The Incidence of Pulmonary Hypertension and the Association with Bronchopulmonary Dysplasia in Preterm Infants of Extremely Low Gestational Age: Single Centre Study at the Maternity Hospital of University Medical Centre Ljubljana, Slovenia
Highlights
- •
- In the first few days of life, preterm infants with a high clinical probability of pulmonary hypertension (PH) may not yet show echocardiographic signs of PH.
- •
- In preterm infants with bronchopulmonary dysplasia (BPD), late screening prior to NICU discharge (≤36 weeks PMA) may still be too early to detect PH that develops later in the course of BPD.
- •
- When there is a clear clinical indication, the absence of echocardiographic signs of PH should not be a contraindication to starting inhaled nitric oxide (iNO) or a reason to delay treatment.
- •
- Echocardiographic screening for PH should be considered beyond the neonatal period and after NICU discharge.
Abstract
1. Introduction
1.1. Definition of Pulmonary Hypertension
1.2. Diagnostic Approach to PH in Extremely Premature Infants
1.3. Definition of Bronchopulmonary Dysplasia (BPD)
1.4. Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension (BPD-PH)
2. Materials and Methods
2.1. Study Participants
2.2. Echocardiographic Studies and Measurements
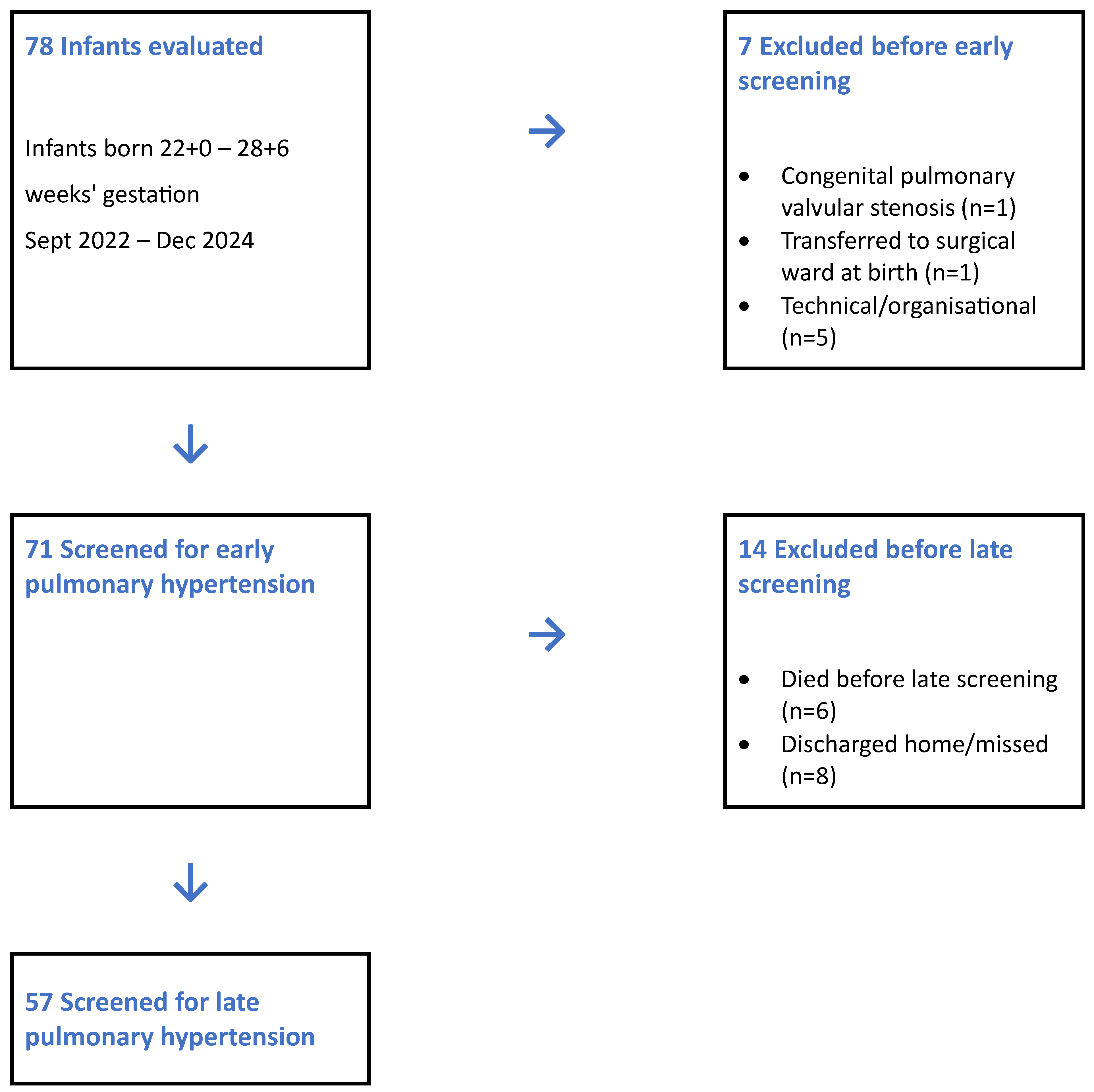
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| ATS | American Thoracic Society |
| BPD | Bronchopulmonary Dysplasia |
| BPD-PH | Bronchopulmonary Dysplasia-associated Pulmonary Hypertension |
| ELGAN | Extremely Low Gestational Age Newborn (<28 weeks) |
| FAC | Fractional Area Change (the percentage change in the right ventricular area between end-diastole and end-systole) |
| iNO | Inhaled nitric oxide |
| IVH | Intraventricular hemorrhage |
| mPAP | Mean Pulmonary Arterial Pressure |
| NEC | Necrotising enterocolitis |
| NICHD | Eunice Kennedy Shriver National Institute of Child Health and Human Development |
| NICU | Neonatal Intensive Care Unit |
| PaCO2 | Arterial partial pressure of carbon dioxide |
| PaO2 | Arterial partial pressure of oxygen |
| PDA | Patent ductus arteriosus |
| PFO | Patent foramen ovale |
| PH | Pulmonary Hypertension |
| PMA | Postmenstrual age |
| PVL | Periventricular leukomalacia |
| PVR | Pulmonary Vascular Resistance |
| ROP | Retinopathy of prematurity |
| RV | Right ventricle; right ventricular |
| RV strain | Right Ventricular Strain (advanced echocardiographic measure of right ventricular systolic function) |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TR | Tricuspid regurgitation |
