Ultrasound Evaluation of the Deep Cerebral Venous System in Term and Preterm Neonates: Normal Features and Correlations with the Occurrence of Germinal Matrix/Intraventricular Haemorrhage
Abstract
1. Introduction
- -
- To identify to what extent the veins of the deep venous system of the brain can be identified by ultrasound in term and preterm neonates;
- -
- To determine the normal values of the angles at the confluence between the terminal vein and the internal cerebral vein;
- -
- To assess if there is a correlation between the previously mentioned angles or an abnormal venous drainage pattern observed in preterm neonates and the presence of germinal matrix haemorrhage.
2. Materials and Methods
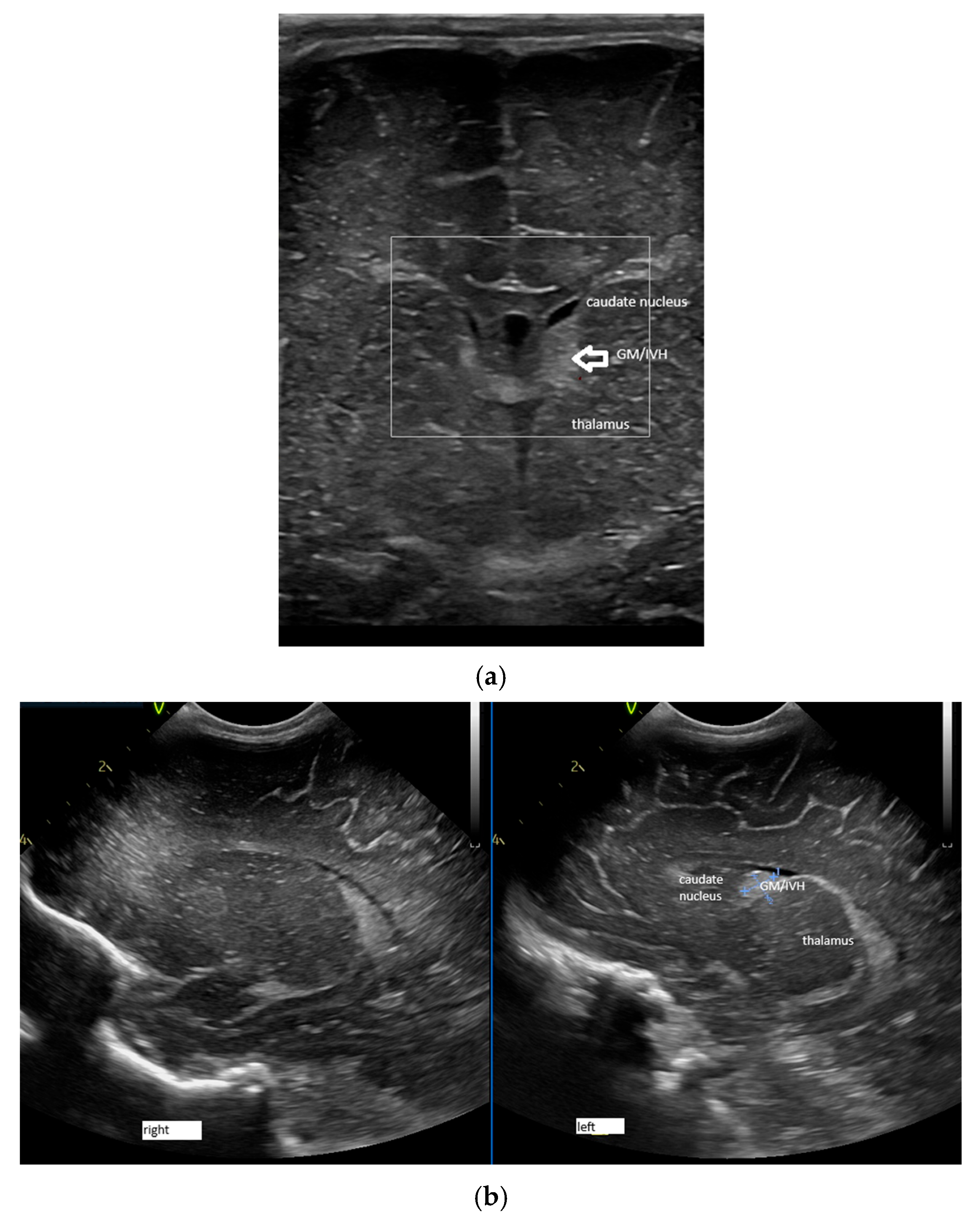
2.1. Ultrasound Technique and Measurements
- -
- The structures investigated were closer to the fontanel, and to better visualise them, we needed a high-frequency probe. The frequency used was 7.5–10 Hz. Linear probes have higher frequencies than microconvex ones [25].
