Cardiac Manifestations and Persistent Myocardial Dysfunction in Multisystem Inflammatory Syndrome in Children: Insights from Conventional and Strain Echocardiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Case Definition
Clinical and Laboratory Evaluation
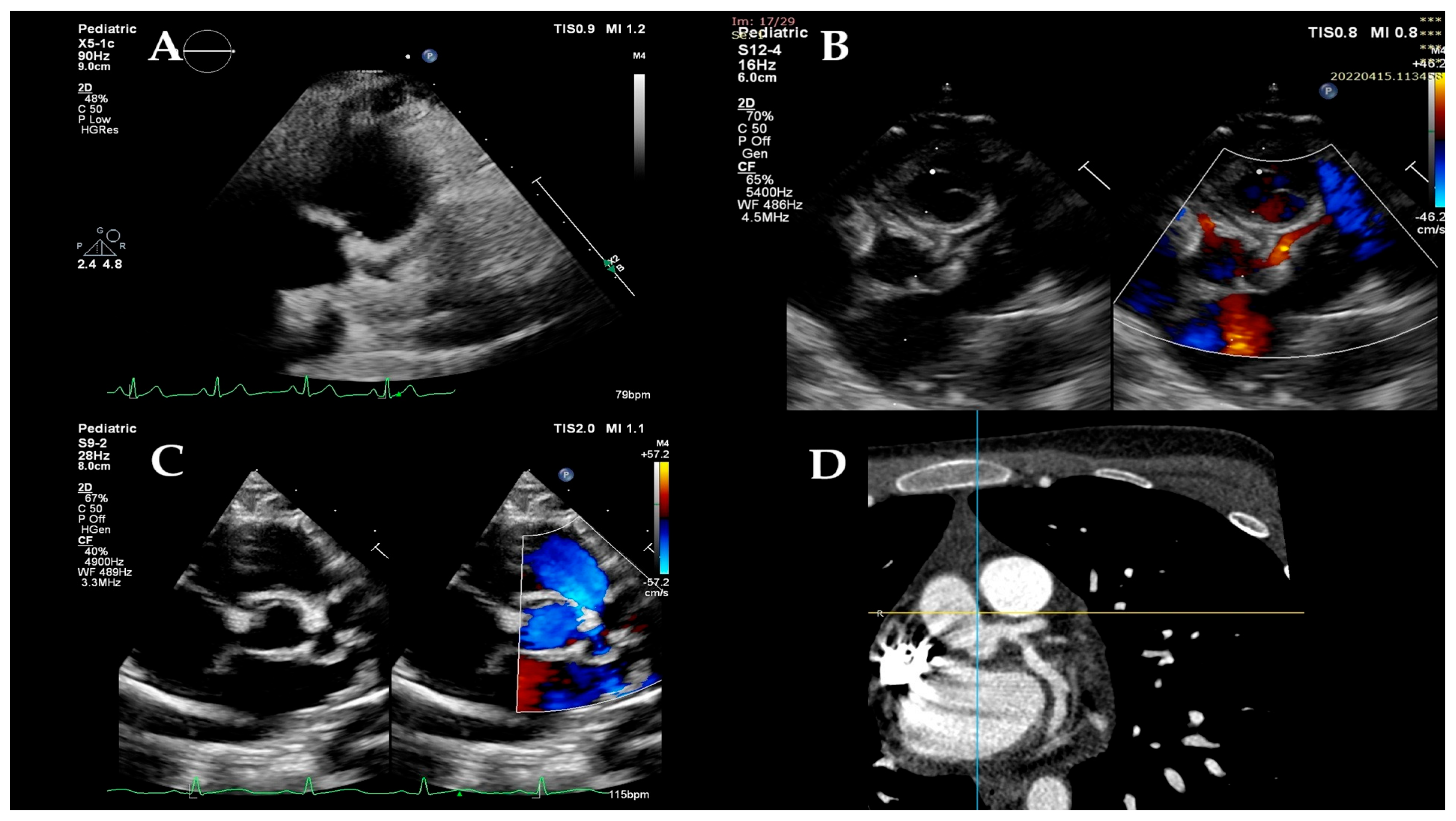
2.2. Conventional Echocardiographic Examination
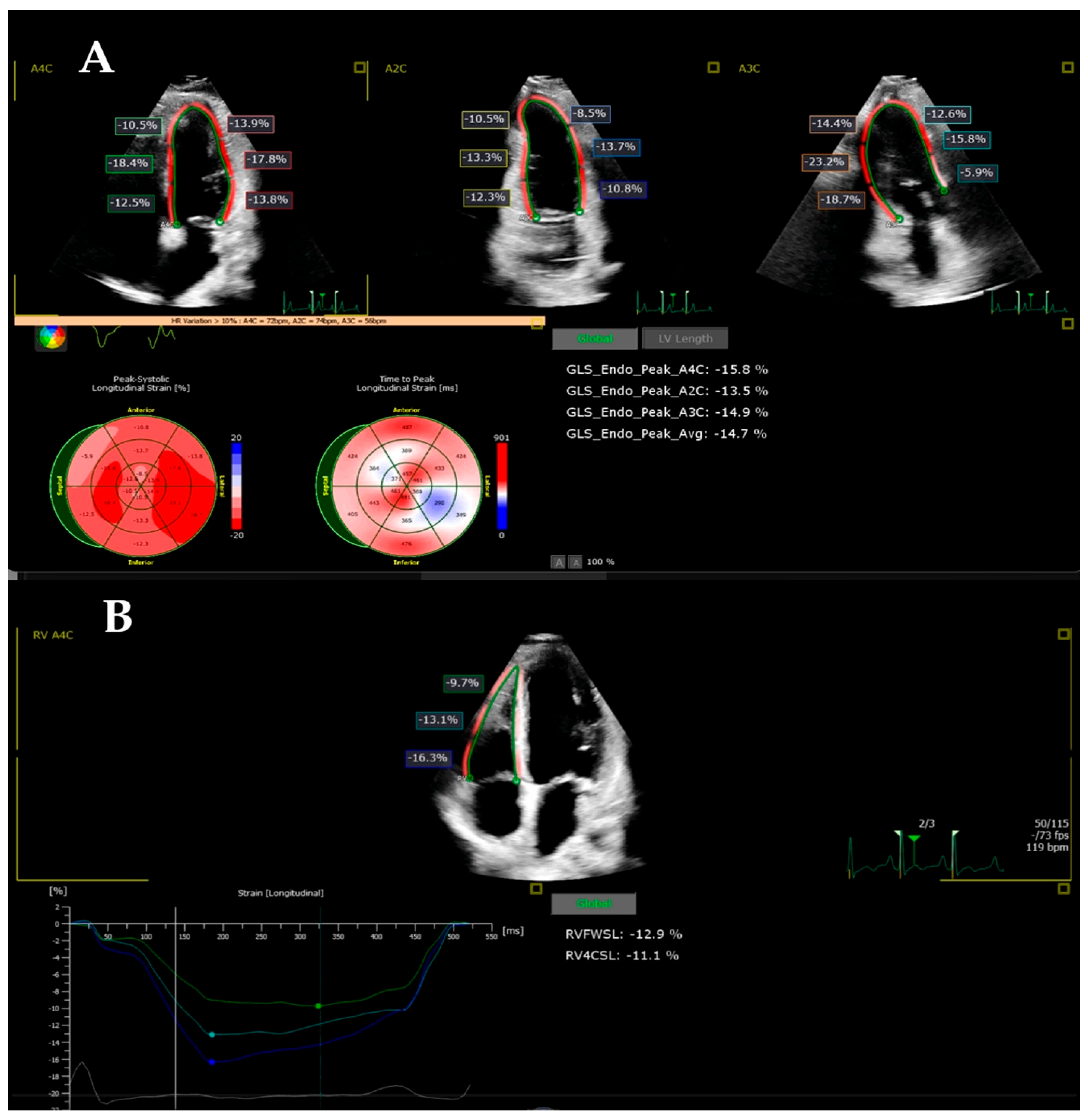
2.3. Strain Imaging
2.4. Statistical Analysis
3. Results
3.1. Description of the Children from the MIS-C Group on the DWD
3.2. Follow-Up of the Echocardiographic Strain Indices in the MIS-C Patients (DWD Versus DD)
3.3. The Impact of LV EF on the Echocardiographic Strain Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KD | Kawasaki disease |
| CDC | Centers for Disease Control and Prevention |
| WHO | World Health Organization |
| MIS-C | Multisystem inflammatory syndrome in children |
| PIMS-TS | Pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 |
| RT-PCR | Reverse-transcriptase polymerase chain reaction |
| IgG | Immunoglobulin G |
| CA | Coronary artery |
| LV | Left ventricular |
| BMI | Body mass index |
| BP | Blood pressure |
| BSA | Body surface area |
| D | Diastole |
| HR | Heart rate |
| LV-EF | Left ventricular ejection fraction |
| S | Systole |
| Y | Year |
| Z | z-score |
| DWD | Day of worst dysfunction |
| NT-proBNP | N-terminal pro b-type natriuretic peptide |
| 2D | 2-dimensional |
| DD | Echocardiogram on the discharge day |
| MAPSE | Lateral mitral annular plane systolic excursion by M-mode |
| TAPSE | Lateral tricuspid annular plane systolic excursion by M-mode |
| TDI | Peak pulsed wave tissue Doppler imaging |
| RV | Right ventricle |
| LVGLS | LV global longitudinal strain |
| RVFWLS | RV free-wall longitudinal strain |
| RV4CLS | RV four-chamber longitudinal strain |
| BIS | Basal infero-septal |
| MIS | Medium infero-septal |
| AIS | Apical infero-septal |
| BAL | Basal antero-lateral |
| MAL | Medium antero-lateral |
| AAL | Apical antero-lateral |
| BRV | Basal RV |
| MRV | Medium RV |
| ARV | Apical RV |
| hsTnI | High-sensitivity troponin I |
| IVIG | Intravenous immunoglobulin |
| ACEi | Angiotensin-converting enzyme inhibitor |
| ALT | Alanine transaminase |
| CRP | C-reactive protein |
