Management of Severe Pediatric Lower Lip Defect After Canine Bite with Polyhexamethylene Biguanide (PHMB), Full-Thickness Skin Graft (FTSG) and Compression Foam: A Case Report
Abstract
Highlights
- Primary closure was achieved in a large, lower-lip defect after a dog bite using an integrated approach (topical polyhexanide, FTSG secured with a polyurethane compression foam, oral antibiotics).
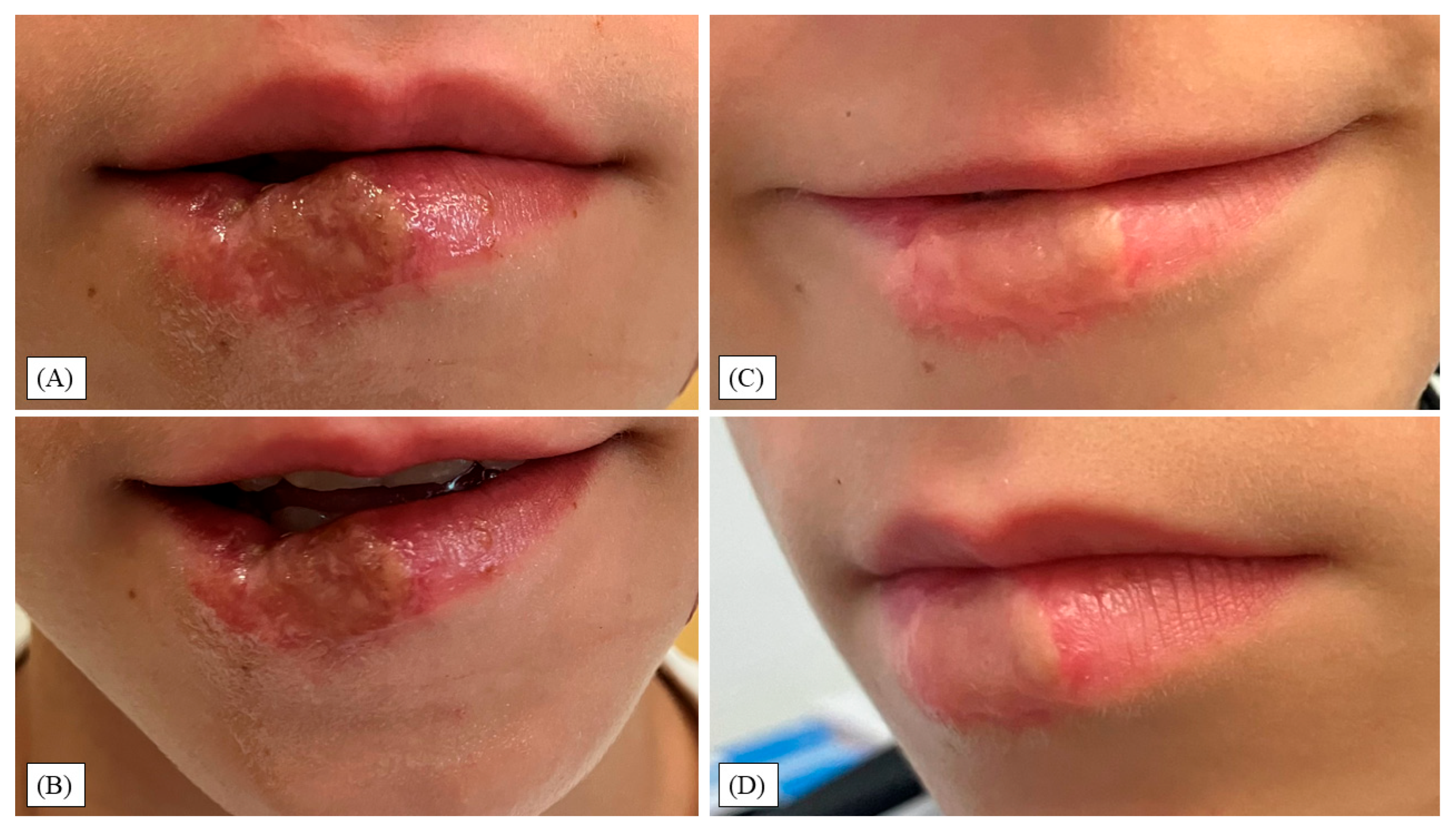
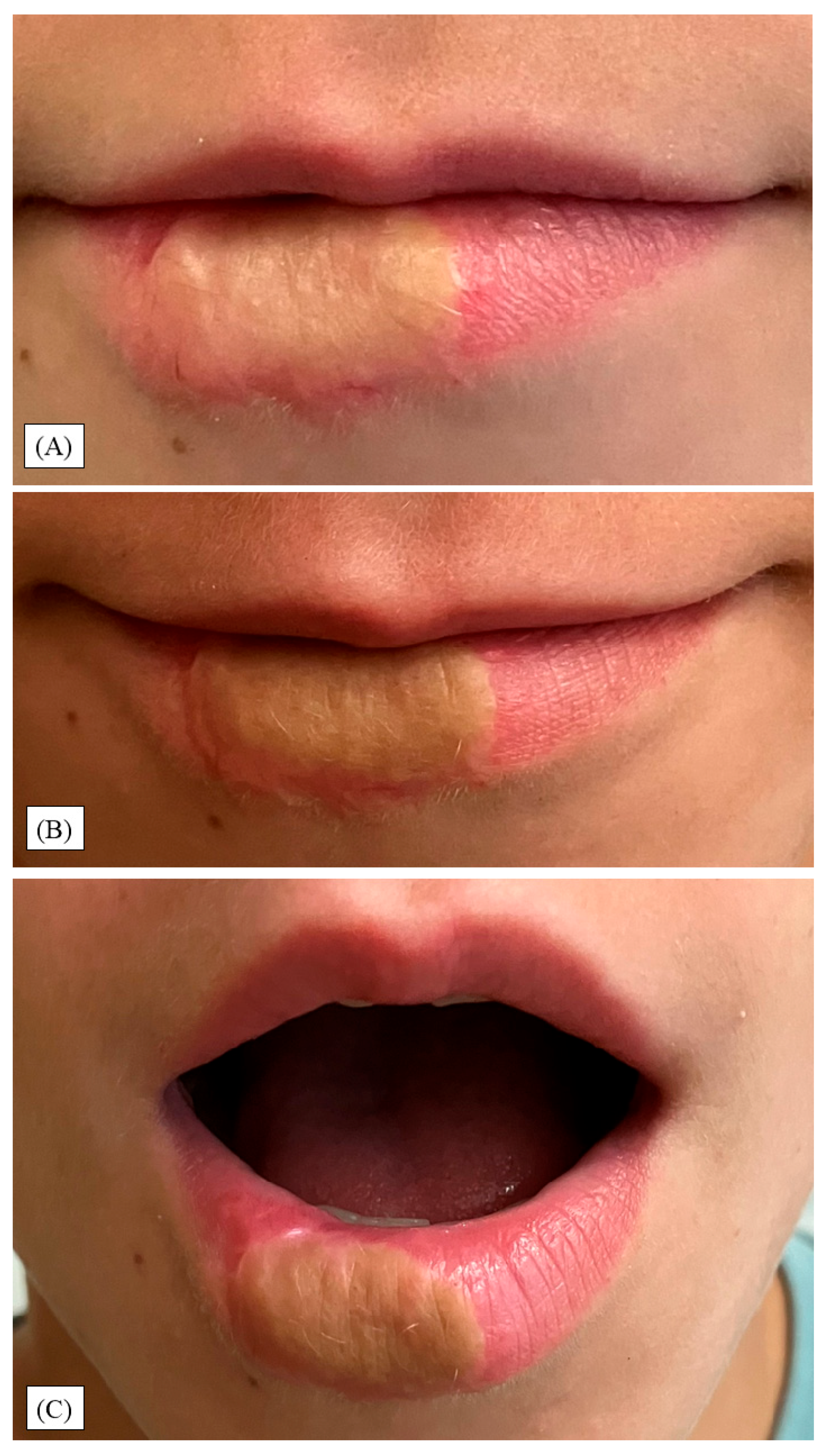
- Function remained intact; at 12 months, modest esthetic sequelae persisted (dyschromia, shallow border indentation, vellus hairs).