- -
- -
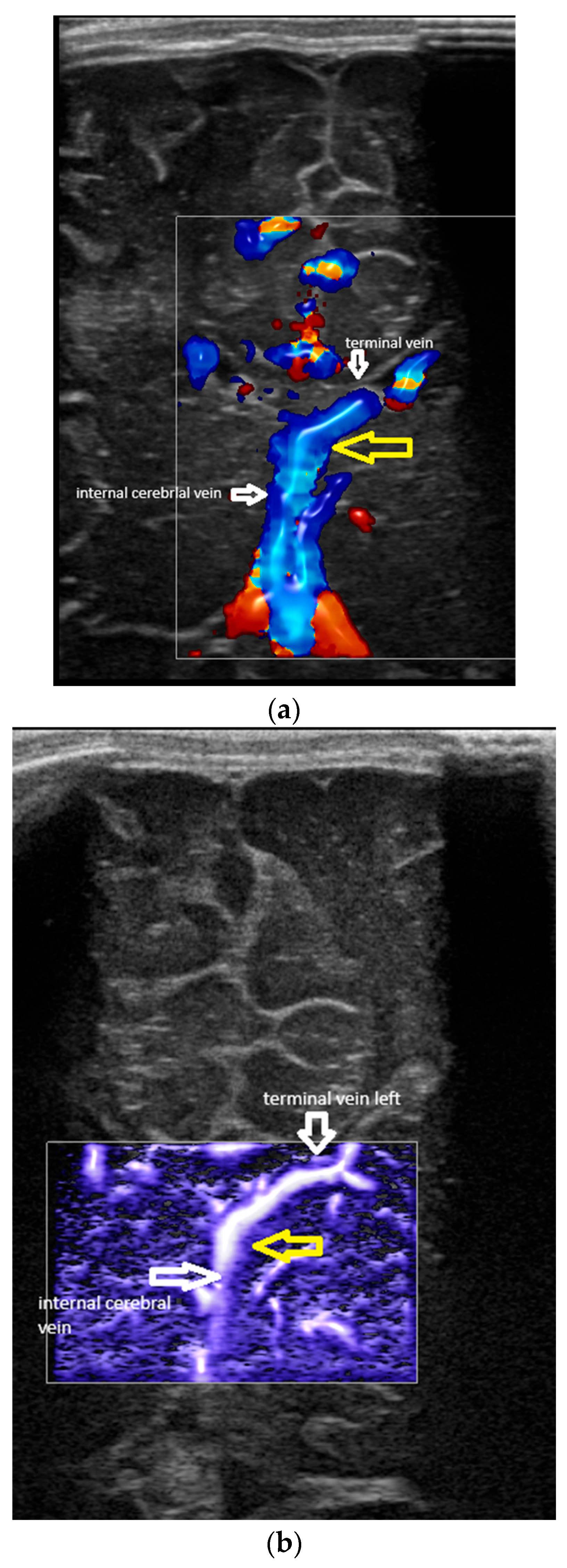
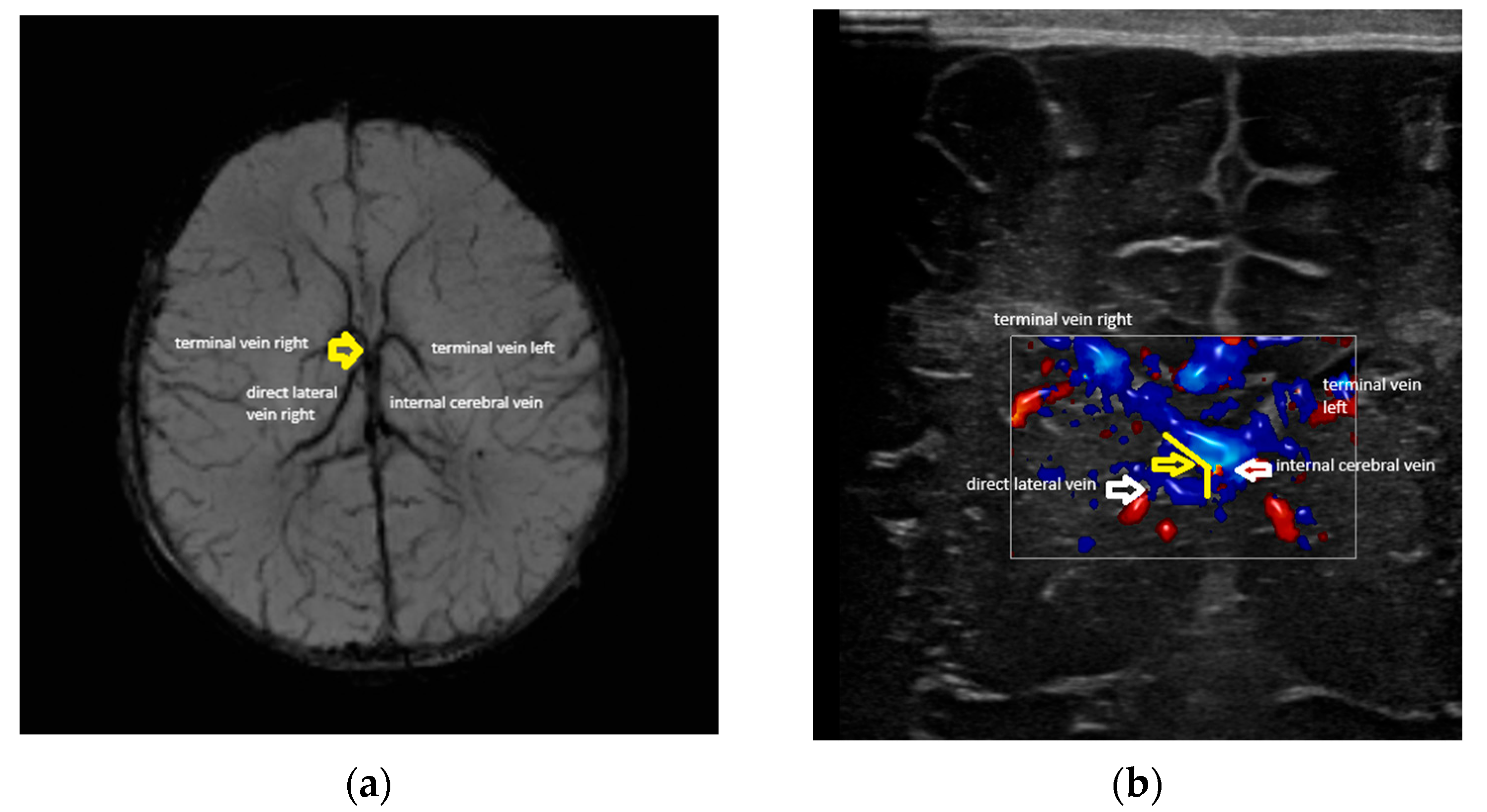
- Confluence posterior to the foramen of Monro, the most frequent variant (Figure 1).
- -
- Confluence at the level of the foramen of Monro (Figure 2).
