Furosemide-Induced Nephrocalcinosis in Premature Neonates: A Critical Review of Observational Data
Highlights
- •
- Furosemide is linked to a dose-related risk of nephrocalcinosis in preterm infants.
- •
- Although the condition resolves with discontinuation of medication in most cases, it can persist especially with prolonged exposure.
- •
- Cautious dosing and close monitoring are crucial to minimizing renal complications.
- •
- Further research is needed to establish optimal dosing and long-term safety.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol Registration
2.2. Study Eligibility Criteria
- •
- Studies on premature infants who received at least one dose of furosemide in the NICU.
- •
- The studies reported nephrocalcinosis (NC) confirmed by ultrasound or similar imaging.
- •
- The studies had a comparator group of premature infants without furosemide exposure (when available).
- •
- Animal studies, narrative reviews, and editorials.
- •
- Conference abstracts without full data.
- •
- Studies that did not assess NC as an outcome.
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Extraction
2.5. Risk of Bias and Quality Assessment
2.6. Data Synthesis
2.7. Ethical Considerations
2.8. Statistical Analysis
3. Results
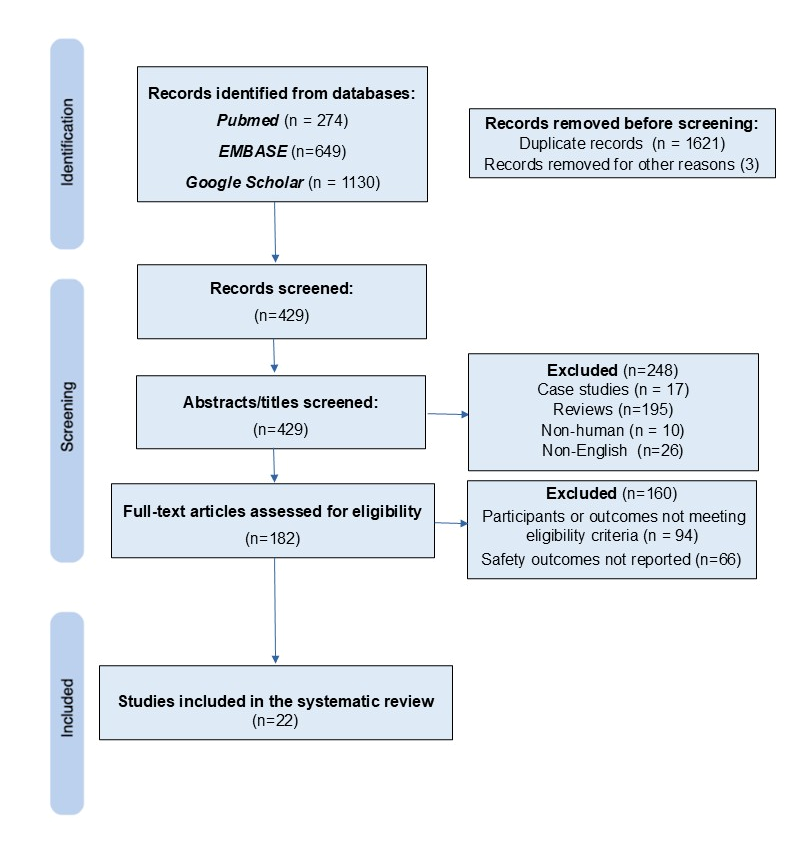
3.1. Studies Identified Through the Search Strategy
3.2. Findings on Furosemide and NC
3.3. Overall Prevalence and Dosing
3.4. Resolution and Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NC | Nephrocalcinosis |
| NICU | Neonatal Intensive Care Unit |
| RUS | Renal Ultrasonography |
| RCT | Randomized Controlled Trial |
References
- Thompson, E.J.; Benjamin, D.K.; Greenberg, R.G.; Kumar, K.R.; Zimmerman, K.O.; Laughon, M.; Clark, R.H.; Smith, P.B.; Hornik, C.P. Pharmacoepidemiology of Furosemide in the Neonatal Intensive Care Unit. Neonatology 2020, 117, 780–784. [Google Scholar] [CrossRef]
- Greenberg, R.G.; Lang, J.; Smith, P.B.; Shekhawat, P.; Courtney, S.E.; Hudak, M.L.; Moya, F.; Iyengar, A.; Eldemerdash, A.; Bloom, B.; et al. Furosemide Safety in Preterm Infants at Risk for Bronchopulmonary Dysplasia: A Randomized Clinical Trial. J. Pediatr. 2025, 283, 114629. [Google Scholar] [CrossRef]
- Garunkstiene, R.; Levuliene, R.; Cekuolis, A.; Cerkauskiene, R.; Drazdiene, N.; Liubsys, A. A Prospective Study of Nephrocalcinosis in Very Preterm Infants: Incidence, Risk Factors and Vitamin D Intake in the First Month. Medicina 2024, 60, 1910. [Google Scholar] [CrossRef]
- Jackson, W.; Taylor, G.; Selewski, D.; Smith, P.B.; Tolleson-Rinehart, S.; Laughon, M.M. Association between furosemide in premature infants and sensorineural hearing loss and nephrocalcinosis: A systematic review. Matern. Health Neonatol. Perinatol. 2018, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Al Madhoob, A.; Al Jufairi, M. Cumulative Doses Predict the Risk of Furosemide-Induced Electrolyte Abnormalities in Critically Ill Neonates. Ther. Clin. Risk Manag. 2022, 18, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, G.M. Clinical Pharmacology of Furosemide in Neonates: A Review. Pharmaceuticals 2013, 6, 1094–1129. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Owens, D.K.; Lohr, K.N.; Atkins, D.; Treadwell, J.R.; Reston, J.T.; Bass, E.B.; Chang, S.; Helfand, M. AHRQ series paper 5: Grading the strength of a body of evidence when comparing medical interventions-agency for healthcare research and quality and the effective health-care program. J. Clin. Epidemiol. 2010, 63, 513–523. [Google Scholar] [CrossRef]