- This pediatric case shows that the combined intervention can yield stable function with moderate esthetic compromise in a contaminated, extensive lower-lip injury.
- Component-level effects are undetermined; comparative studies with validated outcomes are needed.
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design and Patient Selection
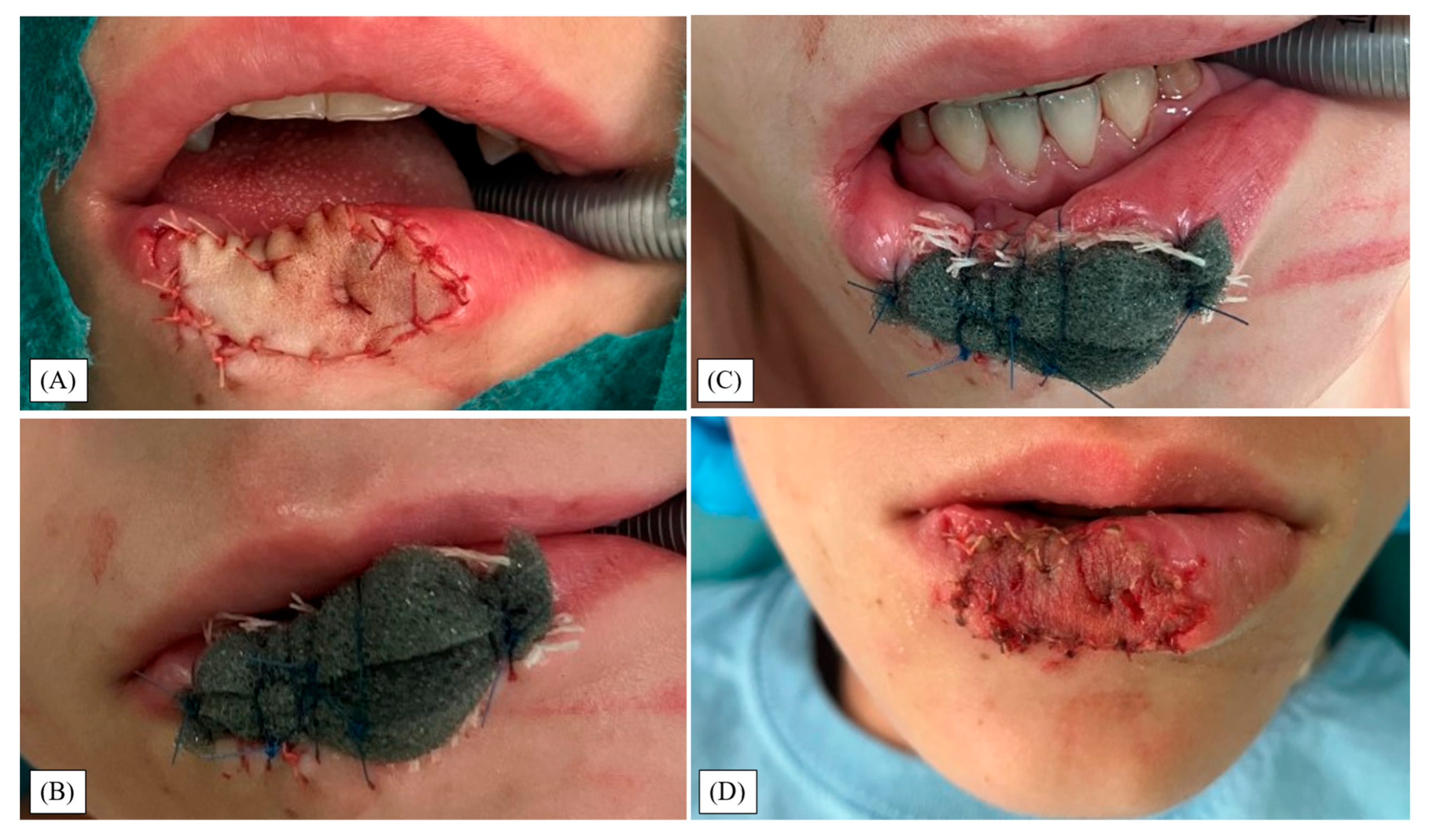
2.2. Operative Algorithm
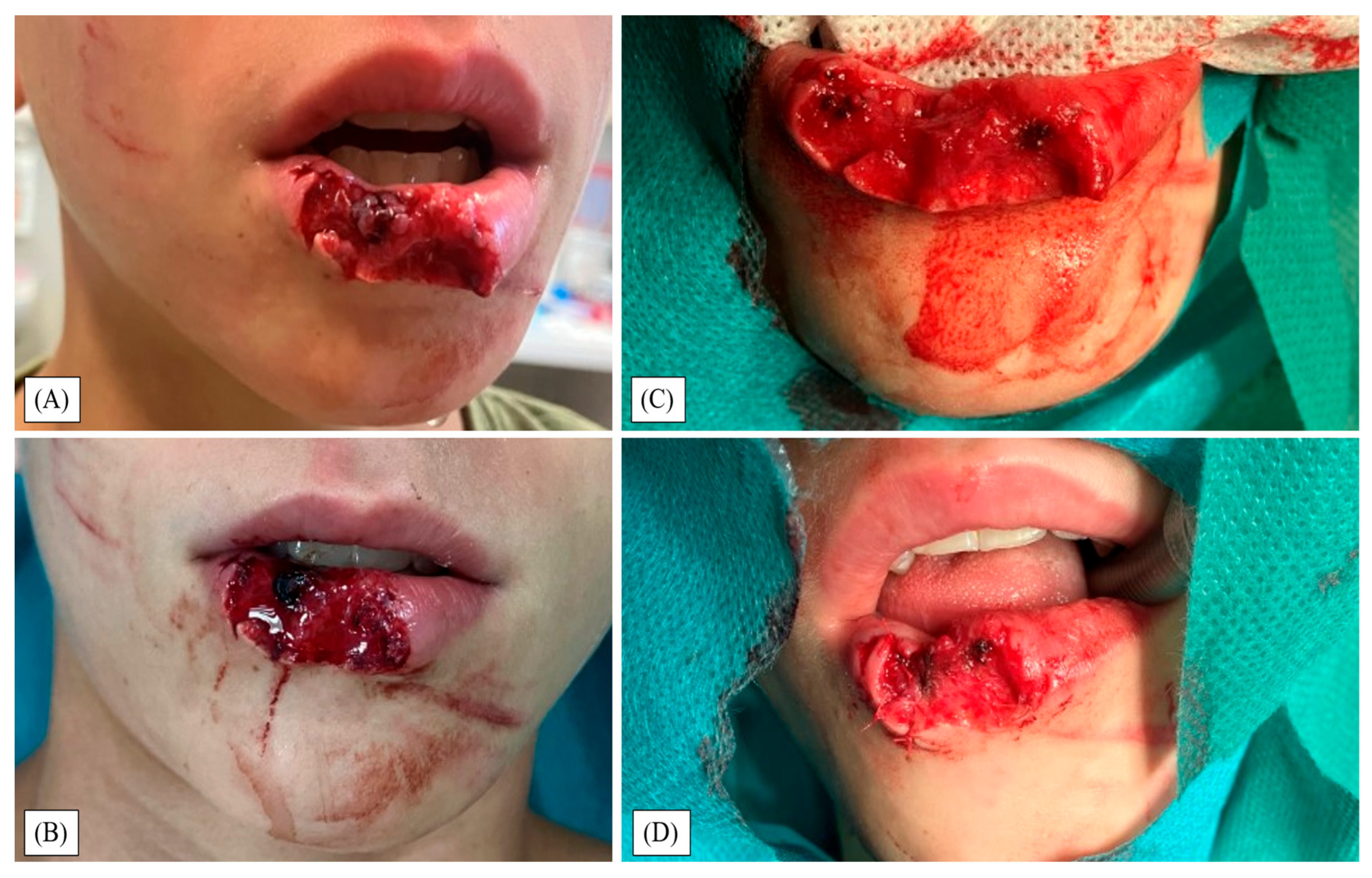
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMGs | Buccal mucosa grafts |
| FAMM | Facial artery musculomucosal (flap) |
| FTSG | Full-thickness skin grafting |
| µg/ml | micrograms per milliliter |
| mm | millimeter |
| NPWT | Negative-Pressure Wound Therapy |
| PedsQL | Pediatric Quality-of-Life Inventory |
| PHMB | Polyhexamethylene biguanide (polyhexanide) |
| POSAS | Patient and Observer Scar Assessment Scale |
| PT | Physical therapy |
| PU | Polyurethane |
| TIVA | Total intravenous anesthesia |
| US | Ultrasound |
| VSS | Vancouver Scar Scale |
References
- McLoughlin, R.J.; Cournoyer, L.; Hirsh, M.P.; Cleary, M.A.; Aidlen, J.T. Hospitalizations for Pediatric Dog Bite Injuries in the United States. J. Pediatr. Surg. 2020, 55, 1228–1233. [Google Scholar] [CrossRef]
- Plana, N.M.; Kalmar, C.L.; Cheung, L.; Swanson, J.W.; Taylor, J.A. Pediatric Dog Bite Injuries: A 5-Year Nationwide Study and Implications of the COVID-19 Pandemic. J. Craniofac. Surg. 2022, 33, 1436. [Google Scholar] [CrossRef]
- Piccart, F.; Dormaar, J.; Coropciuc, R.; Schoenaers, J.; Bila, M.; Politis, C. Dog Bite Injuries in the Head and Neck Region: A 20-Year Review. Craniomaxillofac. Trauma. Reconstr. 2019, 12, 199–204. [Google Scholar] [CrossRef]