| DD | Day of discharge |
| EKG | Electrocardiogram |
| ICU | Intensive care unit |
| LDH | Lactate dehydrogenase |
| LMCA | Left main coronary artery |
| LAD | Left anterior descending |
| RCA | Right coronary artery |
| LCx | Left circumflex artery |
| Mi | Mitral |
| LVGLS | Left ventricle global longitudinal strain |
| ARV | Apical right ventricular |
| BRV | Basal right ventricular |
| LV Max D | Maximum left ventricular diameter in diastole |
| LV max S | Maximum left ventricular diameter in systole |
| MRV | Medium right ventricular |
| RVFWLS | Right ventricular free-wall longitudinal strain |
| RV4CLS | Right ventricular four-chamber longitudinal strain |
References
- Lee, P.-I.; Hsueh, P.-R. Multisystem Inflammatory Syndrome in Children: A Dysregulated Autoimmune Disorder Following COVID-19. J. Microbiol. Immunol. Infect. 2023, 56, 236–245. [Google Scholar] [CrossRef]
- Chan, S.Y.; Tsai, Y.F.; Yen, M.Y.; Yu, W.R.; Hung, C.C.; Kuo, T.L.; Chen, C.C.; Yen, Y.F.; Huang, S.H.; Huang, T.C.; et al. Out-of-Hospital Cardiac Arrest and in-Hospital Mortality among COVID-19 Patients: A Population-Based Retrospective Cohort Study. J. Microbiol. Immunol. Infect. 2022, 55, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M. Multisystem Inflammatory Syndrome in Children (MIS-C). Curr. Allergy Asthma Rep. 2022, 22, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Gupta, S.; Sood, M.; Sharma, S.; Verma, S. A Systematic Review of Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection. Pediatr. Infect. Dis. J. 2020, 39, e340–e346. [Google Scholar] [CrossRef]
- Clarke, K.E.N.; Kim, Y.; Jones, J.; Lee, A.; Deng, Y.; Nycz, E.; Iachan, R.; Gundlapalli, A.V.; MacNeil, A.; Hall, A. Pediatric Infection-Induced SARS-CoV-2 Seroprevalence Increases and Seroprevalence by Type of Clinical Care—September 2021 to February 2022. J. Infect. Dis. 2023, 227, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children During COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An Outbreak of Severe Kawasaki-like Disease at the Italian Epicentre of the SARS-CoV-2 Epidemic: An Observational Cohort Study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). CDC Health Alert Netw. 2020. Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 21 June 2020).