- Hufnagle, K.G.; Khan, S.N.; Penn, D.; Cacciarelli, A.; Williams, P. Renal calcifications: A complication of long-term furosemide therapy in preterm infants. Pediatrics 1982, 70, 360–363. [Google Scholar] [CrossRef]
- Woolfield, N.; Haslam, R.; Le Quesne, G.; Chambers, H.M.; Hogg, R.; Jureidini, K. Ultrasound diagnosis of nephrocalcinosis in preterm infants. Arch. Dis. Child. 1988, 63, 86–88. [Google Scholar] [CrossRef]
- Jacinto, J.S.; Modanlou, H.D.; Crade, M.; Strauss, A.A.; Bosu, S.K. Renal calcification incidence in very low birth weight infants. Pediatrics 1988, 81, 31–35. [Google Scholar]
- Ezzedeen, F.; Adelman, R.D.; Ahlfors, C.E. Renal calcification in preterm infants: Pathophysiology and long-term sequelae. J. Pediatr. 1988, 113, 532–539. [Google Scholar] [CrossRef]
- Short, A.; Cooke, R.W. The incidence of renal calcification in preterm infants. Arch. Dis. Child. 1991, 66, 412–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Downing, G.J.; Egelhoff, J.C.; Daily, D.K.; Alon, U. Furosemide-related renal calcifications in the premature infant: A longitudinal ultrasonographic study. Pediatr. Radiol. 1991, 21, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Downing, G.J.; Egelhoff, J.C.; Daily, D.K.; Thomas, M.K.; Alon, U. Kidney function in very low birth weight infants with furosemide-related renal calcifications at ages 1 to 2 years. J. Pediatr. 1992, 120 Pt 1, 599–604. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Gilmore, H.E.; Kurtin, P.S. Nephrocalcinosis complicating medical treatment of posthemorrhagic hydrocephalus. Pediatr. Neurol. 1992, 8, 179–182. [Google Scholar] [CrossRef]
- Pope, J.C.; Trusler, L.A.; Klein, A.M.; Walsh, W.F.; Yared, A.; Brock, J.W. 3rd. The natural history of nephrocalcinosis in premature infants treated with loop diuretics. J. Urol. 1996, 156 Pt 2, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Saarela, T.; Vaarala, A.; Lanning, P.; Koivisto, M. Incidence, ultrasonic patterns and resolution of nephrocalcinosis in very low birthweight infants. Acta. Paediatr. 1999, 88, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Schell-Feith, E.A.; Kist-van Holthe, J.E.; Conneman, N.; van Zwieten, P.H.; Holscher, H.C.; Zonderland, H.M.; Brand, R.; van der Heijden, B.J. Etiology of nephrocalcinosis in preterm neonates: Association of nutritional intake and urinary parameters. Kidney Int. 2000, 58, 2102–2110. [Google Scholar] [CrossRef]
- Narendra, A.; White, M.P.; Rolton, H.A.; Alloub, Z.I.; Wilkinson, G.; McColl, J.H.; Beattie, J. Nephrocalcinosis in preterm babies. Arch. Dis. Child. Fetal. Neonatal Ed. 2001, 85, F207–F213. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Duran, I.; Martin, A.; Kribs, A.; Benz-Bohm, G.; Michalk, D.V.; Roth, B. Nephrocalcinosis in preterm infants: A single center experience. Pediatr. Nephrol. 2002, 17, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Hein, G.; Richter, D.; Manz, F.; Weitzel, D.; Kalhoff, H. Development of nephrocalcinosis in very low birth weight infants. Pediatr. Nephrol. 2004, 19, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Ketkeaw, K.; Thaithumyanon, P.; Punnahitananda, S. Nephrocalcinosis in very low birth weight infants: A single center experience. J. Med. Assoc. Thail. 2004, 87 (Suppl. S2), S72–S77. [Google Scholar]
- Cranefield, D.J.; Odd, D.E.; Harding, J.E.; Teele, R.L. High incidence of nephrocalcinosis in extremely preterm infants treated with dexamethasone. Pediatr. Radiol. 2004, 34, 138–142. [Google Scholar] [CrossRef]
- Gimpel, C.; Krause, A.; Franck, P.; Krueger, M.; von Schnakenburg, C. Exposure to furosemide as the strongest risk factor for nephrocalcinosis in preterm infants. Pediatr. Int. 2010, 52, 51–56. [Google Scholar] [CrossRef]