- Boyd, L.C.; Chang, J.; Ajmera, S.; Wallace, R.D.; Alvarez, S.M.; Konofaos, P. Pediatric Dog Bites: A Review of 1422 Cases Treated at a Level One Regional Pediatric Trauma Center. J. Craniofac. Surg. 2022, 33, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ali, S.S. Dog Bite Injuries to the Face: A Narrative Review of the Literature. World J. Otorhinolaryngol. Head Neck Surg. 2022, 8, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.A.; Chen, H.H.; Hink, E.M.; Durairaj, V.D. Pediatric Facial Fractures From Dog Bites. Ophthal. Plast. Reconstr. Surg. 2013, 29, 179. [Google Scholar] [CrossRef]
- Chen, H.H.; Neumeier, A.T.; Davies, B.W.; Durairaj, V.D. Analysis of Pediatric Facial Dog Bites. Craniomaxillofac. Trauma. Reconstr. 2013, 6, 225–232. [Google Scholar] [CrossRef]
- Jakeman, M.; Oxley, J.A.; Owczarczak-Garstecka, S.C.; Westgarth, C. Pet Dog Bites in Children: Management and Prevention. BMJ Paediatr. Open 2020, 4, e000726. [Google Scholar] [CrossRef]
- Wu, P.S.; Beres, A.; Tashjian, D.B.; Moriarty, K.P. Primary Repair of Facial Dog Bite Injuries in Children. Pediatr. Emerg. Care 2011, 27, 801. [Google Scholar] [CrossRef]
- Macedo, J.L.S.; Rosa, S.C.; Queiroz, M.N.D.; Gomes, T.G.A.C.B. Reconstruction of Face and Scalp after Dog Bites in Children. Rev. Colégio Bras. Cir. 2016, 43, 452–457. [Google Scholar] [CrossRef]
- Tarantola, A. Four Thousand Years of Concepts Relating to Rabies in Animals and Humans, Its Prevention and Its Cure. Trop. Med. Infect. Dis. 2017, 2, 5. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Firdessa, R.; Good, L. Bactericidal Effects of Polyhexamethylene Biguanide against Intracellular Staphylococcus Aureus EMRSA-15 and USA 300. J. Antimicrob. Chemother. 2016, 71, 1252–1259. [Google Scholar] [CrossRef]
- Gray, D.; Barrett, S.; Battacharyya, M.; Butcher, M.; Enoch, S.; Fumerola, S.; Stephen-Haynes, J.; Edwards-Jones, V.; Leaper, D.; Strohal, R.; et al. PHMB and Its Potential Contribution to Wound Management. Wounds UK 2010, 6, 40–46. [Google Scholar]