- World Health Organization. Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19. Sci. Brief. 2020. Available online: https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 21 June 2020).
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; Bhutta, Z.A. COVID-19 and Multisystem Inflammatory Syndrome in Children and Adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef]
- Blatz, A.M.; Randolph, A.G. Severe COVID-19 and Multisystem Inflammatory Syndrome in Children in Children and Adolescents. Crit. Care Clin. 2022, 38, 571–586. [Google Scholar] [CrossRef]
- Dionne, A.; Son, M.B.F.; Randolph, A.G. An Update on Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Pediatr. Infect. Dis. J. 2022, 41, e6–e9. [Google Scholar] [CrossRef]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA 2022, 327, 2452. [Google Scholar] [CrossRef]
- Son, M.B.F.; Friedman, K. COVID-19: Multisystem Inflammatory Syndrome in Children (MIS-C): Clinical Features, Evaluation, and Diagnosis. In UpToDate; Fulton, D.R., Ed.; Wolters Kluwer: Waltham, MA, USA, 2024; Available online: https://www.uptodate.com/contents/covid-19-multisystem-inflammatory-syndrome-in-children-mis-c-clinical-features-evaluation-and-diagnosis (accessed on 16 August 2025).
- Miller, A.D.; Zambrano, L.D.; Yousaf, A.R.; Abrams, J.Y.; Meng, L.; Wu, M.J.; Melgar, M.; Oster, M.E.; Godfred Cato, S.E.; Belay, E.D.; et al. Multisystem Inflammatory Syndrome in Children-United States, February 2020–July 2021. Clin. Infect. Dis. 2022, 75, e1165–e1175, Erratum in: Clin. Infect. Dis. 2022, 75, 186. https://doi.org/10.1093/cid/ciac253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belot, A.; Antona, D.; Renolleau, S.; Javouhey, E.; Hentgen, V.; Angoulvant, F.; Delacourt, C.; Iriart, X.; Ovaert, C.; Bader-Meunier, B.; et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro. Surveill. 2020, 25, 2001010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsubara, D.; Kauffman, H.L.; Wang, Y.; Calderon-Anyosa, R.; Nadaraj, S.; Elias, M.D.; White, T.J.; Torowicz, D.L.; Yubbu, P.; Giglia, T.M.; et al. Echocardiographic Findings in Pediatric Multisystem Inflammatory Syndrome Associated With COVID-19 in the United States. J. Am. Coll. Cardiol. 2020, 76, 1947–1961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, R.; Dionne, A.; Ferraro, A.; Harrild, D.; Newburger, J.; VanderPluym, C.; Gauvreau, K.; Son, M.B.; Lee, P.; Baker, A.; et al. Detailed Assessment of Left Ventricular Function in Multisystem Inflammatory Syndrome in Children, Using Strain Analysis. CJC Open 2021, 3, 880–887. [Google Scholar] [CrossRef]