| WU | Wood Units (mmHg·min/L; mmHg/(L/min)) |
References
- Ito, M.; Kato, S.; Saito, M.; Miyahara, N.; Arai, H.; Namba, F.; Ota, E.; Nakanishi, H. Bronchopulmonary Dysplasia in Extremely Premature Infants: A Scoping Review for Identifying Risk Factors. Biomedicines 2023, 11, 553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhat, R.; Salas, A.A.; Foster, C.; Carlo, W.A.; Ambalavanan, N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012, 129, e682–e689. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.F.; Omer, B.; Vachharajani, A.; Panchangam, C. Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension: Screening and Management. Neoreviews 2025, 26, e316–e327. [Google Scholar] [CrossRef] [PubMed]
- Ploegstra, M.J.; Ivy, D.D.; Beghetti, M.; Bonnet, D.; Alehan, D.; Ablonczy, L.; Mattos, S.; Bowers, D.; Humpl, T.; Berger, R.M.F.; et al. Long-term outcome of children with newly diagnosed pulmonary arterial hypertension: Results from the global TOPP registry. Eur. Heart J.-Qual. Care Clin. Outcomes 2024, 10, 66–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abman, S.H.; Hansmann, G.; Archer, S.L.; Ivy, D.D.; Adatia, I.; Chung, W.K.; Hanna, B.D.; Rosenzweig, E.B.; Raj, J.U.; Cornfield, D.; et al. Pediatric Pulmonary Hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015, 132, 2037–2099. [Google Scholar] [CrossRef]
- Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar]
- Condon, D.F.; Nickel, N.P.; Anderson, R.; Mirza, S.; de Jesus Perez, V.A. The 6th World Symposium on Pulmonary Hypertension: What’s old is new. F1000Research 2019, 8, F1000 Faculty Rev-888. [Google Scholar] [CrossRef]
- Lee, A.B.; Kumar, C.D. Continuous SpO2 and PaCO2 monitoring in neonatal pulmonary hypertension: A comprehensive approach. Neonatol. Today 2025, 18, 123–128. [Google Scholar]
- Mani, S.; Mirza, H.; Ziegler, J.; Chandrasekharan, P. Early Pulmonary Hypertension in Preterm Infants. Clin. Perinatol. 2024, 51, 171–193. [Google Scholar] [CrossRef]
- Berkelhamer, S.K.; Mestan, K.K.; Steinhorn, R.H. Pulmonary hypertension in bronchopulmonary dysplasia. Semin. Perinatol. 2013, 37, 124–131. [Google Scholar] [CrossRef]
- Kumar, V.H.S. Diagnostic approach to pulmonary hypertension in infants: Echocardiography as the mainstay for non-invasive diagnosis. Clin. Perinatol. 2025, 26, e316–e325. [Google Scholar]
- Hansmann, G.; Sallmon, H.; Roehr, C.C.; Kourembanas, S.; Austin, E.D.; Koestenberger, M.; European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr. Res. 2021, 89, 446–455. [Google Scholar] [CrossRef]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Ivy, D.D.; Abman, S.H. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008, 121, 317–325. [Google Scholar] [CrossRef]