- Rippon, M.G.; Rogers, A.A.; Ousey, K. Polyhexamethylene Biguanide and Its Antimicrobial Role in Wound Healing: A Narrative Review. J. Wound Care 2023, 32, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, A.; Nudelman, H.; Lamberti, A.G.; Garami, A.; Tiborcz, K.A.; Kovács, T.Z.; Józsa, G. Management of Pediatric Superficial Partial-Thickness Burns with Polyhexamethylene Biguanide: Outcomes and Influencing Factors. J. Clin. Med. 2024, 13, 3074. [Google Scholar] [CrossRef]
- Józsa, G.; Dávidovics, K.; Lőrincz, A.; Gabriella, A.; András, G.; Zsolt, J. Management of Pediatric Partial-Thickness Burns with Lavanid Gel (Polyhexamethylen Biguanide, Polyhexanide) “Case Reports”. J. Orthop. Res. Ther. 2022, 7, 1213. [Google Scholar]
- Elzinga, G.; van Doorn, J.; Wiersema, A.M.; Klicks, R.J.; Andriessen, A.; Alblas, J.G.; Spits, H.; Post, A.; van Gent, M. Clinical Evaluation of a PHMB-Impregnated Biocellulose Dressing on Paediatric Lacerations. J. Wound Care 2011, 20, 280–284. [Google Scholar] [CrossRef]
- Lee, C.K.; Chua, Y.P.; Saw, A. Antimicrobial Gauze as a Dressing Reduces Pin Site Infection: A Randomized Controlled Trial. Clin. Orthop. 2012, 470, 610–615. [Google Scholar] [CrossRef]
- Matiasek, J.; Djedovic, G.; Kiehlmann, M.; Verstappen, R.; Rieger, U.M. Negative Pressure Wound Therapy with Instillation: Effects on Healing of Category 4 Pressure Ulcers. Plast. Aesthet. Res. 2018, 5, 36. [Google Scholar] [CrossRef]
- Patel, N.V.; Mathur, U.; Sawant, S.; Acharya, M.; Gandhi, A. Three Consecutive Cases of Ocular Polyhexamethylene Biguanide (PHMB) Toxicity Due to Compounding Error. Cureus 2023, 15, e38540. [Google Scholar] [CrossRef]
- Rutter, N. The Immature Skin. Eur. J. Pediatr. 1996, 155 (Suppl. 2), S18–S20. [Google Scholar] [CrossRef]
- Drumright, B.; Borg, B.; Rozzelle, A.; Donoghue, L.; Shanti, C. Pediatric Dog Bite Outcomes: Infections and Scars. Trauma. Surg. Acute Care Open 2020, 5, e000445. [Google Scholar] [CrossRef]
- Chávez-Serna, E.; Andrade-Delgado, L.; Martínez-Wagner, R.; Altamirano-Arcos, C.; Espino-Gaucín, I.; Nahas-Combina, L. Experiencia En El Manejo de Heridas Por Mordedura de Perro En Un Hospital de Tercer Nivel de Cirugía Plástica y Reconstructiva En México. Cir. Cir. 2019, 87, 528–539. [Google Scholar] [CrossRef]