- Capone, C.A.; Subramony, A.; Sweberg, T.; Schneider, J.; Shah, S.; Rubin, L.; Schleien, C.; Northwell Health COVID-19 Research Consortium; Epstein, S.; Johnson, J.C.; et al. Characteristics, Cardiac Involvement, and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. J. Pediatr. 2020, 224, 141–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grimaud, M.; Starck, J.; Levy, M.; Marais, C.; Chareyre, J.; Khraiche, D.; Leruez-Ville, M.; Quartier, P.; Léger, P.L.; Geslain, G.; et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann. Intensive Care 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valverde, I.; Singh, Y.; Sanchez-de-Toledo, J.; Theocharis, P.; Chikermane, A.; Di Filippo, S.; Kuciñska, B.; Mannarino, S.; Tamariz-Martel, A.; Gutierrez-Larraya, F.; et al. Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation 2021, 143, 21–32. [Google Scholar] [CrossRef]
- Theocharis, P.; Wong, J.; Pushparajah, K.; Mathur, S.K.; Simpson, J.M.; Pascall, E.; Cleary, A.; Stewart, K.; Adhvaryu, K.; Savis, A.; et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 896–903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.E.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaitonde, M.; Ziebell, D.; Kelleman, M.S.; Cox, D.E.; Lipinski, J.; Border, W.L.; Sachdeva, R. COVID-19-Related Multisystem Inflammatory Syndrome in Children Affects Left Ventricular Function and Global Strain Compared with Kawasaki Disease. J. Am. Soc. Echocardiogr. 2020, 33, 1285–1287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sperotto, F.; Friedman, K.G.; Son, M.B.F.; VanderPluym, C.J.; Newburger, J.W.; Dionne, A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur. J. Pediatr. 2021, 180, 307–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, P.; Evans, C.; Kanthimathinathan, H.K.; Lillie, J.; Brierley, J.; Waters, G.; Johnson, M.; Griffiths, B.; du Pré, P.; Mohammad, Z.; et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc. Health 2020, 4, 669–677, Erratum in: Lancet Child Adolesc. Health 2020, 4, e35. https://doi.org/10.1016/S2352-4642(20)30233-9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) Compared with Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651, Erratum in: Circulation 2018, 138, e652. https://doi.org/10.1161/CIR.0000000000000632. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999, Erratum in: Circulation 2019, 140, e181–e184. https://doi.org/10.1161/CIR.0000000000000703. [Google Scholar] [CrossRef] [PubMed]
- Gozar, L.; Iancu, M.; Gozar, H.; Sglimbea, A.; Cerghit Paler, A.; Gabor-Miklosi, D.; Toganel, R.; Făgărășan, A.; Iurian, D.R.; Toma, D. Assessment of Biventricular Myocardial Function with 2-Dimensional Strain and Conventional Echocardiographic Parameters: A Comparative Analysis in Healthy Infants and Patients with Severe and Critical Pulmonary Stenosis. J. Pers. Med. 2022, 12, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsaied, T.; Tremoulet, A.H.; Burns, J.C.; Saidi, A.; Dionne, A.; Lang, S.M.; Newburger, J.W.; de Ferranti, S.; Friedman, K.G. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021, 143, 78–88. [Google Scholar] [CrossRef]