- -
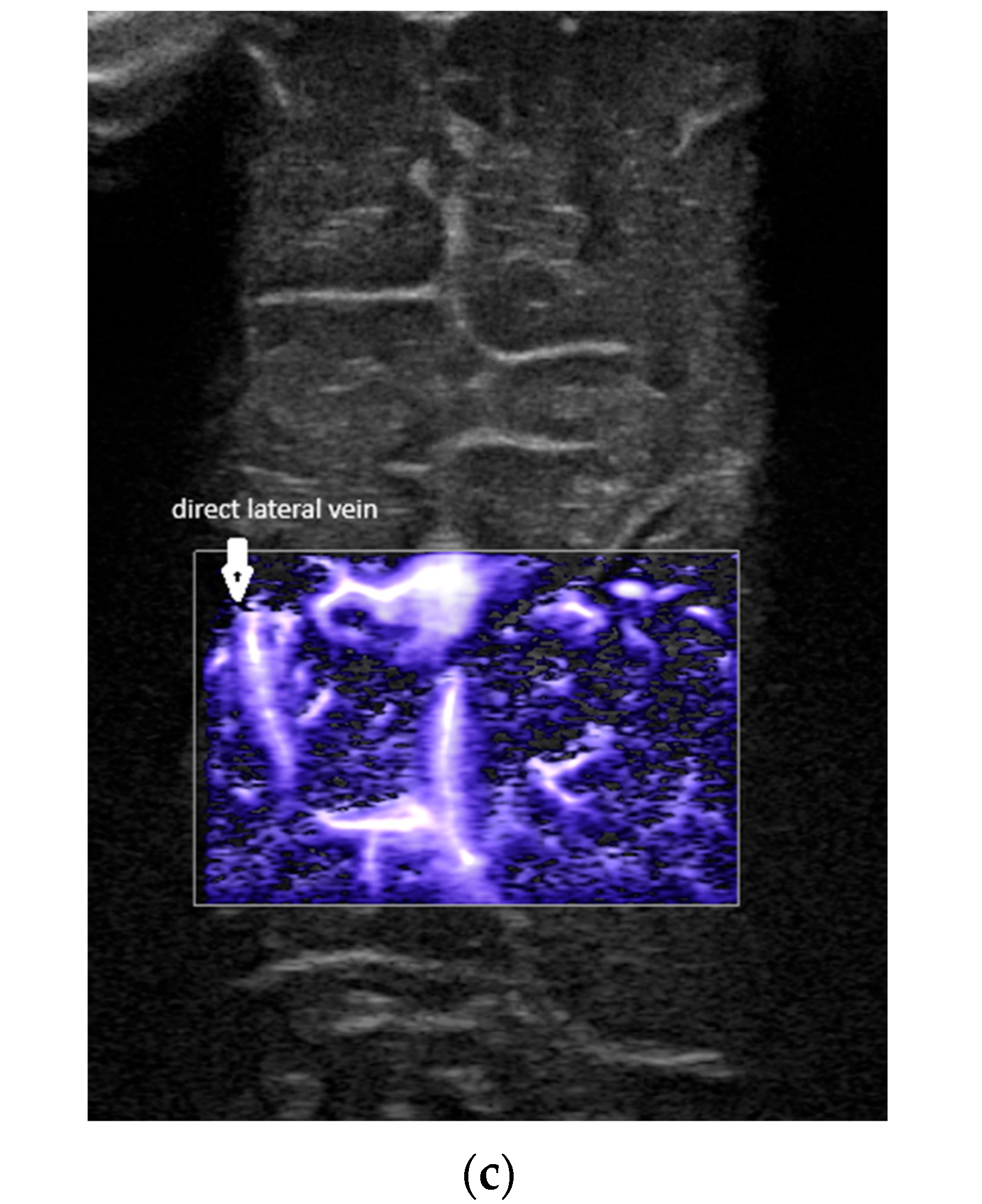
- A lack of identification of the confluence—wherein the terminal vein was absent on one or both sides—an example is the case presented in Figure 3, where the terminal vein was most probably draining in a direct lateral vein on the left side.
- -
- The type of venous pattern (normal or atypical) on the left and right;
- -
- The angles at the confluence of the terminal and internal cerebral vein on the left and right.
2.2. Rationale for Using Ultrasound: Advantages and Disadvantages
2.3. Other Variables Studied
2.4. Statistics
3. Results
3.1. Venous Drainage Patterns
3.2. Angle at Confluence Between Terminal Vein and Internal Cerebral Vein
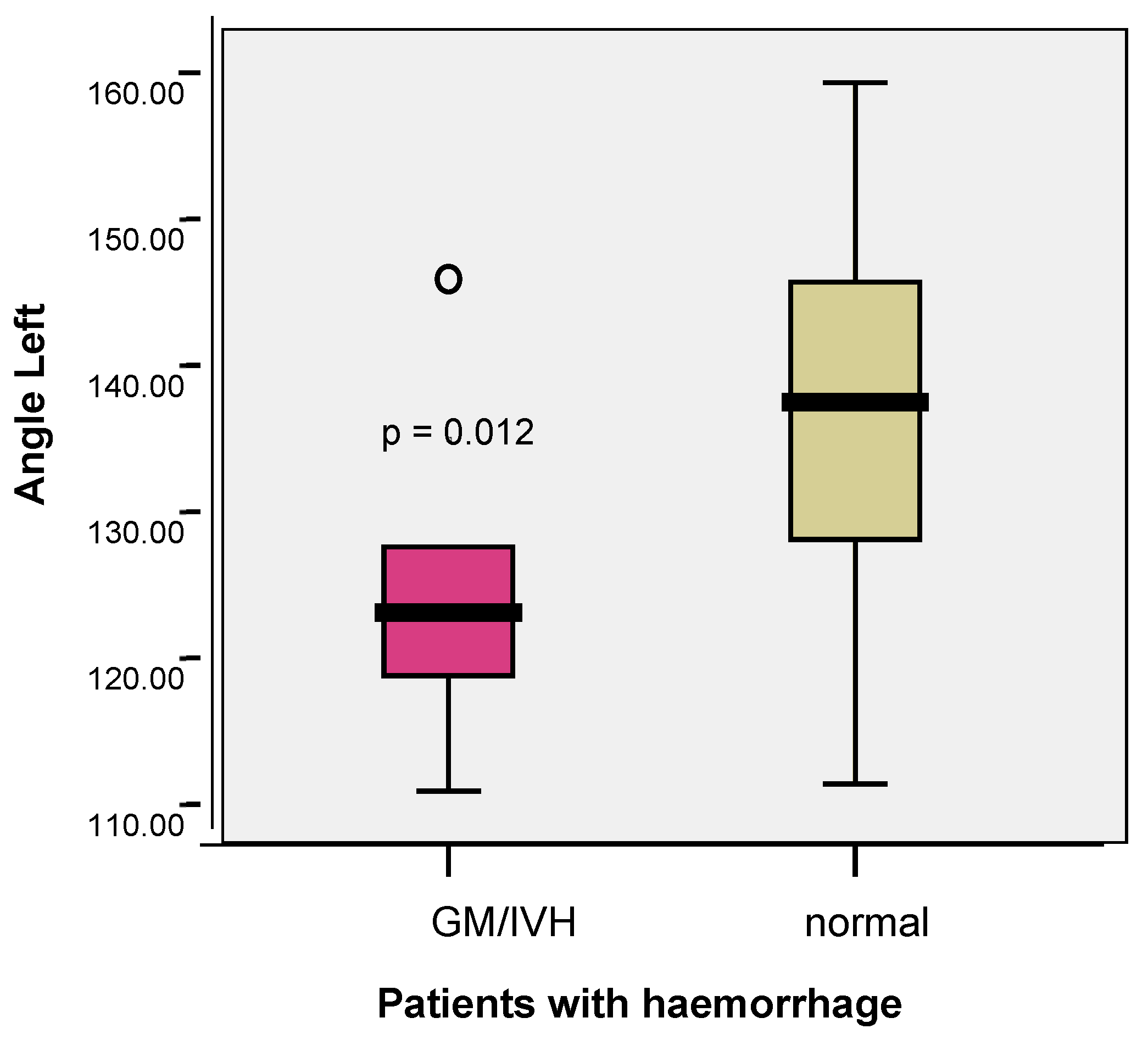
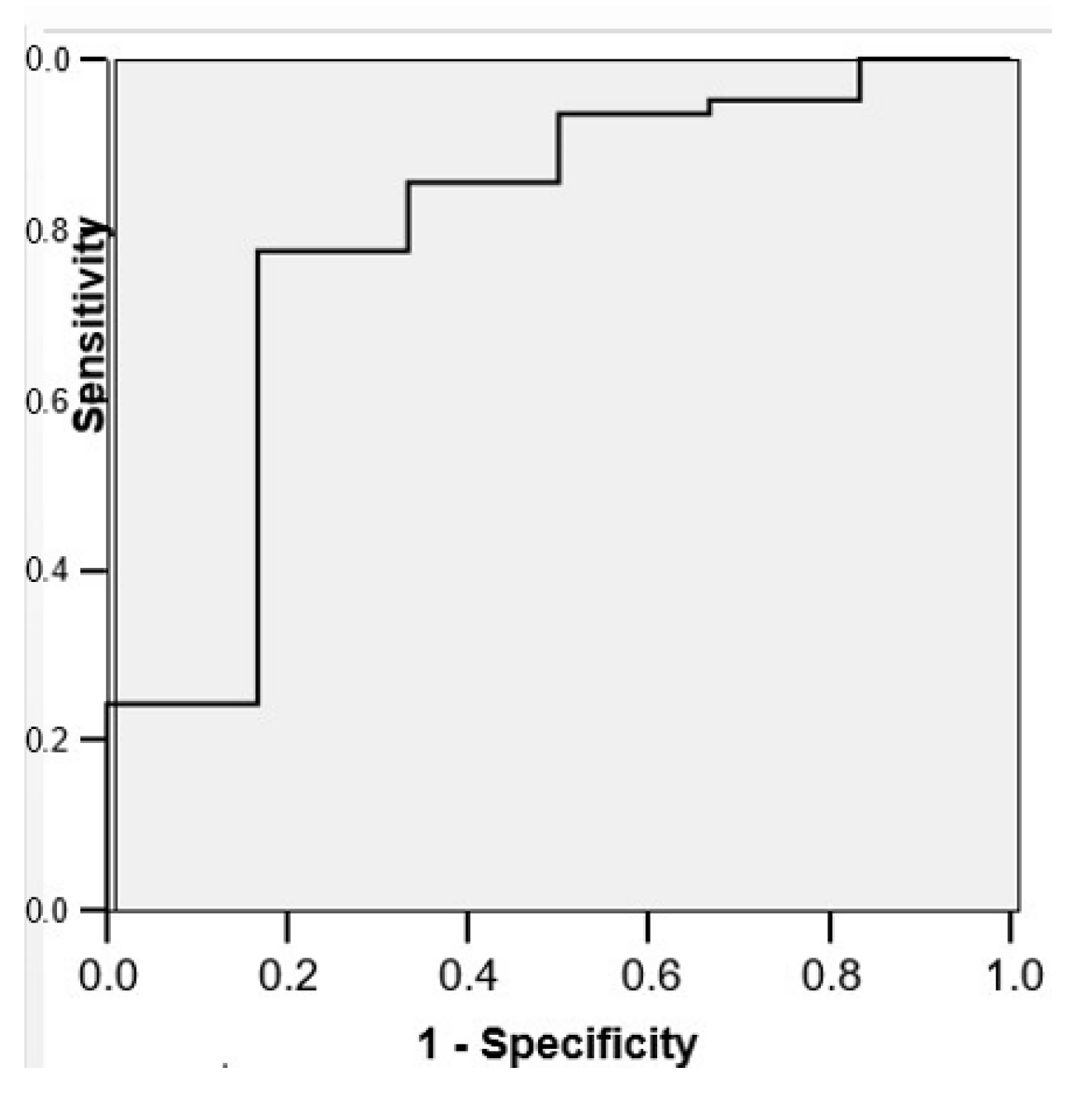
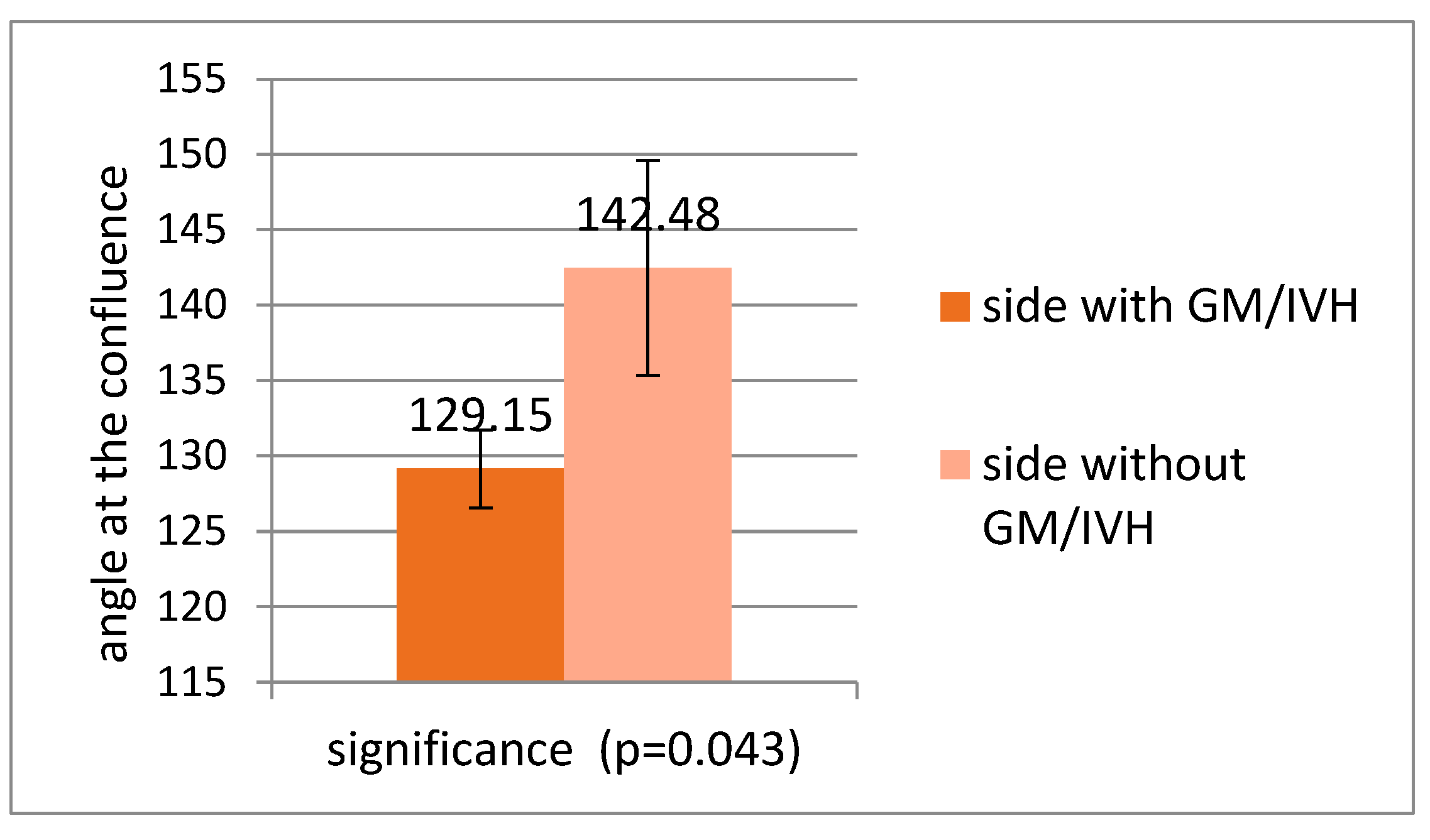
3.3. Correlations Between Venous Patterns and Angles and Risk of Intraventricular Haemorrhage in Premature Neonates
4. Discussion
- -
- In the medical literature, a slight left-sided predominance of IVH is noted. It should be mentioned that the studies did not look for this particular feature. In the study of Tortora and co-workers comparing the angles between the internal cerebral vein and the thalamostriate vein [4], a slight predominance of haemorrhage on the left was noticed, with 8/14 cases of unilateral haemorrhage occurring on the left. Also, in our database, the number of IVH cases was double on the left compared with the right.
- -
- As is already known from the medical literature, certain cerebrovascular diseases of neonates (arterial ischemic stroke) occur more often on the left than on the right due to the hemodynamic particularities of the neonatal circulation [18]. Although the asymmetries in the arterial system of the brain are well known and associated with pathologies, the venous system of the brain also presents with asymmetries of structure and calibre, especially on the left, due to developmental particularities [36]. We could speculate that these structural particularities could play a role in the occurrence of IVH more on the left than on the right, and that the vascular factors are more involved in the appearance of this lesion.
- -
- Several of the components of the bundle of care for the prevention of GM/IVH in premature infants are related to venous return and, particularly, to avoidance of increased venous pressure (maintaining the head on the midline and the avoidance of placing the head lower than the body position [37,38]), a fact underlying, once more, the importance of venous factors in the appearance and progression of the lesion.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CPAP | Continuous positive airway pressure |
| GM/IVH | Germinal matrix/intraventricular haemorrhage |
| ICV | Internal cerebral vein |
| MI | Mechanical index |
| MRI | Magnetic resonance imaging |
| MVI | Microvascular imaging |
| ROC curve | Receiving operating characteristic curve |
| TV | Terminal vein |
| TI | Thermal index |
References
