Development of the Medial Longitudinal Arch of the Foot in Czech Pre- and Primary School Children—A Cross-Sectional and Longitudinal Approach
Abstract
1. Introduction
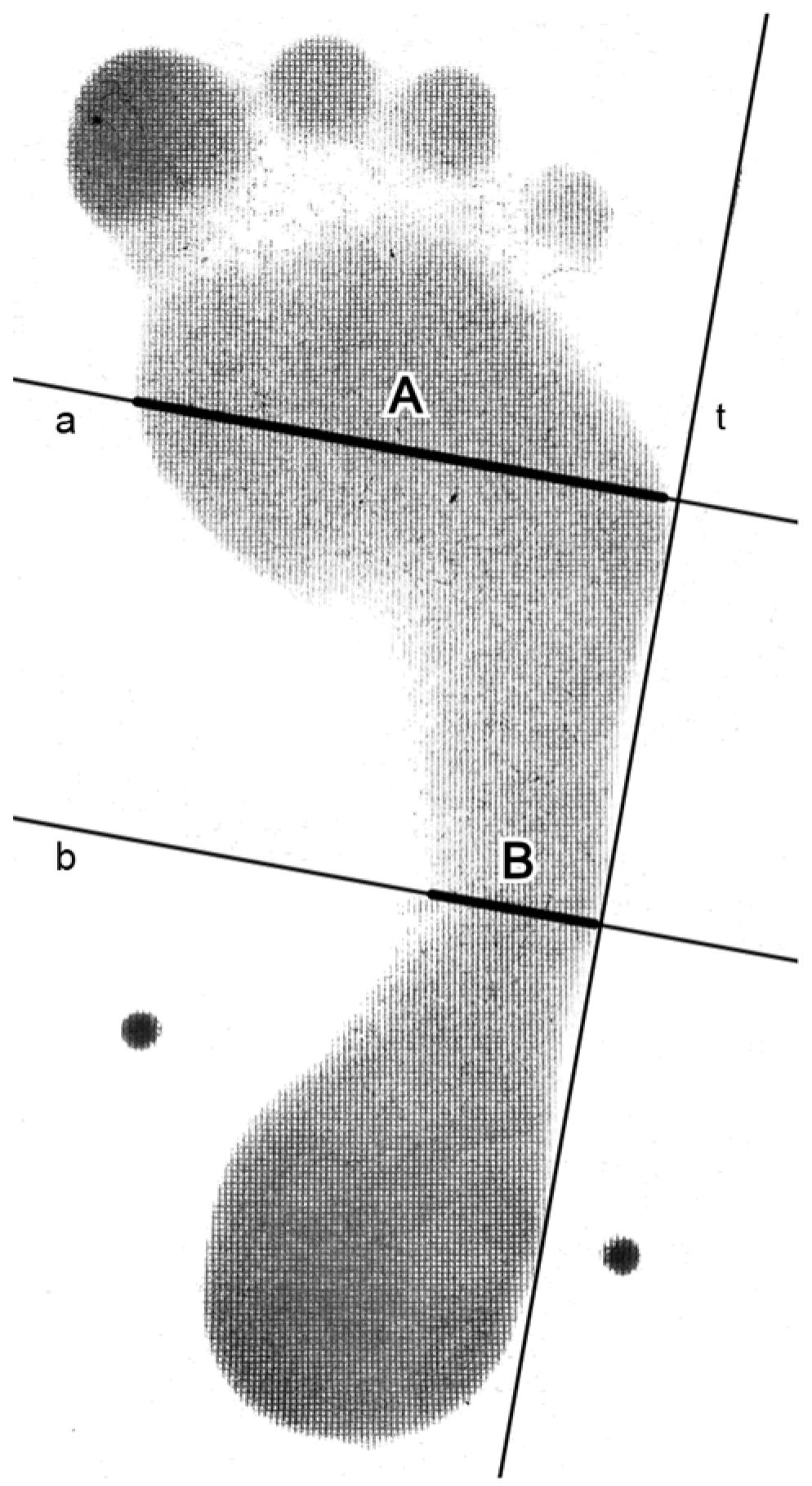
2. Materials and Methods
3. Results
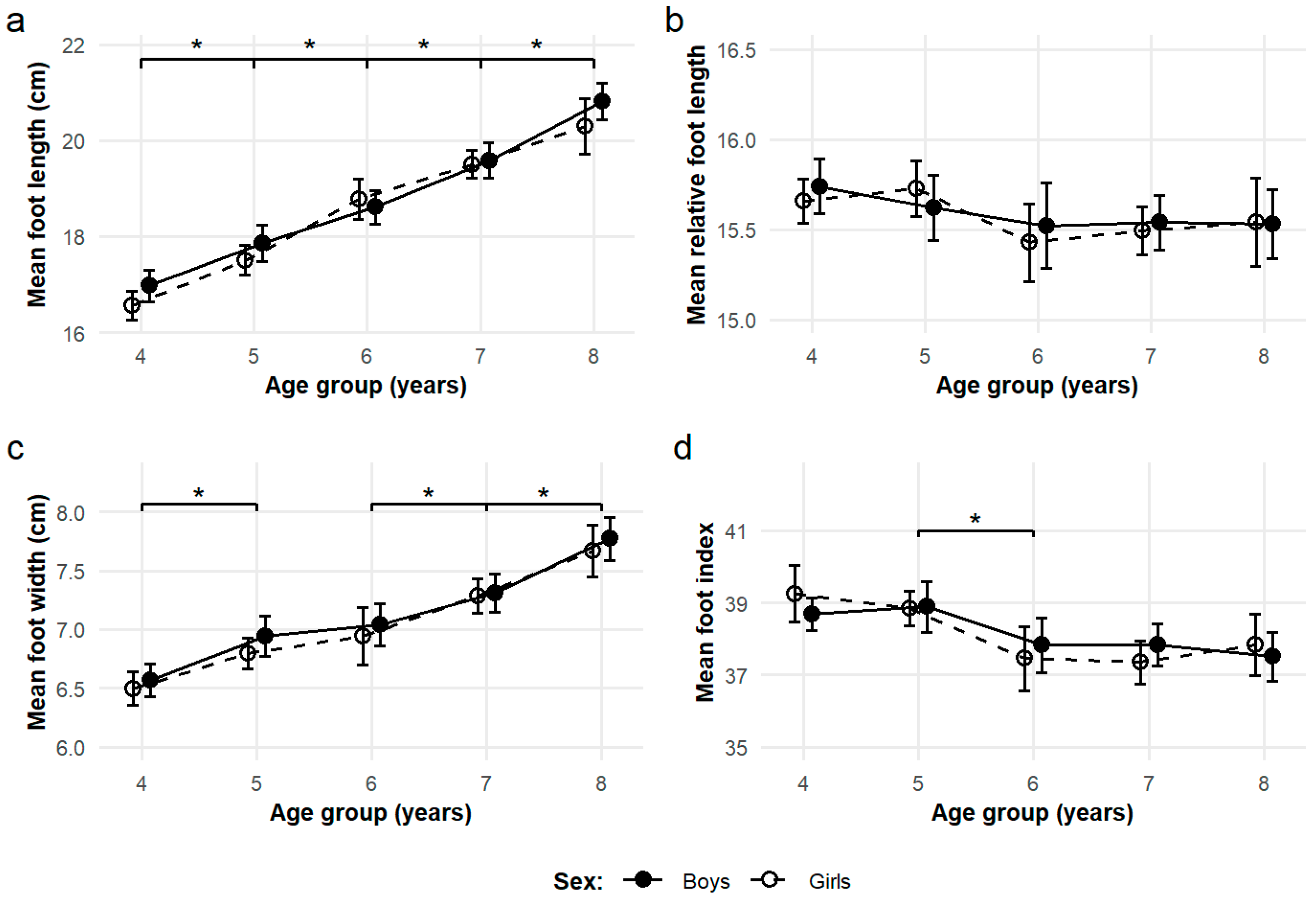
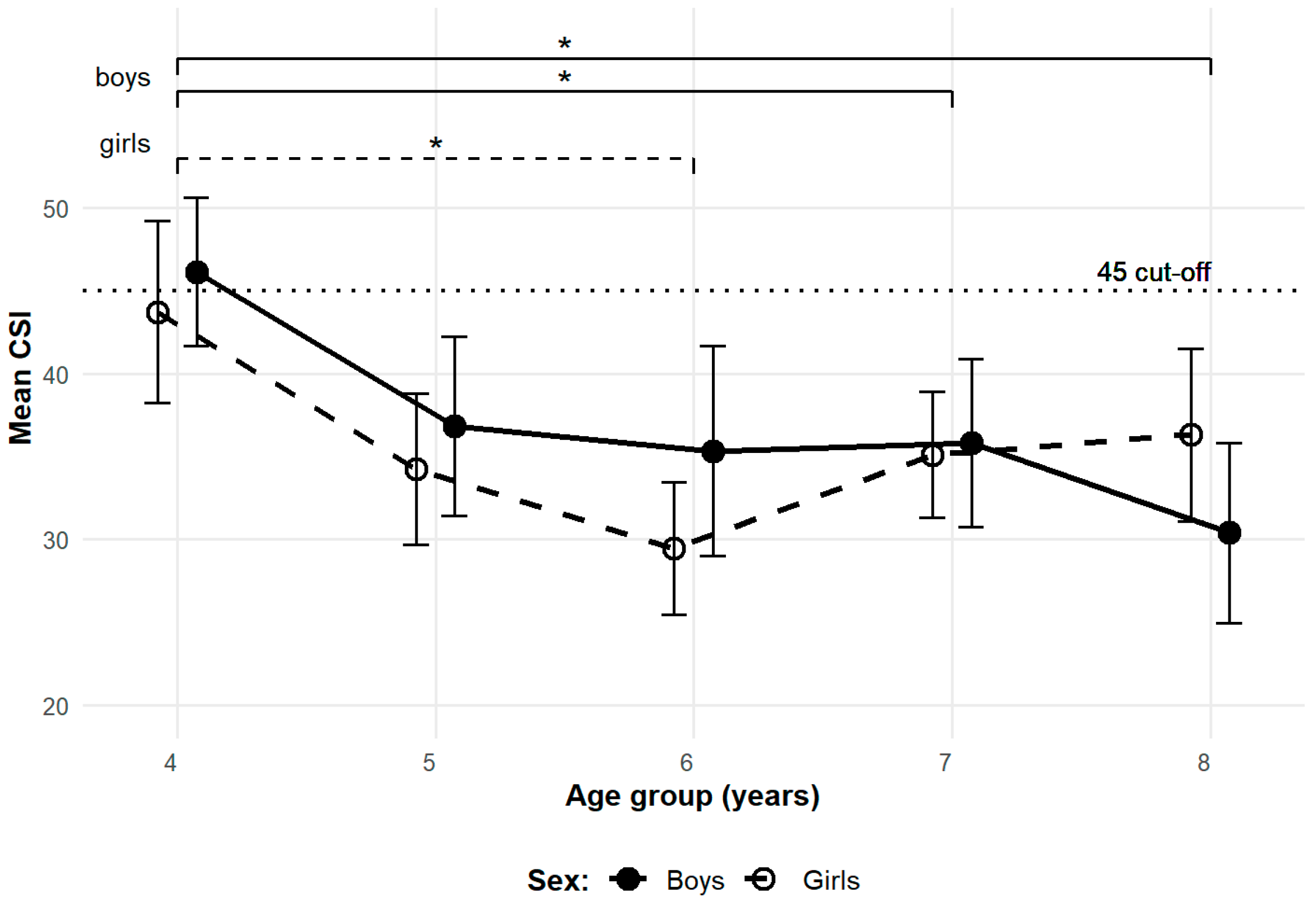
3.1. Cross-Sectional Observations
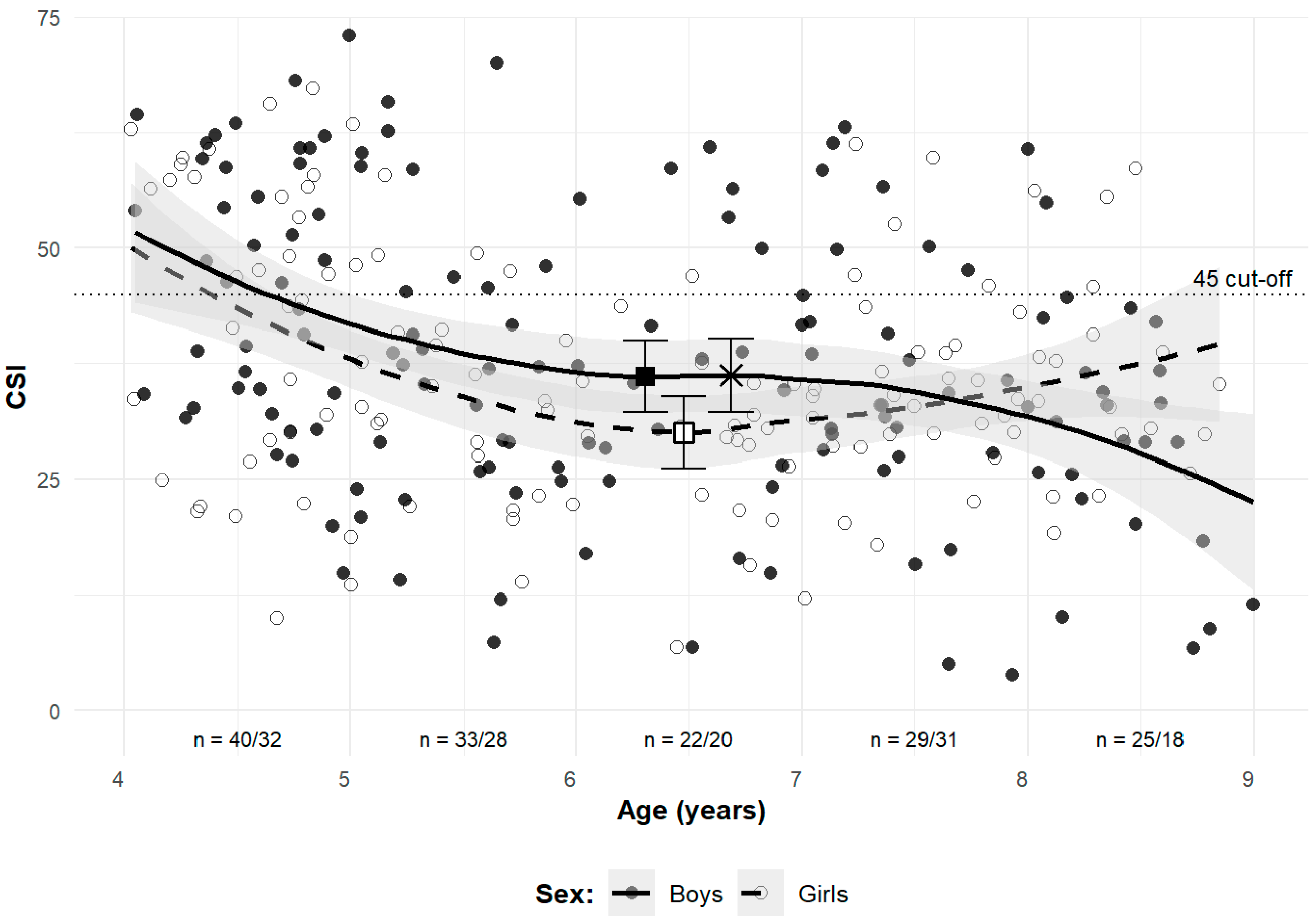
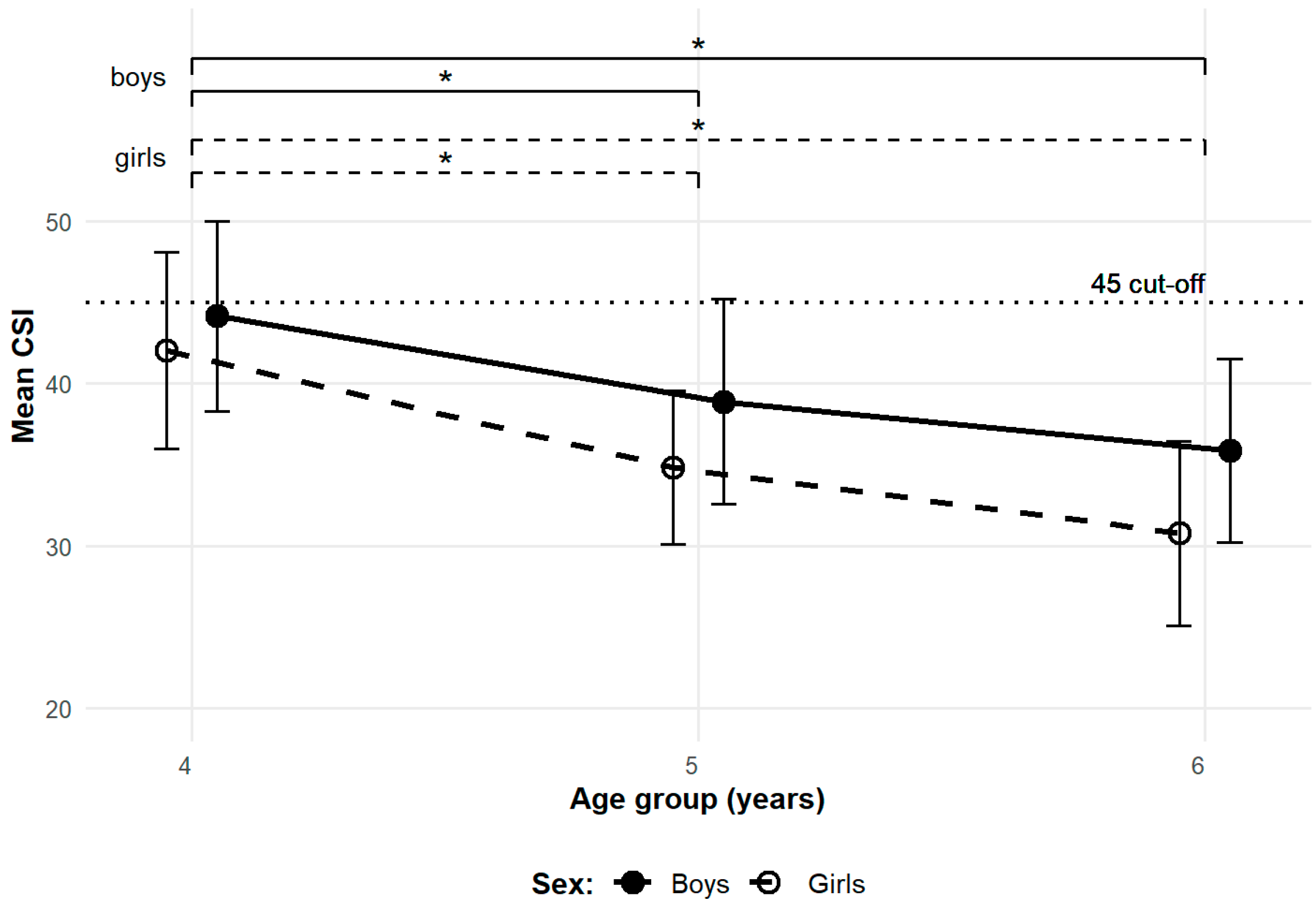
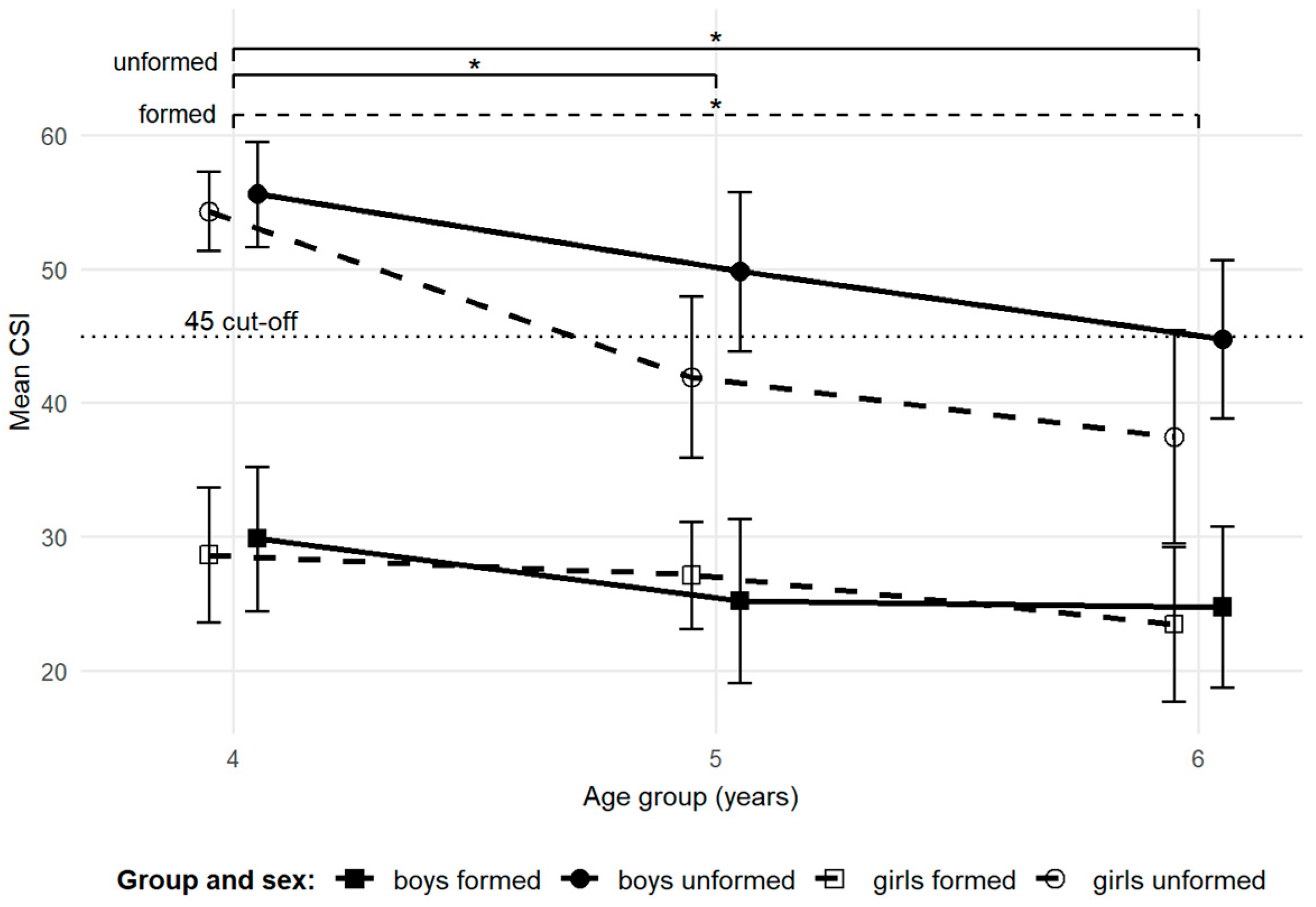
3.2. Longitudinal Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLA | Medial Longitudinal Arch |
| BMI | Body Mass Index |
| CSI | Chippaux–Smirak Index |
| CI | Confidence Intervals |
| SD | Standard Deviation |
| ICC | Intraclass Correlation Coefficient |