- Dhanalakshmi, K.; Venkataraman, A.; Balasubramanian, S.; Madhusudan, M.; Amperayani, S.; Putilibai, S.; Sadasivam, K.; Ramachandran, B.; Ramanan, A.V. Epidemiological and Clinical Profile of Pediatric Inflammatory Multisystem Syndrome—Temporally Associated with SARS-CoV-2 (PIMS-TS) in Indian Children. Indian Pediatr. 2020, 57, 1010–1014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jashvanth, H.J.; Rudrappa, S.; Patagar, P.M. Cardiovascular manifestations and echocardiographic findings in pediatric multisystemic inflammatory syndrome associated with COVID-19 (MIS-C): A retrospective study. Indian J. Child Health 2021, 8, 391–393. [Google Scholar]
- Godfred-Cato, S.; Bryant, B.; Leung, J.; Oster, M.E.; Conklin, L.; Abrams, J.; Roguski, K.; Wallace, B.; Prezzato, E.; Koumans, E.H.; et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children—United States, March–July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1074–1080, Erratum in: MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1229. https://doi.org/10.15585/mmwr.mm6935a6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khoo, N.S.; Smallhorn, J.F.; Atallah, J.; Kaneko, S.; Mackie, A.S.; Paterson, I. Altered left ventricular tissue velocities, deformation and twist in children and young adults with acute myocarditis and normal ejection fraction. J. Am. Soc. Echocardiogr. 2012, 25, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Rached-D’Astous, S.; Boukas, I.; Fournier, A.; Raboisson, M.J.; Dahdah, N. Coronary Artery Dilatation in Viral Myocarditis Mimics Coronary Artery Findings in Kawasaki Disease. Pediatr. Cardiol. 2016, 37, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Muniz, J.C.; Dummer, K.; Gauvreau, K.; Colan, S.D.; Fulton, D.R.; Newburger, J.W. Coronary artery dimensions in febrile children without Kawasaki disease. Circ. Cardiovasc. Imaging 2013, 6, 239–244. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Control n= 22 | Patient DWD n = 22 | p |
|---|---|---|---|
| Age (year) | 5 (3.00, 15.00) | 4.65 (2.37, 15.25) | 0.762 |
| Gender (male%) | 16 (72.72%) | 13 (59.09%) | |
| Weight (kg) | 18.5 (13.62, 55.75) | 21 (12.3, 63.00) | 0.763 |
| Height (cm) | 103.5 (87.25, 158.75) | 118.04 ± 43.05 | 0.763 |
| BSA (m2) | 0.74 (0.59, 1.58) | 0.79 (0.56, 1.75) | 1 |
| BSAz | 0.04 ± 0.66 | 0.29 ± 0.69 | 0.223 |
| BMI (kg/m2) | 17.88 ± 3.02 | 18.89 ± 4.75 | 0.403 |
| BMIz | 0.10 ± 1.07 | 0.10 ± 1.90 | 0.993 |
| SaO2 (%) | 99 (99.00, 100.00) | 98 (96.75, 99.00) | 0.037 |
| sBP (mmHg) | 101.00 ± 13.35 | 102.54 ± 14.57 | 0.716 |
| sBPz | 0.08 ± 0.55 | 0.24 ± 0.90 | 0.479 |
| dBP (mmHg) | 53.00 ± 10.04 | 57.13 ± 10.32 | 0.185 |
| dBPz | −0.03 ± 0.65 | 0.42 (−0.74, 1.26) | 0.132 |
| HR (b/min) | 88.54 ± 15.54 | 115.40 ± 23.37 | 0.0001 |
| HRz | −0.05 ± 0.76 | 1.88 ± 1.27 | 0.0001 |
| LV-EF (%) | 58 (53.90, 67.50) | 48.02 ± 12.04 | 0.035 |
| Parameters | n/Mean/Median | % |
|---|---|---|
| General | ||
| overweight/obesity | 10 | 45.45 |
| other comorbidities | 2 | 9.09 |
| total day fever | 5 (4.00, 6.00) | |
| day presentation | 3.00 (2.00, 5.00) | |
| DWD | 6.00 (4.00, 7.00) | |
| ICU admission | 4 | 18.18 |
| DD | 15.00 (11.00, 21.00) | |
| Organ system involvement | ||
| hypotension/sock | 1 | 4.54 |
| cardiac | 22 | 100.00 |