- Roldán, J.C.; Teschke, M.; Fritzer, E.; Dunsche, A.; Härle, F.; Wiltfang, J.; Terheyden, H. Reconstruction of the Lower Lip: Rationale to Preserve the Aesthetic Units of the Face. Plast. Reconstr. Surg. 2007, 120, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Westgarth, C.; Provazza, S.; Nicholas, J.; Gray, V. Review of Psychological Effects of Dog Bites in Children. BMJ Paediatr. Open 2024, 8, e000922. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.S.M.; Poh, P.-F.; Heng, K.Y.C.; Sultana, R.; Murphy, B.; Ng, R.W.L.; Lee, J.H. Assessment of Long-Term Psychological Outcomes After Pediatric Intensive Care Unit Admission: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2022, 176, e215767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pandya, S.; Alessandri Bonetti, M.; Costantino, A.; Egro, F.M. Comparison of Split Thickness Skin Graft versus Full Thickness Skin Graft for Radial Forearm Flap Donor Site Closure: A Systematic Review and Meta-Analysis. Am. J. Otolaryngol. 2024, 45, 104156. [Google Scholar] [CrossRef]
- Prasetyono, T.O.H.; Sadikin, P.M.; Saputra, D.K.A. The Use of Split-Thickness versus Full-Thickness Skin Graft to Resurface Volar Aspect of Pediatric Burned Hands: A Systematic Review. Burn. J. Int. Soc. Burn. Inj. 2015, 41, 890–906. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, C.H.; Kim, S.; Lee, W.S.; Oh, S.-H. Effective Method for Reconstruction of Remaining Lower Lip Vermilion Defect after a Mental V-Y Advancement Flap. Arch. Craniofac. Surg. 2019, 20, 76–83. [Google Scholar] [CrossRef]
- Pribaz, J.J.; Meara, J.G.; Wright, S.; Smith, J.D.; Stephens, W.; Breuing, K.H. Lip and Vermilion Reconstruction with the Facial Artery Musculomucosal Flap. Plast. Reconstr. Surg. 2000, 105, 864–872. [Google Scholar] [CrossRef]