| ANOVA | Analysis of Variance |
| LOESS | Locally Estimated Scatterplot Smoothing |
References
- Bertsch, C.; Unger, H.; Winkelmann, W.; Rosenbaum, D. Evaluation of Early Walking Patterns from Plantar Pressure Distribution Measurements. First Year Results of 42 Children. Gait Posture 2004, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hallemans, A.; De Clercq, D.; Dongen, S.V.; Aerts, P. Changes in Foot-Function Parameters during the First 5 Months after the Onset of Independent Walking: A Longitudinal Follow-up Study. Gait Posture 2006, 23, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hennig, E.M.; Rosenbaum, D. Pressure Distribution Patterns under the Feet of Children in Comparison with Adults. Foot Ankle 1991, 11, 306–311. [Google Scholar] [CrossRef]
- Mickle, K.J.; Steele, J.R.; Munro, B.J. Is the Foot Structure of Preschool Children Moderated by Gender? J. Pediatr. Orthop. 2008, 28, 593–596. [Google Scholar] [CrossRef]
- Uden, H.; Scharfbillig, R.; Causby, R. The Typically Developing Paediatric Foot: How Flat Should It Be? A Systematic Review. J. Foot Ankle Res. 2017, 10, 37. [Google Scholar] [CrossRef]
- Gijon-Nogueron, G.; Marchena-Rodriguez, A.; Montes-Alguacil, J.; Evans, A.M. Evaluation of the Paediatric Foot Using Footprints and Foot Posture Index: A Cross-sectional Study. J. Paediatr. Child Health 2020, 56, 201–206. [Google Scholar] [CrossRef]