- Chang, H.Y.; Hsu, C.H.; Tsai, J.D.; Li, S.T.; Hung, H.Y.; Kao, H.A.; Chang, J.H.; Chung, H.Y.; Wang, H.K. Renal calcification in very low birth weight infants. Pediatr. Neonatol. 2011, 52, 145–149. [Google Scholar] [CrossRef]
- Lee, H.S.; Sung, I.K.; Kim, S.J.; Youn, Y.A.; Lee, J.Y.; Lim, G.Y.; Im, S.A.; Ku, Y.M.; Lee, J.H.; Kim, S.Y. Risk factors associated with nephrocalcinosis in preterm infants. Am. J. Perinatol. 2014, 31, 279–286. [Google Scholar] [CrossRef]
- Mohamed, G.B.; Ibrahiem, M.A.; Abdel Hameed, W.M. Nephrocalcinosis in pre-term neonates: A study of incidence and risk factors. Saudi J. Kidney Dis. Transpl. 2014, 25, 326–332. [Google Scholar] [CrossRef]
- Fayard, J.; Pradat, P.; Lorthois, S.; Bacchetta, J.; Picaud, J.C. Nephrocalcinosis in very low birth weight infants: Incidence, associated factors, and natural course. Pediatr. Nephrol. 2022, 37, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
| Ref. No (Year) | Country | Study Design | Population and Sample Size | Primary Outcome | Key Findings |
|---|---|---|---|---|---|
| [11] (1982) | USA | Cohort | 10 premature infants with NC | RUS during NICU admission | All infants were exposed to furosemide for at least 12 days at a dose of at least 2 mg/kg/day before their NC diagnosis. |
| [12] (1988) | USA | Cohort | 36 infants with BW ≤ 1500 g | RUS at 12 months of age | NC occurred in 3 of 32 infants (9%) receiving chronic furosemide therapy and resolved in 67% of cases. |
| [13] (1988) | USA | Cohort | 31 infants with BW < 1500 g | RUS in the third week of life and every three weeks thereafter | NC was diagnosed in 64% of infants. Furosemide exposure was significantly more common in the NC group (65% vs. 9%; p < 0.001). |
| [14] (1988) | USA | Cohort | 17 premature infants with NC treated with furosemide; 3 premature infants treated with furosemide without NC (control group) | RUS during NICU admission | There was no difference in the average daily dose or duration of furosemide between the groups. Resolution of NC observed in 44% of cases. |
| [15] (1991) | USA | Cohort | 79 infants with GA < 32 weeks | Serial RUS | NC was diagnosed in 27% of infants. No significant difference was found in the mean total dose of furosemide. |
| [16] (1991) | USA | Cohort | 117 infants with BW < 1750 g and BPD treated with furosemide | RUS prior to discharge and at 3–6 month intervals | 17% of infants showed evidence of NC before discharge. Infants who remained on furosemide were more likely to have persistent NC compared to those who stopped the medication (p < 0.001). |
| [17] (1992) | USA | Cohort | 27 infants with BW < 1500 g divided into 3 groups | RUS and laboratory testing for glomerular and tubular kidney function | Infants with NC had lower creatinine clearance and higher tubular dysfunction compared to the other groups. Resolution observed in 60% of cases. |
| [18] (1992) | USA | Cohort | 11 premature infants with post-hemorrhagic hydrocephalus treated with furosemide and acetazolamide | Serial RUS | 45% of infants showed evidence of NC. No correlation was found between treatment duration, total dosage, and the development of renal calculi. |
| [19] (1996) | USA | Cohort | 13 premature infants with NC exposed to furosemide, divided into 2 groups: resolution of NC and persistent NC | Serial RUS | There was no difference in the duration or cumulative dose of furosemide between the groups. Resolution observed in 46% of cases |