- Chen, Z.; Qiao, H.; Guo, Y.; Li, J.; Miao, H.; Wen, C.; Wen, X.; Zhang, X.; Yang, X.; Chen, C. Visualization of Anatomic Variation of the Anterior Septal Vein on Susceptibility-Weighted Imaging. PLoS ONE 2016, 11, e0164221. [Google Scholar] [CrossRef]
- Taoka, T.; Fukusumi, A.; Miyasaka, T.; Kawai, H.; Nakane, T.; Kichikawa, K.; Naganawa, S. Structure of the Medullary Veins of the Cerebral Hemisphere and Related Disorders. RadioGraphics 2017, 37, 281–297. [Google Scholar] [CrossRef]
- Tortora, D.; Severino, M.; Malova, M.; Parodi, A.; Morana, G.; Ramenghi, L.; Rossi, A. Variability of Cerebral Deep Venous System in Preterm and Term Neonates Evaluated on MR SWI Venography. Am. J. Neuroradiol. 2016, 37, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Tortora, D.; Severino, M.; Malova, M.; Parodi, A.; Morana, G.; Sedlacik, J.; Govaert, P.; Volpe, J.J.; Rossi, A.; Ramenghi, L.A. Differences in subependymal vein anatomy may predispose preterm infants to GMH–IVH. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 103, F59–F65. [Google Scholar] [CrossRef] [PubMed]
- Couture, A.P.; Veyrac, C. Transfontanellar Doppler Imaging in Neonates; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Dean, L.M.; A Taylor, G. The Intracranial Venous System in Infants: Normal and Abnormal Findings on Duplex and Color Doppler Sonography. Am. J. Roentgenol. 1995, 164, 151–156. [Google Scholar] [CrossRef]
- Taylor, G.A. Intracranial venous system in the newborn: Evaluation of normal anatomy and flow characteristics with color Doppler US. Radiology 1992, 183, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Horsch, S.; Schwarz, S.; Arnaez, J.; Steggerda, S.; Arena, R.; Govaert, P. Cerebral Doppler imaging in neonates: A guide for clinical applications and diagnosis. Dev. Med. Child Neurol. 2024, 66, 1570–1589. [Google Scholar] [CrossRef]
- Huang, Y.P.; Wolf, B.S. Veins of the white matter of the cerebral hemispheres (the medullary veins). Am. J. Roentgenol. Radium Ther. Nucl. Med. 1964, 92, 739–755. [Google Scholar]
- Okudera, T.; Huang, Y.P.; Fukusumi, A.; Nakamura, Y.; Hatazawa, J.; Uemura, K. Micro-angiographical studies of the medullary venous system of the cerebral Hemisphere. Neuropathology 1999, 19, 93–111. [Google Scholar] [CrossRef]
- Inder, T.E.; Perlman, J.M.; Volpe, J.J. Preterm Intraventricular Hemorrhage/Posthemorrhagic Hydrocephalus. In Volpe’s Neurology of the Newborn, 7th ed.; Volpe, J.J., Ed.; Elsevier: Philadelphia, PA, USA, 2025; pp. 777–846. [Google Scholar]
- Pape, K.E.; Wigglesworth, J.S. Haemorrhage, Ischemia and the Perinatal Brain. Clin. Dev. Med. 1979, 69, 100–117. [Google Scholar]
- Ramenghi, L.A. Germinal Matrix—Intraventricular Haemorrhage: Still a very important brain lesion in premature infants! J. Matern.-Fetal Neonatal Med. 2015, 28, 2259–2260. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional and global estimates of preterm birth in 2020, with trends from 2010. A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hintz, S.R.; Hibbs, A.M.; Walsh, M.C.; Vohr, B.R.; Bann, C.M.; Wilson-Costello, D.E. Neurodevelopmental outcomes of extremely Low-Gestational-Age neonates with Low-Grade Periventricular-Intraventricular hemorrhage. JAMA Pediatr. 