- Weiner, J.S.; Lourie, J.A. Human Biology: A Guide to Field Methods; International Biological Programme: London, UK; IBP Handbook: London, UK; Blackwell Scientific: Oxford, UK, 1969; ISBN 978-0-632-05550-0. [Google Scholar]
- Martin, R.; Saller, K. Lehrbuch der Anthropologie in Systematischer Darstellung Mit Besonderer Berücksichtigung der Anthropologischen Methoden. Band I; Dritte, Völlig Umgearbeitete und Erweiterte Auflage; Gustav Fischer Verlag: Stuttgart, Germany, 1957. [Google Scholar]
- Bláha, P. Anthropometric Studies of the Czech Pre-School Children from 3 to 7 Years; Institute of Sport Medicine: Prague, Czech Republic, 1990. [Google Scholar]
- Eston, R.; Reilly, T. Kinanthropometry and Exercise Physiology Laboratory Manual. Volume 1: Anthropometry, 3rd ed.; Eston, R., Reilly, T., Eds.; Taylor and Francis Group: London, UK; Routledge: New York, NY, USA, 2009; ISBN 978-0-415-43722-6. [Google Scholar]
- National Institute of Public Health. RůstCZ. 2005. Available online: https://szu.gov.cz/publikace-szu/data/hodnoceni-rustu-a-vyvoje/program-rustcz-ke-stazeni/ (accessed on 11 August 2025).
- Vignerová, J.; Riedlová, J.; Bláha, P.; Kobzová, J.; Krejčovský, L.; Brabec, M.; Hrušková, M. 6th Nation-Wide Anthropological Survey of Children and Adolescents 2001: Summary Results, 1st ed.; SZÚ: Prague, Czech Republic, 2006; ISBN 978-80-86561-30-1. [Google Scholar]
- GeoGebra Team. GeoGebra. 2025. Available online: https://www.geogebra.org/ (accessed on 11 August 2025).