- Baj, A.; Rocchetta, D.; Beltramini, G.; Giannì, A.B. FAMM Flap Reconstruction of the Inferior Lip Vermilion: Surgery during Early Infancy. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2008, 61, 425–427. [Google Scholar] [CrossRef]
- Lizbeth, D.; Luis, V.; Hugo, G.; Gibran, R.; Jesús, V.; Emmanuel, G.; Esteban, G.; Yilzett, R.; Laura, R.; Alexis, O. V-Y Flap as a Treatment for Lower Lip Bite. Int. Surg. J. 2023, 10, 2006–2009. [Google Scholar] [CrossRef]
- Vignesh, K.; Sivakumar, S.; Thenmozhi, M.; Kumar, S.M. Use Of Estlander Flap In Small To Large Lip And Commissure Defects—A Case Series. Int. J. Adv. Res. 2025, 12, 576–583. [Google Scholar] [CrossRef]
- Sumarroca, A.; Vega, C.; López, S.; Leidinger, A.; Pedemonte, G.; León, X.; Costa-González, J.M. Lower Lip Reconstruction with the Karapandzic Flap or the Colmenero Flap: Description of Results. Acta Otorrinolaringol. Esp. 2025, 76, 83–90. [Google Scholar] [CrossRef]
- Shipkov, C.; Simov, R.; Bukov, Y.; Piral, T.; Anastassov, Y. The nasolabial flap and the buccinator flap. Anatomic study and 2 cases reports. Ann. Chir. Plast. Esthet. 2003, 48, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lengelé, B.G.; Testelin, S.; Bayet, B.; Devauchelle, B. Total Lower Lip Functional Reconstruction with a Prefabricated Gracilis Muscle Free Flap. Int. J. Oral. Maxillofac. Surg. 2004, 33, 396–401. [Google Scholar] [CrossRef]
- Murray-Douglass, A.; Romeo, P.; Fox, C. Free Flap Reconstruction of the Lower Lip: A Systematic Review and Meta-Analysis. J. Reconstr. Microsurg. 2024, 41, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Davis, K.P.; Anderson, Z.; Hartzell, L. Repair of Facial Dog Bite Wound Utilizing Cryopreserved Umbilical Cord Allograft. Wound Manag. Prev. 2024, 70. [Google Scholar] [CrossRef]
- Vallejo, A.; Wallis, M.; McMillan, D. Use of Low-Frequency Contact Ultrasonic Debridement with and without Polyhexamethylene Biguanide in Hard-to-Heal Leg Ulcers: An RCT. J. Wound Care 2022, 31, 670–681. [Google Scholar] [CrossRef]