2013, 167, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Filip, C.; Zonda, G.I.; Vasilache, I.-A.; Scripcariu, I.S.; Vicoveanu, P.; Dima, V.; Socolov, D.; Paduraru, L. Neonatal Cerebral Sinovenous Thrombosis and the Main Perinatal Risk Factors—A Retrospective Unicentric Study. Children 2022, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Ramenghi, L.A.; Gill, B.J.; Tanner, S.F.; Martinez, D.; Arthur, R.; Levene, M.I. Cerebral venous thrombosis, intraventricular haemorrhage and white matter lesions in a preterm newborn with factor V (Leiden) mutation. Neuropediatrics 2002, 33, 97–99. [Google Scholar] [CrossRef]
- Srivastava, R.; Mailo, J.; Dunbar, M. Perinatal Stroke in Fetuses, Preterm and Term Infants. Semin. Pediatr. Neurol. 2022, 43, 100988. [Google Scholar] [CrossRef]
- Larroque, B.; Marret, S.; Ancel, P.-Y.; Arnaud, C.; Marpeau, L.; Supernant, K.; Pierrat, V.; Rozé, J.-C.; Matis, J.; Cambonie, G.; et al. White matter damage and intraventricular hemorrhage in very preterm infants: The EPIPAGE study. J. Pediatr. 2003, 143, 477–483. [Google Scholar] [CrossRef]
- Cust, A.E.; A Darlow, B.; A Donoghue, D. Outcomes for high risk New Zealand newborn infants in 1998–1999: A population based, national study. Arch. Dis. Child. 2003, 88, 15–22. [Google Scholar] [CrossRef]
- Heibel, M.; Heber, R.; Bechinger, D.; Kornhuber, H.H. Early diagnosis of perinatal cerebral lesions in apparently normal full-term newborns by ultrasound of the brain. Neuroradiology 1993, 35, 85–91. [Google Scholar] [CrossRef]
- Perlman, J.M.; McMenamin, J.B.; Volpe, J.J. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. N. Engl. J. Med. 1983, 309, 204–209. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okudera, T.; Fukuda, S.; Hashimoto, T. Germinal Matrix Hemorrhage of venous origin in preterm neonates. Hum. Pathol. 1990, 21, 1059–1062. [Google Scholar] [CrossRef]
- Dudink, J.; Steggerda, S.J.; Horsch, S. State-of-the-art neonatal cerebral ultrasound: Technique and reporting. Pediatr. Res. 2020, 87, 3–12. [Google Scholar] [CrossRef]
- Meijler, G.; Steggerda, S.J. Neonatal Cranial Ultrasonography, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Shankar, H.; Pagel, P.S. Potential Adverse Effects of Ultrasound- related Biological Effects. A Critical Review. Anesthesiology 2011, 115, 1109–1124. [Google Scholar] [CrossRef]
- Fowlkes, J.B. Bioeffects Committee of the American Institute of Ultrasound in Medicine: American Institute of Ultrasoun in Medicine consensus report on potential bioeffects of diagnostic ultrasound: Executive summary. J. Ultrasound Med. 2008, 27, 503–515. [Google Scholar] [PubMed]
- Aziz, M.U.; Eisenbrey, J.R.; Deganello, A.; Zahid, M.; Sharbidre, K.; Sidhu, P.; Robbin, M.L. Microvascular Flow Imaging: A State-of-the-Art Review of Clinical Use and Promise. Radiology 2012, 305, 250–264. [Google Scholar] [CrossRef]