- Fisher, M.R.; Forfia, P.R.; Chamera, E.; Housten-Harris, T.; Champion, H.C.; Girgis, R.E.; Corretti, M.C.; Hassoun, P.M. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 615–621. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary dysplasia: Executive summary of a workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The diagnosis of bronchopulmonary dysplasia in very Preterm Infants. An evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Miller, J.L.; Kinsella, J.P.; Baker, C.D.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015, 191, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doyle, L.W.; Davis, P.G.; Morley, C.J.; McPhee, A.; Carlin, J.B.; DART Study Investigators. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: A multicenter, international, randomized, controlled trial. Pediatrics 2006, 117, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, D.; Al-Balushi, M.; Al-Sabahi, A.; Weisz, D.E.; Jain, A.; Jasani, B. Pulmonary hypertension in preterm neonates with bronchopulmonary dysplasia: A meta-analysis. Arch. Dis. Child.-Fetal Neonatal Ed. 2025, 110, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Branescu, I.; Shetty, S.; Richards, J.; Vladareanu, S.; Kulkarni, A. Pulmonary hypertension in preterm infants with moder-ate-to-severe bronchopulmonary dysplasia (BPD). Acta Paediatr. 2023, 112, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Arjaans, S.; Zwart, E.A.H.; Roofthooft, M.; Kooi, E.M.W.; Bos, A.F.; Berger, R.M.F. Pulmonary hypertension in extremely preterm infants: A call to standardize echocardiographic screening and follow-up policy. Eur. J. Pediatr. 2021, 180, 1855–1865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dasgupta, S.; Richardson, J.C.; Aly, A.M.; Jain, S.K. Role of functional echocardiographic parameters in the diagnosis of bronchopulmonary dysplasia-associated pulmonary hypertension. J. Perinatol. 2022, 42, 19–30. [Google Scholar] [CrossRef]
- Mullaly, R.; Smith, A.; Murphy, C.; Armstrong, S.; Franklin, O.; McCallion, N.; El-Khuffash, A. Characteristics and outcomes of preterm infants with early pulmonary hypertension. J. Perinatol. 2025, 45, 1395–1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canadian Neonatal Network. Canadian Neonatal Network 2023 Annual Report. Toronto (ON): Canadian Neonatal Network. 2024. Available online: https://www.canadianneonatalnetwork.org (accessed on 15 August 2025).