| [20] (1999) | Finland | Cohort | 129 infants with BW < 1500 g | RUS at 2, 6, and 12 weeks of age | 20% of infants were diagnosed with NC. The mean cumulative doses of furosemide were significantly higher in infants with NC. Resolution observed in 84% of cases. |
| [21] (2000) | The Netherlands | Cohort | 215 infants with GA < 32 weeks | RUS at 4 weeks of life and at term | NC was diagnosed in 33% of infants at 4 weeks and 41% at term. At term, furosemide exposure was higher in the group with NC (32% vs. 18%). |
| [22] (2001) | USA | Cohort | 101 infants with GA < 32 weeks or BW < 1500 g | RUS at 1 month of age and at term or NICU discharge | 16% of infants were diagnosed with NC. The median total dose of furosemide was not significantly different between the groups. |
| [23] (2002) | Germany | Cohort | 16 infants with GA < 37 weeks with an NC diagnosis | RUS during NICU admission and every 3–6 months following discharge | NC persisted in 33% of infants who received follow-up. Infants whose NC resolved received lower dosages of furosemide. Resolution observed in 69% of cases. |
| [24] (2004) | Germany | Cohort | 114 infants with BW < 1500 g, divided into 2 groups: (1) with NC; (2) without NC | RUS every 2 weeks during NICU admission | There was no difference in the duration of furosemide therapy between the two groups. |
| [25] (2004) | Thailand | Cohort | 36 infants with GA < 32 weeks and BW < 1250 g | RUS prior to NICU discharge | 39% of infants were diagnosed with NC. The mean cumulative dose and mean duration of furosemide were significantly higher in infants with NC. |
| [26] (2004) | USA | Cohort | 33 infants with GA < 28 weeks and BW < 990 g, δexamethasone trial group | RUS on study entry, day of life 28, and at discharge or 36 weeks postmenstrual age | 83% of infants with complete data were diagnosed with NC. Furosemide was used infrequently, and 88% of infants who never received it still developed NC. |
| [27] (2010) | Germany | Cohort | 55 infants with GA < 32 weeks and BW < 1500 g | RUS obtained after the first month of life | 27% of infants were diagnosed with NC. The strongest independent risk factor was furosemide therapy with a cumulative dose over 10 mg/kg. Resolution observed in 100% of cases during 2–3 years of follow-up. |
| [28] (2011) | USA | Cohort | 102 infants with GA < 34 weeks and BW < 1500 g | RUS at term or prior to NICU discharge | 6% of infants were diagnosed with NC. Furosemide exposure was more common in the NC group (33% vs. 3%). Resolution observed in 50% of cases. |
| [29] (2014) | North Korea | Cohort | 52 infants with BW < 1500 g | RUS at 4 and 8 weeks of life | Furosemide exposure did not significantly differ between the groups with and without NC. |
| [30] (2014) | Saudi Arabia | Cohort | 97 infants with GA ≤ 34 weeks | RUS at the first week of life, at term, and at one year corrected age | Furosemide exposure was more common in the NC group (50% vs. 16%). Resolution observed in 57% of cases. |
| [31] (2022) | USA | Retrospective case–control study | 265 infants with GA < 32 + 6 weeks and ≤1500 g, 80 infants with NC | RUS at 35 weeks corrected GA | Furosemide exposure and postnatal growth were identified as independent risk factors for NC. Resolution observed in 61% of cases. |
| [2] (2025) | USA | Randomized clinical trial | 80 infants with GA < 29 weeks | RUS prior to NICU discharge | Furosemide did not increase the overall incidence of adverse events, including NC. |
| Ref. No (Year) | Risk of Bias | Main Limitation/Strength |
|---|---|---|
| [11] (1982) | Critical | Limitation: Critical risk of bias due to lack of control group and no statistical analysis of NC-furosemide association |