- Riccabona, M. (Ed.) Imaging in Neonates; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Riccabona, M. (Ed.) Pediatric Ultrasound. Requisites and Applications; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Shackleford, G.D.; Vople, J.J. Cranial ultrasonography in the evaluation of neonatal intracranial haemorrhage and its complications. J. Perinat. Med. 1983, 13, 293. [Google Scholar]
- Leijser, L.M.; Srinivasan, L.; Rutherford, M.A.; Counsell, S.J.; Allsop, J.M.; Cowan, F.M. Structural linear measurements in the newborn brain: Accuracy of cranial ultrasound compared to MRI. Pediatr. Radiol. 2007, 37, 640–648. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (Updated June 2017); Higgins, J.P.T., Churchill, R., Chandler, J., Cumpston, M.S., Eds.; Cochrane: London, UK, 2017. [Google Scholar]
- Bendrea, C. Biostatistică. Elemente de Teorie si Modelare Probabilistică; Universitatea “Dunarea de Jos” Galați: Galați, Romania, 2019; ISBN 978-606-25-0471-7. [Google Scholar]
- Swinscow, T.D.V. Statistics at Square One, 9th ed.; BMJ Publishing Group: London, UK, 1997. [Google Scholar]
- Padget, D.H. The cranial venous system in man in reference to development, adult configuration, and relation to the arteries. Am. J. Anat. 1956, 98, 307–355. [Google Scholar] [CrossRef]
- Gross, M.; Engel, C.; Trotter, A. Evaluating the Effect of a Neonatal Care Bundle for the Prevention of Intraventricular Hemorrhage in Preterm Infants. Children 2021, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- de Bijl-Marcus, K.; Brouwer, A.J.; De Vries, L.S.; Groendaal, F.L.; van Wezel-Meijler, G. Neonatal care bundles are associated with a reduction in the incidence of intraventricular haemorrhage in preterm infants: A multicentre cohort study. Arch. Dis. Child.- Fetal Neonatal Ed. 2020, 105, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Raets, M.; Dudink, J.; Raybaud, C.; Ramenghi, L.; Lequin, M.; Govaert, P. Brain vein disorders in newborn infants. Dev. Med. Child Neurol. 2015, 57, 229–240. [Google Scholar] [CrossRef]
- Çagatay Çimşit, N.; Türe, U.; Ekinci, G.; Necmettin Pamir, M.; Erzen, C. Venous variations in the region ofthe third ventricle: The role of MRvenography. Neuroradiology 2003, 45, 900–904. [Google Scholar] [CrossRef]
- Fujii, S.; Kanasaki, Y.; Matsusue, E.; Kakite, S.; Kminou, T.; Ogawa, T. Demonstration of cerebral venous variations in the region of the third ventricle on phase-sensitive imaging. Am. J. Neuroradiol. 2010, 31, 55–59. [Google Scholar] [CrossRef]
- Mokrohisky, J.F.; Paul, R.E.; Lin, P.M.; Stauffer, H.M. The diagnostic importance of normal variants in deep cerebral phlebography, with special emphasis on the true and false venous angles of the brain and evaluation of venous angle measurements. Radiology 1956, 67, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Benders, M.; Lazeyras, F.; Borradori-Tolsa, C.; Leuchter, R.H.-V.; Mangin, J.; Hüppi, P. Structural asymmetries of perisylvian regions in the preterm newborn. NeuroImage 2010, 52, 32–42. [Google Scholar] [CrossRef]
- Lin, P.Y.; Roche-Labarbe, N.; Dehaes, M.; Fenoglio, A.; Grant, P.E.; Franceschini, M.A. Regional and hemispheric asymmetries of cerebral hemodynamic and oxygen metabolism in newborns. Cereb. Cortex 2013, 23, 339–348. [Google Scholar] [CrossRef] [PubMed]