| renal | 3 | 13.63 |
| respiratory | 11 | 50.00 |
| hematologic | 19 | 86.36 |
| gastrointestinal | 21 | 95.45 |
| dermatologic | 12 | 54.54 |
| neurologic | 2 | 9.09 |
| total systems | 1 | 4.54 |
| Symptoms | ||
| fever | 22 | 100.00 |
| conjunctivitis | 9 | 40.90 |
| stomatitis | 8 | 36.36 |
| rash | 12 | 54.54 |
| edema | 10 | 45.45 |
| lymphadenopathy | 13 | 59.09 |
| digestive symptoms | 21 | 95.45 |
| respiratory symptoms | 11 | 50.00 |
| arthralgia | 9 | 40.90 |
| myalgia | 6 | 27.27 |
| limping | 2 | 9.09 |
| fatigue | 19 | 86.36 |
| shock | 1 | 4.54 |
| Cardiac manifestations | ||
| LV dysfunction (LV-EF: <50/49–40/<39) | 12 (n = 7/n = 2/n = 3) | 54.54 (31.81/9.09/13.63) |
| mitral regurgitation | 20 | 90.90 |
| pericardial effusion | 21 | 95.45 |
| coronary involvement | 8 | 36.36 |
| arrhythmias | 1 | 4.54 |
| EKG changes | 22 | 100.00 |
| sinus tachycardia | 21 | 95.45 |
| diffuse T wave inversions | 22 | 100.00 |
| ST segment elevation | 12 | 54.54 |
| COVID testing | ||
| RT PCR | 3 | 13.63 |
| serology | 19 | 86.36 |
| Laboratories | ||
| hsTnI (ng/L) | 84.00 (16.00, 385.00) | |
| NT-proBNP (pg/mL) | 953.00 (425.00, 8776.00) | |
| leukocytes count (cells/μL) | 9144.44 ± 4485.88 | |
| lymphocyte count (cells/μL) | 1570.00 (1050.00, 2440.00) | |
| neutrophil count (cells/μL) | 6487.22 ± 3965.78 | |
| erythrocyte count (cells/μL) | 4.32 ± 0.70 | |
| platelet count (cells/μL) | 250,977.77 ± 151,885.12 | |
| hemoglobin (g/dL) | 11.45 (10.40, 14.10) | |
| CRP (mg/L) | 113.81 ± 84.22 | |
| interleukin-6 (pg/mL) | 15.39 (11.50, 65.60) | |
| ferritin (ng/mL) | 389.25 (185.00, 593.00) | |
| fibrinogen (mg/dL) | 394.70 (330.19, 464.90) | |
| D-dimer (μg/mL) | 1785.00 (867.00, 2560.00) | |
| albumin (g/dL) | 3.57 ± 0.81 | |
| creatinine (mg/dL) | 0.59 ± 0.27 | |
| ALT (Ui/mL) | 36.75 (20.00, 44.00) | |
| LDH (Ui/mL) | 279.50 (220.00, 365.00) | |
| Treatment | ||
| Immunoglobulin infusion | 22 | 100.00 |
| Intravenous steroids | 18 | 81.81 |
| Anticoagulation Enoxaparin | 17 | 77.27 |
| Aspirin | 17 + 1 clopidogrel | 81.81 |
| Inotropic support | 3 | 13.63 |
| Interleukin receptor antagonist | 0 | 0.00 |
| ACEi Lisinopril | 13 + 1 captopril | 63.63 |
| Spironolactone | 20 | 90.90 |
| Hydrochlorothiazide | 6 | 27.27 |
| Furosemide | 3 | 13.63 |
| Beta-blockers | 3 | 13.63 |
| Parameters | n/Mean/Median | % |
|---|---|---|
| LVEF | 48.02 ± 12.04 | |
| LVEF < 50% | 12 | 54.54 |
| MAPSE (mm) | 11.12 ± 4.09 | |
| TAPSE (mm) | 15.35 ± 5.17 | |
| Mi E/A ratio | 1.45 ± 0.49 | |
| Mi E/e`lat ratio | 0.06 (0.06, 0.08) | |
| Mi s`lat | 7.57 ± 1.94 | |
| Mi s`lat (z-score) | −0.61 ± 0.97 | |
| Mi e`lat | 13.15 ± 4.00 | |
| Mi e`lat (z-score) | −0.75 (−1.77, −0.02) | |
| Mi a`lat | 7.04 ± 2.38 | |
| Mi a`lat (z-score) | 0.73 ± 1.54 | |
| basal septal dyskinesia | 22 | 100.00 |
| mitral valve regurgitation | 20 | 90.90 |
| mitral valve regurgitation > mild | 6 | 27.27 |
| tricuspid valve regurgitation | 17 | 77.27 |
| tricuspid valve regurgitation > mild | 2 | 9.09 |