- Šmiřák, J. Contribution to the Problem of Flat Feet in School and Working Youth; SPN: Prague, Czech Republic, 1960. (In Czech) [Google Scholar]
- Queen, R.M.; Mall, N.A.; Hardaker, W.M.; Nunley, J.A. Describing the Medial Longitudinal Arch Using Footprint Indices and a Clinical Grading System. Foot Ankle Int. 2007, 28, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Onodera, A.N.; Sacco, I.C.N.; Morioka, E.H.; Souza, P.S.; Sá, M.R.D.; Amadio, A.C. What Is the Best Method for Child Longitudinal Plantar Arch Assessment and When Does Arch Maturation Occur? Foot 2008, 18, 142–149. [Google Scholar] [CrossRef]
- Forriol, F.; Maiques, J.P.; Dankloff, C.; Pellico, G.L. Foot Morphology Development with Age. Gegenbaurs Morphol. Jahrb. 1990, 136, 669–676. [Google Scholar]
- Nikolaidou, M.E.; Boudolos, K.D. A Footprint-Based Approach for the Rational Classification of Foot Types in Young Schoolchildren. Foot 2006, 16, 82–90. [Google Scholar] [CrossRef]
- Chen, K.-C.; Yeh, C.-J.; Kuo, J.-F.; Hsieh, C.-L.; Yang, S.-F.; Wang, C.-H. Footprint Analysis of Flatfoot in Preschool-Aged Children. Eur. J. Pediatr. 2011, 170, 611–617. [Google Scholar] [CrossRef]
- Gill, S.V.; Keimig, S.; Kelty-Stephen, D.; Hung, Y.-C.; DeSilva, J.M. The Relationship between Foot Arch Measurements and Walking Parameters in Children. BMC Pediatr. 2016, 16, 15. [Google Scholar] [CrossRef]
- Chang, C.-H.; Yang, W.-T.; Wu, C.-P.; Chang, L.-W. Would Foot Arch Development in Children Characterize a Body Maturation Process? A Prospective Longitudinal Study. Biomed. J. 2022, 45, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Forriol, F.; Pascual, J. Footprint Analysis Between Three and Seventeen Years of Age. Foot Ankle 1990, 11, 101–104. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 11 August 2025).
- The Jamovi Project. Jamovi, Version 2.3.28; The Jamovi Project: Sydney, Australia, 2024. Available online: https://www.jamovi.org/ (accessed on 11 August 2025).
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Martínez-Nova, A.; Gijón-Noguerón, G.; Alfageme-García, P.; Montes-Alguacil, J.; Evans, A.M. Foot Posture Development in Children Aged 5 To11 Years: A Three-Year Prospective Study. Gait Posture 2018, 62, 280–284. [Google Scholar] [CrossRef]
- Stavlas, P.; Grivas, T.B.; Michas, C.; Vasiliadis, E.; Polyzois, V. The Evolution of Foot Morphology in Children Between 6 and 17 Years of Age: A Cross-Sectional Study Based on Footprints in a Mediterranean Population. J. Foot Ankle Surg. 2005, 44, 424–428. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Kotz, R.; Ledl, T.; Hauser, G.; Sluga, M. Prevalence of Flat Foot in Preschool-Aged Children. Pediatrics 2006, 118, 634–639. [Google Scholar] [CrossRef]
- Chang, J.-H.; Wang, S.-H.; Kuo, C.-L.; Shen, H.C.; Hong, Y.-W.; Lin, L.-C. Prevalence of Flexible Flatfoot in Taiwanese School-Aged Children in Relation to Obesity, Gender, and Age. Eur. J. Pediatr. 2010, 169, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.W.K.; Kong, P.W. Medial Longitudinal Arch Development of Children Aged 7 to 9 Years: Longitudinal Investigation. Phys. Ther. 2016, 96, 1216–1224. [Google Scholar] [CrossRef]
- Bosch, K.; Gerß, J.; Rosenbaum, D. Development of Healthy Children’s Feet—Nine-Year Results of a Longitudinal Investigation of Plantar Loading Patterns. Gait Posture 2010, 32, 564–571. [Google Scholar] [CrossRef]
- Staheli, L.T.; Chew, D.E.; Corbett, M. The Longitudinal Arch. A Survey of Eight Hundred and Eighty-Two Feet in Normal Children and Adults. J. Bone Jt. Surg. 1987, 69, 426–428. [Google Scholar] [CrossRef]
- Unger, H.; Rosenbaum, P.D.D.D. Gender-Specific Differences of the Foot During the First Year of Walking. Foot Ankle Int. 2004, 25, 582–587. [Google Scholar] [CrossRef]
- Gijon-Nogueron, G.; Martinez-Nova, A.; Alfageme-Garcia, P.; Montes-Alguacil, J.; Evans, A.M. International Normative Data for Paediatric Foot Posture Assessment: A Cross-Sectional Investigation. BMJ Open 2019, 9, e023341. [Google Scholar] [CrossRef]