- The Joint Committee on Infant Hearing. Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. J. Early Hear. Detect. Interv. 2019, 4, 1–44. [Google Scholar]
- Dima, V. Actualities in neonatal endocrine and metabolic screening. Acta Endocrinol. 2021, 17, 394–399. [Google Scholar] [CrossRef]
- Wilcken, B.; Rinaldo, P.; Matern, D. Newborn Screening for Inborn Errors of Metabolism. In Inborn Metabolic Diseases. Diagnosis and Treatment, 5th ed.; Saudubray, J.M., van derBerghe, G., Walter, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2025; pp. 75–86. [Google Scholar]



| Preterm | Term | p-Value | |
|---|---|---|---|
| Number | 32 | 68 | NA |
| Gestational age—weeks (+standard deviation) | 34.53 (+2.42) | 38.22 (+1.09) | 0.001 |
| Birth weight—grams (+standard deviation) | 2372 (+639.91) | 3180 (+426.24) | 0.001 |
| Male | 24/32 | 35/68 | 0.087 |
| Female | 8/32 | 33/68 | |
| CPAP | 25/32 | 1/68 | 0.001 |
| Administration of oxygen | 30/32 | 11/68 | 0.004 |
| Hypoglycaemia | 10/32 | 12/68 | 0.103 |
| Mechanical ventilation | 7/32 | 2/68 | 0.004 |
| Category of Neonates | Venous Drainage Pattern | Total | Chi2 Test p | |
|---|---|---|---|---|
| Atypical (n = 35) | Normal (n = 65) | |||
| Term | 23 (65.7%) | 45 (69.2%) | 68 (68.0%) | 0.443 |
| Preterm | 12 (34.3%) | 20 (30.8%) | 32 (32.0%) | |
| Twins | 4 (11.4%) | 10 (15.4%) | 14 (14.0%) | 0.413 |
| Singletons | 31 (88.6%) | 55 (84.6%) | 86 (86.0%) | |
| Number of Cases | Left | Right | |
|---|---|---|---|
| 68 | 60 | ||
| Mean (degrees) | 135.95 | 138.16 | |
| Median (degrees) | 136.90 | 138.20 | |
| Standard deviation (degrees). | 11.37 | 10.22 | |
| Variance | 8.36 | 7.40 | |
| Kurtosis test | −0.659 | 0.450 | |
| Kurtosis standard error | 0.574 | 0.608 | |
| Kolmogorov–Smirnov | 0.200 | 0.200 | |
| Minimum | 110.90 | 105.60 | |
| Maximum | 159.30 | 154.50 | |
| Percentiles | 25 | 126.75 | 131.53 |
| 50 | 136.90 | 138.20 | |
| 75 | 145.60 | 146.13 | |
| Number | Mean | Standard Deviation | Standard Error of Mean | Min. | Max. | t-Test for Independent Samples | ||
|---|---|---|---|---|---|---|---|---|
| T | p | |||||||
| Angles—Left: | ||||||||
| Term | 51 | 136.82 | 11.44 | 1.602 | 110.90 | 159.30 | 1.189 | 0.279 |
| Preterm | 17 | 133.35 | 11.05 | 2.681 | 117.50 | 151.60 | ||
| Total | 68 | 135.95 | 11.37 | 1.378 | 110.90 | 159.30 | ||
| Angles—Right: | ||||||||
| Term | 44 | 138.45 | 10.26 | 1.547 | 105.60 | 154.50 | 0.132 | 0.718 |
| Preterm | 16 | 137.36 | 10.39 | 2.598 | 118.00 | 152.50 | ||
| Total | 60 | 138.16 | 10.22 | 1.319 | 105.60 | 154.50 | ||
| Number | Mean | Standard Deviation | Standard Error of Mean | Min. | Max. | t-Test for Independent Samples | ||
|---|---|---|---|---|---|---|---|---|
| t | p | |||||||
| Angles—Left: | ||||||||
| Male | 40 | 136.49 | 11.49 | 1.816 | 111.40 | 159.30 | 0.216 | 0.643 |
| Female | 28 | 135.18 | 11.36 | 2.146 | 110.90 | 159.00 | ||
| Total | 68 | 135.95 | 11.37 | 1.378 | 110.90 | 159.30 | ||
| Angles—Right: | ||||||||
| Male | 37 | 139.05 | 10.18 | 1.673 | 118.00 | 152.60 | 0.721 | 0.399 |