| pericardial effusion | 21 | 95.45 |
| coronary artery (CA) dilation | 8 | 36.36 |
| LMCA (z-score) | 1.54 ± 1.52 | |
| LAD (z-score) | 1.20 (0.90 ± 3.60) | |
| LCx (z-score) | 0.88 (0.80 ± 1.30) | |
| proximal RCA (z-score) | 1.03 (0.57 ± 1.40) | |
| CA z-score z < 2 | 14 | 63.63 |
| CA z-score 2 ≤ z < 2.5 | 1 | 4.54 |
| CA z-score 2.5 ≤ z < 5 | 6 | 27.27 |
| CA z-score 5 ≤ z < 10 | 0 | 0.00 |
| CA z-score z ≥ 10 | 1 | 4.54 |
| Parameter | Control n= 22 | Patient DWD n = 22 | Patient DD n = 22 | c vs. pDWD | c vs. pDD | pDWD vs. pDD |
|---|---|---|---|---|---|---|
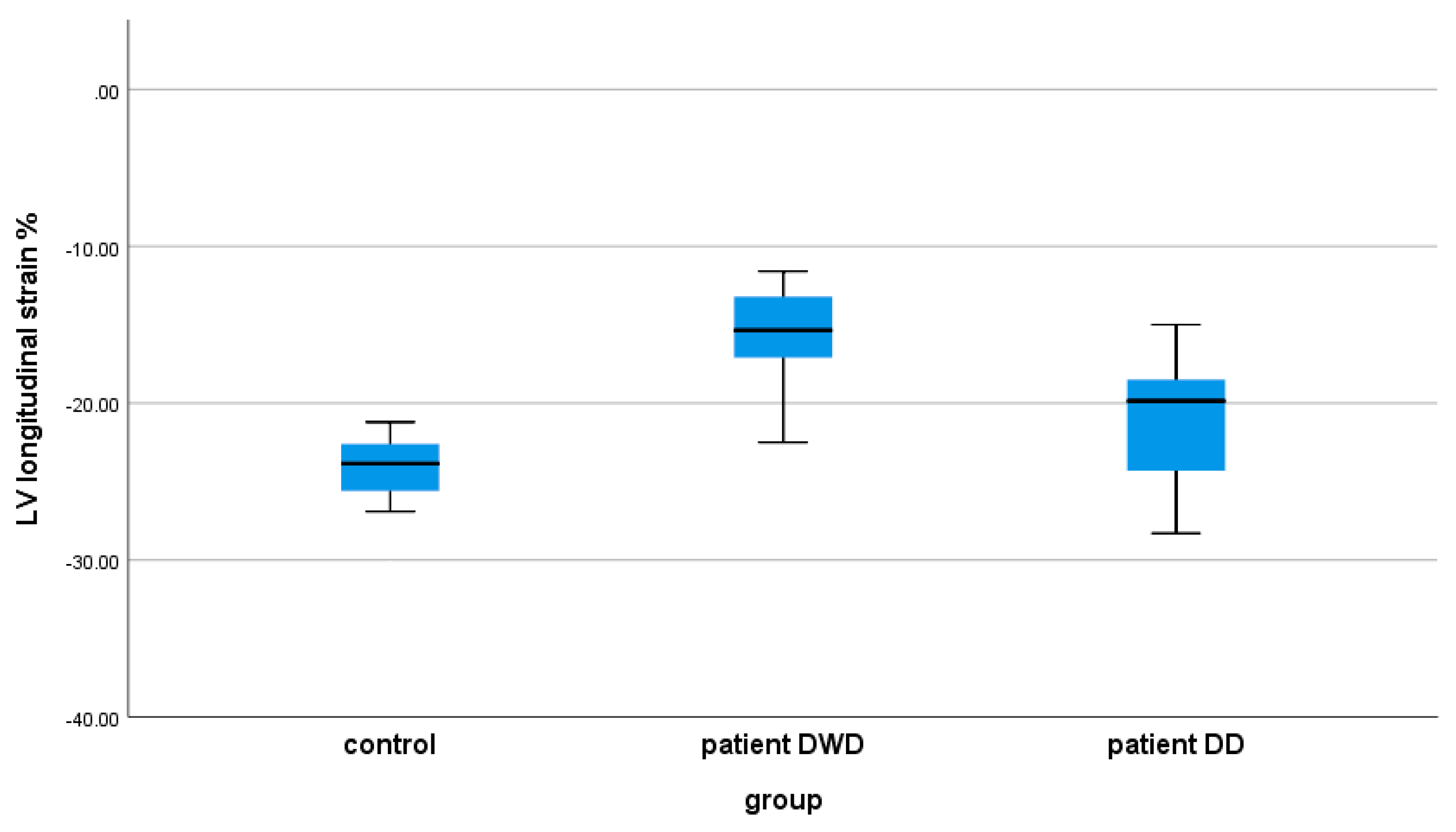
| LV GLS (%) | −24.23 ± 2.23 | −15.45 ± 4.76 | −20.63 ± 4.66 | 0.0001 | 0.014 | 0.0001 |
| LV Max D (mm) | 62.09 ± 17.64 | 63.63 ± 19.15 | 64.04 ± 18.35 | 1.000 | 1.000 | 1.000 |
| LV Max S (mm) | 49.00 ± 14.01 | 53.86 ± 17.58 | 53.13 ± 16.75 | 0.969 | 1.000 | 1.000 |
| RVFWLS (%) | −27.13 ± 13.37 | −21.94 ± 7.86 | −25.90 ± 6.76 | 0.248 | 1.000 | 0.549 |
| RV4CLS (%) | −23.90 ± 10.77 | −18.30 ± 9.90 | −21.34 ± 10.87 | 0.248 | 1.000 | 1.000 |
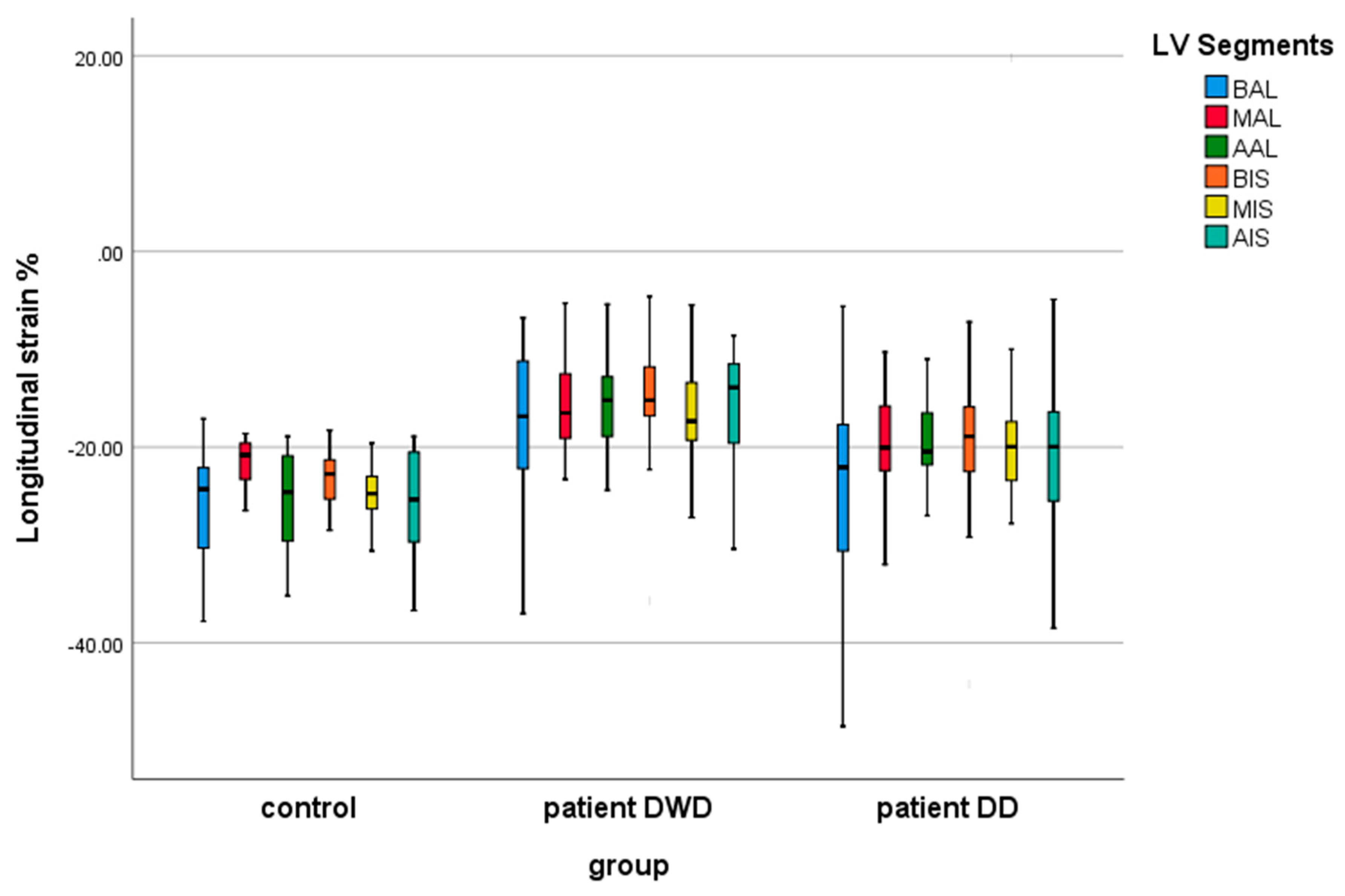
| BIS LS (%) | −23.51 ± 4.02 | −15.54 ± 6.39 | −19.89 ± 7.88 | 0.0001 | 0.183 | 0.077 |
| MIS LS (%) | −25.45 ± 4.10 | −16.70 ± 5.10 | −18.51 ± 9.82 | 0.0001 | 0.004 | 1.00 |
| AIS LS (%) | −25.62 ± 5.33 | −15.38 ± 5.48 | −20.66 ± 7.02 | 0.0001 | 0.024 | 0.015 |
| BAL LS (%) | −26.12 ± 5.56 | −18.19 ± 9.01 | −25.14 ± 10.45 | 0.010 | 1.00 | 0.028 |
| MAL LS (%) | −22.13 ± 3.85 | −15.45 ± 5.08 | −18.83 ± 6.05 | 0.0001 | 0.151 | 0.136 |
| AAL LS (%) | −25.20 ± 4.86 | −15.26 ± 4.90 | −19.30 ± 4.99 | 0.0001 | 0.001 | 0.025 |
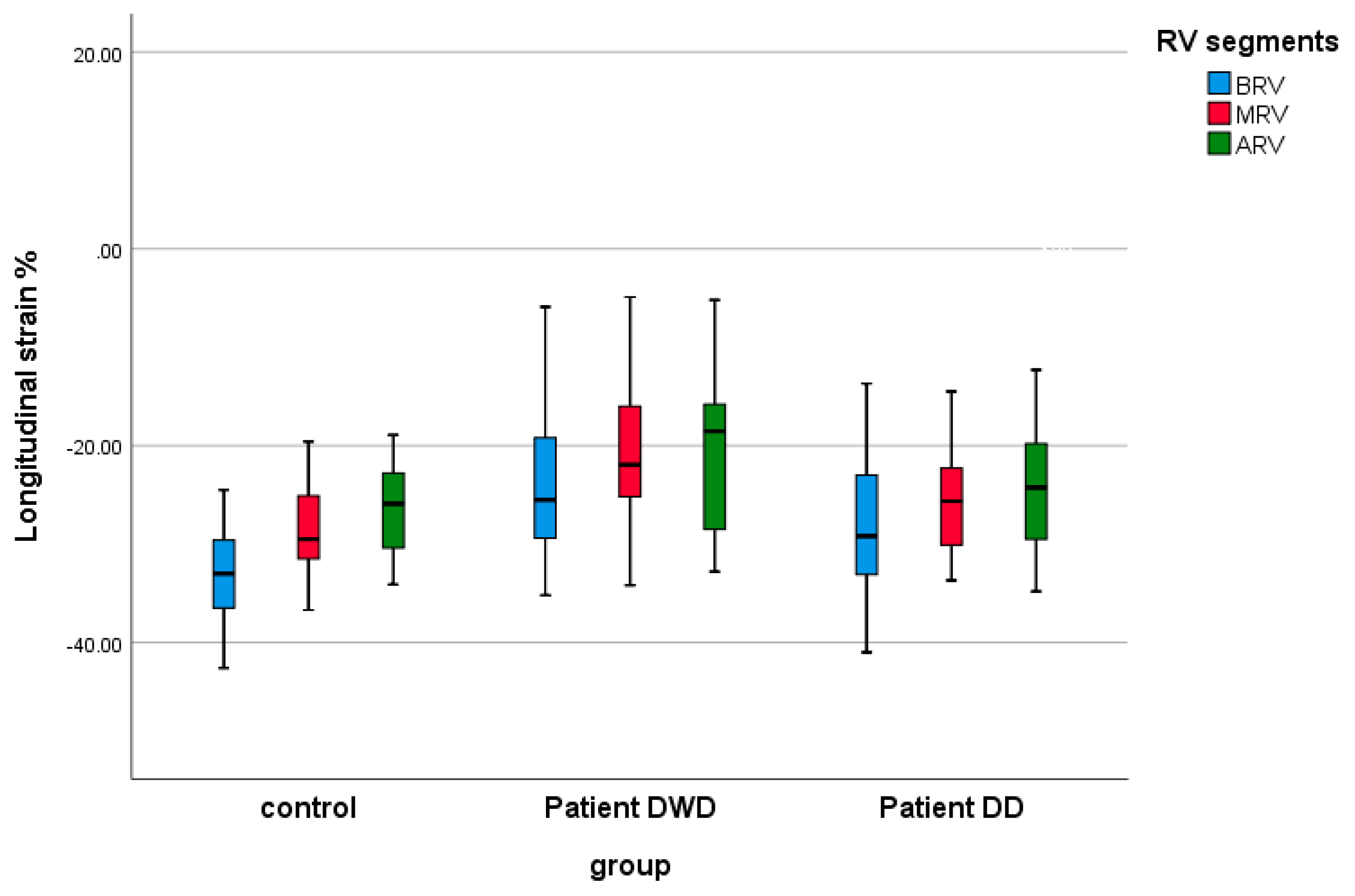
| BRV LS (%) | −33.55 ± 5.32 | −23.85 ± 7.91 | −27.72 ± 8.43 | 0.0001 | 0.032 | 0.259 |
| MRV LS (%) | −28.68 ± 4.55 | −21.11 ± 7.55 | −24.99 ± 6.83 | 0.001 | 0.187 | 0.15 |
| ARV LS (%) | −26.24 ± 4.55 | −18.59 ± 10.26 | −23.64 ± 7.50 | 0.005 | 0.819 | 0.107 |
| Parameter | Control n = 22 | Patient nEF n = 10 | Patient rEF n = 12 | p (c vs. nEF) | p (c vs. rEF) | p (nEF vs. rEF) |
|---|---|---|---|---|---|---|
| LV GLS (%) | −24.23 ± 2.23 | −17.94 ± 4.68 | −13.39 ± 3.90 | 0.0001 | 0.0001 | 0.009 |
| LV Max D (mm) | 62.09 ± 17.64 | 65.00 ± 17.74 | 62.50 ± 17.64 | 1.000 | 1.000 | 1.000 |
| LV Max S (mm) | 49.00 ± 14.01 | 53.70 ± 14.59 | 54.00 ± 20.39 | 1.000 | 1.000 | 1.000 |
| RVFWLS (%) | −27.13 ± 13.37 | −24.46 ± 6.81 | −19.84 ± 8.33 | 1.000 | 0.214 | 0.995 |
| RV4CLS (%) | −23.90 ± 10.77 | −21.69 ± 4.99 | −15.49 ± 12.16 | 1.000 | 0.081 | 0.494 |
| BIS LS (%) | −23.51 ± 4.02 | −18.16 ± 7.58 | −13.36 ± 4.41 | 0.027 | 0.0001 | 0.103 |
| MIS LS (%) | −25.45 ± 4.10 | −17.72 ± 4.83 | −15.85 ± 5.38 | 0.0001 | 0.0001 | 1.000 |
| AIS LS (%) | −25.62 ± 5.33 | −18.39 ± 6.33 | −12.88 ± 3.09 | 0.002 | 0.0001 | 0.047 |
| BAL LS (%) | −26.12 ± 5.56 | −21.21 ± 11.10 | −15.68 ± 6.25 | 0.256 | 0.001 | 0.255 |
| MAL LS (%) | −22.13 ± 3.85 | −17.07 ± 4.14 | −14.10 ± 5.56 | 0.014 | 0.0001 | 0.380 |
