki T, Tzatzarakis M, Vakonaki E, Anagnostatou N, Roumeliotaki T, Panteris E, Markodimitraki M, Kakatsaki I, Kondylakis H, Tsatsakis A, et al. Blood Melatonin in Breast Milk-Fed Preterm Infants: Longitudinal Biomonitoring to 38 Weeks’ Postmenstrual Age (ProMote Study). Children. 2025; 12(11):1490. https://doi.org/10.3390/children12111490
Chicago/Turabian StyleKokkinaki, Theano, Manolis Tzatzarakis, Elena Vakonaki, Nicole Anagnostatou, Theano Roumeliotaki, Eleftherios Panteris, Maria Markodimitraki, Ioanna Kakatsaki, Haridimos Kondylakis, Aristidis Tsatsakis, and et al. 2025. "Blood Melatonin in Breast Milk-Fed Preterm Infants: Longitudinal Biomonitoring to 38 Weeks’ Postmenstrual Age (ProMote Study)" Children 12, no. 11: 1490. https://doi.org/10.3390/children12111490
APA StyleKokkinaki, T., Tzatzarakis, M., Vakonaki, E., Anagnostatou, N., Roumeliotaki, T., Panteris, E., Markodimitraki, M., Kakatsaki, I., Kondylakis, H., Tsatsakis, A., & Hatzidaki, E. (2025). Blood Melatonin in Breast Milk-Fed Preterm Infants: Longitudinal Biomonitoring to 38 Weeks’ Postmenstrual Age (ProMote Study). Children, 12(11), 1490. https://doi.org/10.3390/children12111490