- Sousa, I.; Maia, F.; Silva, A.; Cunha, Â.; Almeida, A.; Evtyugin, D.V.; Tedim, J.; Ferreira, M.G. A Novel Approach for Immobilization of Polyhexamethylene Biguanide within Silica Capsules. RSC Adv. 2015, 5, 92656–92663. [Google Scholar] [CrossRef]
- Patterson, K.N.; Horvath, K.Z.; Minneci, P.C.; Thakkar, R.; Wurster, L.; Noffsinger, D.L.; Bourgeois, T.; Deans, K.J. Pediatric Dog Bite Injuries in the USA: A Systematic Review. World J. Pediatr. Surg. 2022, 5, e000281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lőrincz, A.; Nudelman, H.; Lamberti, A.G.; Vástyán, A.; Molnár, E.; Pavlovics, G.; Józsa, G. Management of Severe Pediatric Lower Lip Defect After Canine Bite with Polyhexamethylene Biguanide (PHMB), Full-Thickness Skin Graft (FTSG) and Compression Foam: A Case Report. Children 2025, 12, 1308. https://doi.org/10.3390/children12101308
Lőrincz A, Nudelman H, Lamberti AG, Vástyán A, Molnár E, Pavlovics G, Józsa G. Management of Severe Pediatric Lower Lip Defect After Canine Bite with Polyhexamethylene Biguanide (PHMB), Full-Thickness Skin Graft (FTSG) and Compression Foam: A Case Report. Children. 2025; 12(10):1308. https://doi.org/10.3390/children12101308
Chicago/Turabian StyleLőrincz, Aba, Hermann Nudelman, Anna Gabriella Lamberti, Attila Vástyán, Enikő Molnár, Gábor Pavlovics, and Gergő Józsa. 2025. "Management of Severe Pediatric Lower Lip Defect After Canine Bite with Polyhexamethylene Biguanide (PHMB), Full-Thickness Skin Graft (FTSG) and Compression Foam: A Case Report" Children 12, no. 10: 1308. https://doi.org/10.3390/children12101308
APA StyleLőrincz, A., Nudelman, H., Lamberti, A. G., Vástyán, A., Molnár, E., Pavlovics, G., & Józsa, G. (2025). Management of Severe Pediatric Lower Lip Defect After Canine Bite with Polyhexamethylene Biguanide (PHMB), Full-Thickness Skin Graft (FTSG) and Compression Foam: A Case Report. Children, 12(10), 1308. https://doi.org/10.3390/children12101308