| Female | 23 | 136.74 | 10.34 | 2.157 | 105.60 | 154.50 | ||
| Total | 60 | 138.16 | 10.22 | 1.319 | 105.60 | 154.50 | ||
| Number | Mean | Standard Deviation | Standard Error of Mean | Min. | Max. | t-Test for Independent Samples | ||
|---|---|---|---|---|---|---|---|---|
| t | p | |||||||
| Angles—Left: | ||||||||
| Twins | 7 | 134.61 | 10.00 | 3.780 | 121.90 | 145.70 | 0.107 | 0.745 |
| Singletons | 61 | 136.11 | 11.58 | 1.482 | 110.90 | 159.30 | ||
| Total | 68 | 135.95 | 11.37 | 1.378 | 110.90 | 159.30 | ||
| Angles—Right: | ||||||||
| Twins | 8 | 135.41 | 7.41 | 2.619 | 120.80 | 142.70 | 0.665 | 0.418 |
| Singletons | 52 | 138.59 | 10.58 | 1.467 | 105.60 | 154.50 | ||
| Total | 60 | 138.16 | 10.22 | 1.319 | 105.60 | 154.50 | ||
| Category | Left (Mean + SD) | Right (Mean + SD) | t-Test for Independent Samples |
|---|---|---|---|
| Total | 135.9 (+10.2) | 139.06 (+15.8) | 0.121 |
| Term neonates | 133.37 (+8.4) | 139.26 (+12.8) | 0.440 |
| Preterm neonates | 133.95 (+9.6) | 138.37 (+15.4) | 0.092 |
| Side | Number of Cases with an Unidentified Angle | Neonates | Chi2 Test p | |
|---|---|---|---|---|
| Term (n = 68) | Preterm (n = 32) | |||
| Left | 32 | 17 (25.0%) | 15 (46.9%) | 0.026 |
| Right | 40 | 24 (35.3%) | 16 (50.0%) | 0.119 |
| GM/IVH | Venous Pattern | Total | Chi2 Test p | |
|---|---|---|---|---|
| Atypical (n = 12) | Normal (n = 20) | |||
| Left | 2 (16.7%) | 6 (30.0%) | 8 (25.0%) | 0.344 |
| Right | 2 (16.7%) | 2 (10.0%) | 4 (12.5%) | 0.485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, A.I.; Năstase, L.; Necula, A.I.; Pavalache-Stoiciu, R.; Harnagea, M.; Gavrilă, E.; Bivoleanu, A.R. Ultrasound Evaluation of the Deep Cerebral Venous System in Term and Preterm Neonates: Normal Features and Correlations with the Occurrence of Germinal Matrix/Intraventricular Haemorrhage. Children 2025, 12, 1347. https://doi.org/10.3390/children12101347
Toma AI, Năstase L, Necula AI, Pavalache-Stoiciu R, Harnagea M, Gavrilă E, Bivoleanu AR. Ultrasound Evaluation of the Deep Cerebral Venous System in Term and Preterm Neonates: Normal Features and Correlations with the Occurrence of Germinal Matrix/Intraventricular Haemorrhage. Children. 2025; 12(10):1347. https://doi.org/10.3390/children12101347
Chicago/Turabian StyleToma, Adrian Ioan, Leonard Năstase, Andreea Ioana Necula, Roxana Pavalache-Stoiciu, Miruna Harnagea, Eduard Gavrilă, and Anca Roxana Bivoleanu. 2025. "Ultrasound Evaluation of the Deep Cerebral Venous System in Term and Preterm Neonates: Normal Features and Correlations with the Occurrence of Germinal Matrix/Intraventricular Haemorrhage" Children 12, no. 10: 1347. https://doi.org/10.3390/children12101347
APA StyleToma, A. I., Năstase, L., Necula, A. I., Pavalache-Stoiciu, R., Harnagea, M., Gavrilă, E., & Bivoleanu, A. R. (2025). Ultrasound Evaluation of the Deep Cerebral Venous System in Term and Preterm Neonates: Normal Features and Correlations with the Occurrence of Germinal Matrix/Intraventricular Haemorrhage. Children, 12(10), 1347. https://doi.org/10.3390/children12101347