- Vanderwilde, R.; Staheli, L.T.; Chew, D.E.; Malagon, V. Measurements on Radiographs of the Foot in Normal Infants and Children. J. Bone Jt. Surg. 1988, 70, 407–415. [Google Scholar] [CrossRef]
- Villarroya, A.M.; Esquivel, M.J.; Tomás, C.; Moreno, L.A.; Buenafé, A.; Bueno, G. Assessment of the Medial Longitudinal Arch in Children and Adolescents with Obesity: Footprints and Radiographic Study. Eur. J. Pediatr. 2009, 168, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kanatli, U.; Yetkin, H.; Cila, E. Footprint and Radiographic Analysis of the Feet. J. Pediatr. Orthop. 2001, 21, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Squibb, M.; Sheerin, K.; Francis, P. Measurement of the Developing Foot in Shod and Barefoot Paediatric Populations: A Narrative Review. Children 2022, 9, 750. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Yu, L.; Gao, Z.; Liu, W.; Mei, Q.; Gu, Y. Understanding the Role of Children’s Footwear on Children’s Feet and Gait Development: A Systematic Scoping Review. Healthcare 2023, 11, 1418. [Google Scholar] [CrossRef]
- Wolf, S.; Simon, J.; Patikas, D.; Schuster, W.; Armbrust, P.; Döderlein, L. Foot Motion in Children Shoes—A Comparison of Barefoot Walking with Shod Walking in Conventional and Flexible Shoes. Gait Posture 2008, 27, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hollander, K.; De Villiers, J.E.; Sehner, S.; Wegscheider, K.; Braumann, K.-M.; Venter, R.; Zech, A. Growing-up (Habitually) Barefoot Influences the Development of Foot and Arch Morphology in Children and Adolescents. Sci. Rep. 2017, 7, 8079. [Google Scholar] [CrossRef]
- Plesek, J.; Freedman Silvernail, J.; Hamill, J.; Jandacka, D. Running Footstrike Patterns and Footwear in Habitually Shod Preschool Children. Med. Sci. Sports Exerc. 2021, 53, 1630–1637. [Google Scholar] [CrossRef]
- Rao, U.B.; Joseph, B. The Influence of Footwear on the Prevalence of Flat Foot. A Survey of 2300 Children. J. Bone Jt. Surg. Br. Vol. 1992, 74, 525–527. [Google Scholar] [CrossRef]
- Sachithanandam, V.; Joseph, B. The Influence of Footwear on the Prevalence of Flat Foot. A Survey of 1846 Skeletally Mature Persons. J. Bone Jt. Surg. Br. Vol. 1995, 77, 254–257. [Google Scholar] [CrossRef]
- Echarri, J.J.; Forriol, F. The Development in Footprint Morphology in 1851 Congolese Children from Urban and Rural Areas, and the Relationship between This and Wearing Shoes. J. Pediatr. Orthop. B 2003, 12, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Mauch, M.; Mickle, K.J.; Munro, B.J.; Dowling, A.M.; Grau, S.; Steele, J.R. Do the Feet of German and Australian Children Differ in Structure? Implications for Children’s Shoe Design. Ergonomics 2008, 51, 527–539. [Google Scholar] [CrossRef]
- Aibast, H.; Okutoyi, P.; Sigei, T.; Adero, W.; Chemjor, D.; Ongaro, N.; Fuku, N.; Konstabel, K.; Clark, C.; Lieberman, D.E.; et al. Foot Structure and Function in Habitually Barefoot and Shod Adolescents in Kenya. Curr. Sports Med. Rep. 2017, 16, 448–458. [Google Scholar] [CrossRef]
| Variable | Sex | Age Group | ||||
|---|---|---|---|---|---|---|
| 4.00–4.99 | 5.00–5.99 | 6.00–6.99 | 7.00–7.99 | 8.00–8.99 | ||
| n | boys | 40 | 33 | 23 | 32 | 25 |
| girls | 32 | 30 | 20 | 31 | 19 | |
| Age (years) | boys | 4.6 (0.3) | 5.4 (0.3) | 6.5 (0.3) | 7.4 (0.3) | 8.4 (0.3) |
| girls | 4.5 (0.3) | 5.4 (0.3) | 6.6 (0.3) | 7.5 (0.3) | 8.4 (0.3) | |
| Body height (cm) | boys | 107.6 (4.8) | 114.4 (6.1) | 119.9 (3.7) | 126.0 (5.6) | 134.0 (5.1) |
| girls | 105.8 (4.9) | 111.7 (3.8) | 121.7 (5.2) | 125.9 (3.9) | 130.5 (6.3) | |
| BMI (kg/m2) | boys | 15.5 (1.5) | 15.5 (1.2) | 14.9 (0.9) | 15.8 (1.5) | 16.0 (1.3) |
| girls | 15.4 (1.2) | 15.3 (1.1) | 14.9 (1.3) | 16.0 (1.8) | 16.5 (1.8) | |
| BMI z-score b | boys | −0.1 (0.9) | 0.0 (0.8) | −0.4 (0.6) | −0.0 (0.9) | −0.0 (0.7) |