- Australian and New Zealand Neonatal Network. ANZNN Annual Report 2022. Australian and New Zealand Neonatal Network. 2023. Available online: https://anznn.net/annual-reports (accessed on 15 August 2025).
- Horbar, J.D.; Edwards, E.M.; Greenberg, L.T.; Morrow, K.A.; Soll, R.F.; Buus-Frank, M.E.; Buzas, J.S. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA Pediatr. 2017, 171, e164396, Erratum in JAMA Pediatr. 2017, 171, 306. [Google Scholar] [CrossRef]
- Löfberg, L.; Abrahamsson, T.; Björklund, L.J.; Hellström Westas, L.; Farooqi, A.; Domellöf, M.; Ådén, U.; Gadsbøll, C.; Källén, K.; Ley, D.; et al. Respiratory support and bronchopulmonary dysplasia in infants born at 22–26 weeks gestation in Sweden, 2004–2007 and 2014–2016. Eur. Respir. J. 2025, 65, 2401203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, C.; Kim, S.; Kim, H.; Hwang, J.; Kim, S.H.; Yang, M.; Ahn, S.Y.; Sung, S.I.; Chang, Y.S. Long-term impact of late pulmonary hypertension requiring medication in extremely preterm infants with severe bronchopulmonary dysplasia. Sci. Rep. 2024, 14, 8705. [Google Scholar] [CrossRef] [PubMed]
- Arjaans, S.; Zwart, E.A.H.; Ploegstra, M.J.; Bos, A.F.; Kooi, E.M.W.; Hillege, H.L.; Berger, R.M.F. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2018, 32, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Gentle, S.J.; Travers, C.P.; Clark, M.; Carlo, W.A.; Ambalavanan, N. Patent Ductus Arteriosus and Development of Bronchopulmonary Dysplasia-associated Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2023, 207, 921–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iacobelli, S.; Allamèle-Moutama, K.; Lorrain, S.; Gouyon, B.; Gouyon, J.B.; Bonsante, F.; Logipren Collaborative Working Group. Postnatal corticosteroid exposure in very preterm infants: A French cohort study. Front. Pharmacol. 2023, 14, 1170842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parikh, S.; Reichman, B.; Kusuda, S.; Adams, M.; Lehtonen, L.; Vento, M.; Norman, M.; San Feliciano, L.; Isayama, T.; Hakansson, S.; et al. Trends, Characteristic, and Outcomes of Preterm Infants Who Received Postnatal Corticosteroid: A Cohort Study from 7 High-Income Countries. Neonatology 2023, 120, 517–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esterman, E.; Goyen, T.A.; Jani, P.; Lowe, G.; Baird, J.; Maheshwari, R.; D’Cruz, D.; Luig, M.; Shah, D. Systemic postnatal corticosteroid use for the prevention of bronchopulmonary dysplasia and its relationship to early neurodevelopment in extremely preterm infants. World J. Pediatr. 2023, 19, 586–594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- le Cras, T.D.; Markham, N.E.; Morris, K.G.; Ahrens, C.R.; McMurtry, I.F.; Abman, S.H. Neonatal dexamethasone treatment increases the risk for pulmonary hypertension in adult rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L822–L829. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, CD001145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.M.; Sie, L.; Liu, J.; Profit, J.; Lee, H.C. Evaluation of Trends in Bronchopulmonary Dysplasia and Respiratory Support Practice for Very Low Birth Weight Infants: A Population-Based Cohort Study. J. Pediatr. 