| [12] (1988) | Serious | Limitation: No statistical tests were performed to assess the association of NC and furosemide |
| [13] (1988) | Serious | Limitation: The study did not control for illness severity, despite lower birth weight and gestational age being associated with the outcome and furosemide exposure |
| [14] (1988) | Serious | Limitation: Lack of adjustment for illness severity and a small control group |
| [15] (1991) | Moderate | Strength: Controlled for confounding with multivariate analyses and assessed dose–response relationship |
| [16] (1991) | Moderate | Strength: High follow-up rate and all infants had chronic lung disease, allowing consistent outcome assessment |
| [17] (1992) | Moderate | Strength: Robust comparator groups and included long-term follow-up |
| [18] (1992) | Serious | Limitation: No statistical tests were performed to assess the NC-furosemide association. The study also failed to report the frequency of NC in unexposed infants |
| [19] (1996) | Moderate | Strength: Study groups had similar illness severity and provided long-term follow-up with serial ultrasounds while also evaluating a dose–response relationship |
| [20] (1999) | Moderate | Strength: Dose–response relationship |
| [21] (2000) | Moderate | Strength: Large sample size with control group without NC |
| [22] (2001) | Moderate | Strength: Multivariate analyses were used to control for other risk factors for NC, and a dose–response relationship was also evaluated |
| [23] (2002) | Serious | Limitation: Absence of a control group without NC |
| [24] (2004) | Moderate | Strength: Large sample size and appropriate control groups |
| [25] (2004) | Moderate | Strength: Appropriate control group included; dose–response evaluated |
| [26] (2004) | Moderate | Strength: Comparable illness severity among all infants |
| [27] (2010) | Moderate | Strength: Multivariate analysis with dose–response assessment |
| [28] (2011) | Serious | Limitation: Lack of adjustment for illness severity and a low incidence of NC in the sample. |
| [29] (2014) | Serious | Limitation: No adjustment was made for the severity of illness |
| [30] (2014) | Serious | Limitation: No adjustment was made for the severity of illness |
| [31] (2022) | Serious | Limitation: The case and control groups differed in terms of morbidity, which introduces a high risk of confounding |
| [2] (2025) | Low | Strength: Multicenter randomized controlled trial; minimizes bias and systematically assessed NC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotis, J.; Skarlatou, A.; Fourikou, M.; Papadopoulou, A.; Chochliourou, E. Furosemide-Induced Nephrocalcinosis in Premature Neonates: A Critical Review of Observational Data. Children 2025, 12, 1442. https://doi.org/10.3390/children12111442
Dotis J, Skarlatou A, Fourikou M, Papadopoulou A, Chochliourou E. Furosemide-Induced Nephrocalcinosis in Premature Neonates: A Critical Review of Observational Data. Children. 2025; 12(11):1442. https://doi.org/10.3390/children12111442
Chicago/Turabian StyleDotis, John, Alexandra Skarlatou, Maria Fourikou, Athina Papadopoulou, and Elpis Chochliourou. 2025. "Furosemide-Induced Nephrocalcinosis in Premature Neonates: A Critical Review of Observational Data" Children 12, no. 11: 1442. https://doi.org/10.3390/children12111442
APA StyleDotis, J., Skarlatou, A., Fourikou, M., Papadopoulou, A., & Chochliourou, E. (2025). Furosemide-Induced Nephrocalcinosis in Premature Neonates: A Critical Review of Observational Data. Children, 12(11), 1442. https://doi.org/10.3390/children12111442