| girls | 0.0 (0.8) | 0.0 (0.7) | −0.4 (0.8) | 0.1 (0.8) | 0.1 (0.9) | |
| CSI | boys | 46.1 (14.4) | 36.8 (15.8) | 35.3 (15.4) | 35.8 (14.6) | 30.4 (13.9) |
| girls | 43.7 (15.9) | 34.2 (12.7) | 29.5 (9.1) | 35.1 (10.8) | 36.3 (11.6) | |
| Foot length (cm) | boys | 17.0 (1.1) | 17.9 (1.1) | 18.6 (0.9) | 19.6 (1.1) | 20.8 (1.0) |
| girls | 16.6 (0.9) | 17.5 (0.9) | 18.8 (0.9) | 19.5 (0.8) | 20.3 (1.3) | |
| Foot width (cm) | boys | 6.6 (0.4) | 6.9 (0.5) | 7.0 (0.4) | 7.3 (0.5) | 7.8 (0.5) |
| girls | 6.5 (0.4) | 6.8 (0.4) | 6.9 (0.6) | 7.3 (0.4) | 7.7 (0.5) | |
| Foot index | boys | 38.7 (1.4) | 38.9 (2.1) | 37.8 (1.8) | 37.8 (1.7) | 37.5 (1.7) |
| girls | 39.3 (2.3) | 38.8 (1.3) | 37.5 (2.0) | 37.3 (1.7) | 37.8 (1.9) | |
| Relative foot length | boys | 15.7 (0.5) | 15.6 (0.5) | 15.5 (0.6) | 15.5 (0.4) | 15.5 (0.5) |
| girls | 15.7 (0.4) | 15.7 (0.4) | 15.4 (0.5) | 15.5 (0.4) | 15.5 (0.5) | |
| Variable | Sex | Measurement b (Age Group) | ||
|---|---|---|---|---|
| 1 (4.00–4.99) | 2 (5.00–5.99) | 3 (6.00–6.99) | ||
| n | boys | 27 | 27 | 27 |
| girls | 23 | 23 | 23 | |
| Age (years) | boys | 4.5 (0.3) | 5.5 (0.3) | 6.5 (0.3) |
| girls | 4.5 (0.3) | 5.5 (0.3) | 6.5 (0.3) | |
| Body height (cm) | boys | 108.3 (6.1) | 115.0 (6.4) | 121.4 (6.8) |
| girls | 105.8 (5.1) | 112.2 (5.2) | 118.7 (5.8) | |
| BMI (kg/m2) | boys | 15.4 (1.1) | 15.2 (1.2) | 15.5 (1.8) |
| girls | 15.1 (1.1) | 15.1 (1.3) | 15.0 (1.4) | |
| BMI z-score c | boys | −0.1 (0.8) | −0.1 (0.7) | −0.1 (0.8) |
| girls | −0.2 (0.8) | −0.1 (0.8) | −0.3 (0.8) | |
| CSI | boys | 44.2 (15.5) | 38.9 (16.7) | 35.9 (15.0) |
| girls | 42.0 (14.8) | 34.8 (11.6) | 30.8 (13.9) | |
| Foot length (cm) | boys | 17.2 (1.1) | 18.0 (1.2) | 18.7 (1.0) |
| girls | 16.6 (1.0) | 17.5 (1.1) | 18.4 (1.1) | |
| Foot width (cm) | boys | 6.6 (0.4) | 6.9 (0.5) | 7.1 (0.5) |
| girls | 6.5 (0.4) | 6.8 (0.4) | 7.1 (0.4) | |
| Foot index | boys | 38.5 (1.4) | 38.6 (1.7) | 37.8 (1.5) |
| girls | 39.3 (1.4) | 38.9 (1.4) | 38.4 (2.0) | |
| Relative foot length | boys | 15.8 (0.4) | 15.6 (0.5) | 15.6 (0.5) |
| girls | 15.7 (0.5) | 15.6 (0.4) | 15.5 (0.4) | |
| Variable | Sex | Measurement | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Unformed arch group | n | boys | 15 | 15 | 15 |
| girls | 12 | 12 | 12 | ||
| Age (years) | boys | 4.4 (0.2) | 5.4 (0.2) | 6.4 (0.2) | |
| girls | 4.4 (0.3) | 5.4 (0.3) | 6.4 (0.3) | ||
| CSI | boys | 55.6 (7.7) | 49.8 (11.7) | 44.8 (11.7) | |
| girls | 54.3 (5.2) | 41.9 (10.6) | 37.5 (14.1) | ||
| Formed arch group | n | boys | 12 | 12 | 12 |
| girls | 11 | 11 | 11 | ||
| Age (years) | boys | 4.7 (0.3) | 5.7 (0.3) | 6.7 (0.3) | |
| girls | 4.6 (0.3) | 5.6 (0.3) | 6.6 (0.3) | ||
| CSI | boys | 29.9 (9.5) | 25.2 (10.8) | 24.8 (10.7) | |
| girls | 28.6 (8.5) | 27.1 (6.8) | 23.5 (9.7) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novák, J.; Novák, J.; Vážná, A.; Sedlak, P. Development of the Medial Longitudinal Arch of the Foot in Czech Pre- and Primary School Children—A Cross-Sectional and Longitudinal Approach. Children 2025, 12, 1407. https://doi.org/10.3390/children12101407
Novák J, Novák J, Vážná A, Sedlak P. Development of the Medial Longitudinal Arch of the Foot in Czech Pre- and Primary School Children—A Cross-Sectional and Longitudinal Approach. Children. 2025; 12(10):1407. https://doi.org/10.3390/children12101407
Chicago/Turabian StyleNovák, Jakub, Jan Novák, Anna Vážná, and Petr Sedlak. 2025. "Development of the Medial Longitudinal Arch of the Foot in Czech Pre- and Primary School Children—A Cross-Sectional and Longitudinal Approach" Children 12, no. 10: 1407. https://doi.org/10.3390/children12101407
APA StyleNovák, J., Novák, J., Vážná, A., & Sedlak, P. (2025). Development of the Medial Longitudinal Arch of the Foot in Czech Pre- and Primary School Children—A Cross-Sectional and Longitudinal Approach. Children, 12(10), 1407. https://doi.org/10.3390/children12101407