2022, 243, 47–52.e2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, Y.; Yuan, L.; Zhou, J.G.; Huang, X.Y.; Lin, S.B.; Yuan, M.; He, Y.; Mao, W.Y.; Ai, D.Y.; Chen, C. Echocardiography evaluation of bronchopulmonary dysplasia-associated pulmonary hypertension: A retrospective observational cohort study. Transl. Pediatr. 2021, 10, 73–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Total (N = 71) | |
|---|---|
| Neonatal | |
| Characteristic | |
| Gestational age, weeks—median (IQR) | 25 (24–27) |
| Sex—Male, n (%) | 40 (56.3%) |
| Sex—Female, n (%) | 31 (43.7%) |
| Small for gestational age (SGA), n (%) | 14 (19.7%) |
| Apgar score at 1 min—median (IQR) | 6 (4–7) |
| Apgar score at 5 min—median (IQR) | 7 (6–8) |
| Postnatal steroids, n (%) | 30 (46.15%) |
| Surfactant, n (%) | 50 (70.4%) |
| Caffeine, n (%) | 71 (100%) |
| Morbidity and mortality | |
| Mortality, n (%) | 6 (8.5%) |
| PDA present, n (%) | 38 (53.5%) |
| PVL, n (%) | 5 (7.0%) |
| NEC, n (%) | 5 (7.0%) |
| ROP, n (%) | 33 (46.5%) |
| IVH grade 3/4, n (%) | 7 (9.9%) |
| BPD, n (%) | 49 (69.0%) |
| Maternal | |
| Cesarean section, n (%) | 28 (39.4%) |
| Prenatal steroids, n (%) | 53 (74.6%) |
| Maternal age >37 years, n (%) | 12 (16.9%) |
| Preeclampsia, n (%) | 4 (5.6%) |
| Eclampsia, n (%) | 0 (0.0%) |
| Gestational diabetes, n (%) | 5 (7.0%) |
| Total N = 71 | |
|---|---|
| Characteristic | |
| Day of early screening (median [IQR]; mean ± SD), days | 7 (7–7); 6.9 ± 2.5 |
| Oxygen need at early screening, n (%) | 45 (63.4%) |
| Noninvasive ventilation (NIV) at early screening, n (%) | 28 (39.4%) |
| Invasive ventilation (IMV) at early screening, n (%) | 30 (42.3%) |
| No ventilatory support (neither NIV nor IMV) at early screening, n (%) | 13 (18.3%) |
| Echocardiography | |
| No tricuspid regurgitation (TR = N), n (%) | 12 (16.9%) |
| TR jet velocity (m/s), mean ± SD | 0.97 ± 0.46 |
| PDA present (any L→R or R→L), n (%) | 38 (53.5%) |
| R→L shunt in PDA: n (%) of total cohort; % of PDA cases | 5 (7.0%); 13.2% |
| PFO present (any L→R or R→L), n (%) | 64 (90.1%) |
| R→L shunt in PFO: n (%) of total cohort; % of PFO cases | 10 (14.1%); 15.6% |
| RV dilatation, IV septum flattened, n (%) | 0 (0%) |
| Total N = 57 | |
|---|---|
| Characteristic | |
| Day of late screening (median [IQR]; mean ± SD), days | 70 (56–77); 66.5 ± 15.2 |
| Oxygen need at late screening, n (%) | 28 (49.1%) |
| Noninvasive ventilation (NIV) at late screening, n (%) | 7 (12.3%) |
| Invasive ventilation (IMV) at late screening, n (%) | 7 (12.3%) |
| No ventilatory support at late screening, n (%) | 43 (75.4%) |
| Echocardiography | |
| No tricuspid regurgitation (TR = N), n (%) | 36 (63.2%) |
| TR jet velocity (m/s), mean ± SD | 0.93 ± 0.47 |
| PDA present (any L→R or R→L), n (%) | 10 (17.5%) |
| R→L shunt in PDA: n (%) of total cohort; % of PDA cases | 0 (0.0%); 0.0% |
| PFO present (any L→R or R→L), n (%) | 20 (35.1%) |