| AAL LS (%) | −25.20 ± 4.86 | −16.72 ± 4.95 | −14.05 ± 4.73 | 0.0001 | 0.0001 | 0.622 |
| BRV LS (%) | −33.55 ± 5.32 | −25.10 ± 7.43 | −22.80 ± 8.46 | 0.006 | 0.0001 | 1.000 |
| MRV LS (%) | −28.68 ± 4.55 | −23.20 ± 6.69 | −19.37 ± 8.06 | 0.074 | 0.0001 | 0.463 |
| ARV LS (%) | −26.24 ± 4.55 | −22.94 ± 6.55 | −14.97 ± 11.59 | 0.762 | 0.0001 | 0.052 |
| Parameter | Control n = 22 | Patient nEF n = 10 | Patient rEF n = 12 | p (c vs. nEF) | p (c vs. rEF) | p (nEF vs. rEF) |
|---|---|---|---|---|---|---|
| LV GLS (%) | −24.23 ± 2.23 | −23.34 ± 2.84 | −18.37 ± 4.76 | 1.000 | 0.0001 | 0.003 |
| LV Max D (mm) | 62.09 ± 17.64 | 65.90 ± 17.28 | 62.50 ± 19.82 | 1.000 | 1.000 | 1.000 |
| LV Max S (mm) | 49.00 ± 14.01 | 53.20 ± 15.20 | 53.08 ± 18.62 | 1.000 | 1.000 | 1.000 |
| RVFWLS (%) | −27.13 ± 13.37 | −28.09 ± 6.13 | −24.09 ± 6.97 | 1.000 | 1.000 | 1.000 |
| RV4CLS (%) | −23.90 ± 10.77 | −25.87 ± 3.40 | −17.57 ± 13.50 | 1.000 | 0.304 | 0.219 |
| BIS LS (%) | −23.51 ± 4.02 | −23.59 ± 8.72 | −16.80 ± 5.79 | 1.000 | 0.008 | 0.029 |
| MIS LS (%) | −25.45 ± 4.10 | −17.80 ± 13.54 | −19.11 ± 5.77 | 0.035 | 0.075 | 1.000 |
| AIS LS (%) | −25.62 ± 5.33 | −23.25 ± 7.85 | −18.51 ± 5.72 | 0.932 | 0.007 | 0.228 |
| BAL LS (%) | −26.12 ± 5.56 | −27.96 ± 10.52 | −22.80 ± 10.23 | 1.000 | 0.807 | 0.457 |
| MAL LS (%) | −22.13 ± 3.85 | −22.44 ± 5.41 | −15.82 ± 6.98 | 1.000 | 0.005 | 0.015 |
| AAL LS (%) | −25.20 ± 4.86 | −20.76 ± 5.22 | −18.09 ± 4.67 | 0.066 | 0.001 | 0.631 |
| BRV LS (%) | −33.55 ± 5.32 | −29.09 ± 8.48 | −26.56 ± 8.59 | 0.317 | 0.026 | 1.000 |
| MRV LS (%) | −28.68 ± 4.55 | −26.19 ± 4.32 | −24.00 ± 8.46 | 0.806 | 0.092 | 1.000 |
| ARV LS (%) | −26.24 ± 4.55 | −26.33 ± 5.15 | −21.40 ± 8.59 | 1.000 | 0.092 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șuteu, C.C.; Gozar, L.; Șuteu, N.; Hack, B.-J.; Muntean, I. Cardiac Manifestations and Persistent Myocardial Dysfunction in Multisystem Inflammatory Syndrome in Children: Insights from Conventional and Strain Echocardiography. Children 2025, 12, 1383. https://doi.org/10.3390/children12101383
Șuteu CC, Gozar L, Șuteu N, Hack B-J, Muntean I. Cardiac Manifestations and Persistent Myocardial Dysfunction in Multisystem Inflammatory Syndrome in Children: Insights from Conventional and Strain Echocardiography. Children. 2025; 12(10):1383. https://doi.org/10.3390/children12101383
Chicago/Turabian StyleȘuteu, Carmen Corina, Liliana Gozar, Nicola Șuteu, Beatrix-Julia Hack, and Iolanda Muntean. 2025. "Cardiac Manifestations and Persistent Myocardial Dysfunction in Multisystem Inflammatory Syndrome in Children: Insights from Conventional and Strain Echocardiography" Children 12, no. 10: 1383. https://doi.org/10.3390/children12101383
APA StyleȘuteu, C. C., Gozar, L., Șuteu, N., Hack, B.-J., & Muntean, I. (2025). Cardiac Manifestations and Persistent Myocardial Dysfunction in Multisystem Inflammatory Syndrome in Children: Insights from Conventional and Strain Echocardiography. Children, 12(10), 1383. https://doi.org/10.3390/children12101383