| R→L shunt in PFO: n (%) of total cohort; % of PFO cases | 0 (0.0%); 0.0% |
| RV dilatation, IV septum flattened, n (%) | 1 (1.75%) |
| Gestational age at late screening (median [IQR]; mean ± SD), weeks | 35 (34–36); 34.9 ± 1.5 |
| Patient ID | GA (Weeks) | Day of Diagnosis | Survival | Diagnosis of PH | BPD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Echocardiography | |||||||||
| FiO2 | iNO/Sildenafil Response | TR Jet (m/s) | PDA | PFO | RV Dilatation/Septum Flattering | |||||
| Early screening | ||||||||||
| 1 | 26 | 4 | S | 1.0 | + | 0.85 | − | − | − | + |
| 2 | 24 | 1 | S | 0.6 | + | − | − | − | − | + |
| 3 | 26 | 5 | S | 1.0 | + | 0.80 | − | − | − | + |
| 4 | 24 | 1 | S | 1.0 | + | 0.72 | L→D | L→D | − | + |
| 5 | 22 | 1 | D | 1.0 | + | 3.06 | D→L | D→L | − | / |
| 6 | 26 | 2 | S | 1.0 | + | 0.90 | D→L | D→L | − | + |
| 7 | 25 | 2 | S | 1.0 | + | − | D→L | D→L | − | + |
| 8 | 27 | 2 | S | 0.7 | + | 1.20 | D→L | D→L | − | + |
| 9 | 25 | 2 | D | 0.6 | + | 0.60 | L→D | D→L | − | / |
| 10 | 23 | 1 | D | 1.0 | + | 3.00 | D→L | D→L | − | / |
| Median (IQR) | 25 (24–26) | 2 (1–2) | / | 1.0 (0.77–1.0) | / | 0.88 (0.78–1.65) | / | / | / | / |
| Late screening | ||||||||||
| 11 | 24 | 96 | S | 0.4 | + | − | L→D | L→D | + | + |
| GA (Weeks) | All n = 57 (%) | BPD n = 42 (%) | % with BPD |
|---|---|---|---|
| 23 | 3 (5.3) | 3 (7.1) | 100.0 |
| 24 | 14 (24.6) | 12 (28.6) | 85.7 |
| 25 | 13 (22.8) | 12 (28.6) | 92.3 |
| 26 | 8 (14.0) | 6 (14.3) | 75.0 |
| 27 | 11 (19.3) | 6 (14.3) | 54.5 |
| 28 | 8 (14.0) | 3 (7.1) | 37.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Križnar, T.; Grosek, Š.; Perme, T. The Incidence of Pulmonary Hypertension and the Association with Bronchopulmonary Dysplasia in Preterm Infants of Extremely Low Gestational Age: Single Centre Study at the Maternity Hospital of University Medical Centre Ljubljana, Slovenia. Children 2025, 12, 1441. https://doi.org/10.3390/children12111441
Križnar T, Grosek Š, Perme T. The Incidence of Pulmonary Hypertension and the Association with Bronchopulmonary Dysplasia in Preterm Infants of Extremely Low Gestational Age: Single Centre Study at the Maternity Hospital of University Medical Centre Ljubljana, Slovenia. Children. 2025; 12(11):1441. https://doi.org/10.3390/children12111441
Chicago/Turabian StyleKrižnar, Tomaž, Štefan Grosek, and Tina Perme. 2025. "The Incidence of Pulmonary Hypertension and the Association with Bronchopulmonary Dysplasia in Preterm Infants of Extremely Low Gestational Age: Single Centre Study at the Maternity Hospital of University Medical Centre Ljubljana, Slovenia" Children 12, no. 11: 1441. https://doi.org/10.3390/children12111441
APA StyleKrižnar, T., Grosek, Š., & Perme, T. (2025). The Incidence of Pulmonary Hypertension and the Association with Bronchopulmonary Dysplasia in Preterm Infants of Extremely Low Gestational Age: Single Centre Study at the Maternity Hospital of University Medical Centre Ljubljana, Slovenia. Children, 12(11), 1441. https://doi.org/10.3390/children12111441







