Acute Neurotoxicity in Children Treated for Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma: A 10-Year Single-Centre Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Population Characteristics
3.2. Acute Neurotoxic Events
3.3. Clinical Manifestations
3.4. Diagnostics
3.4.1. EEG
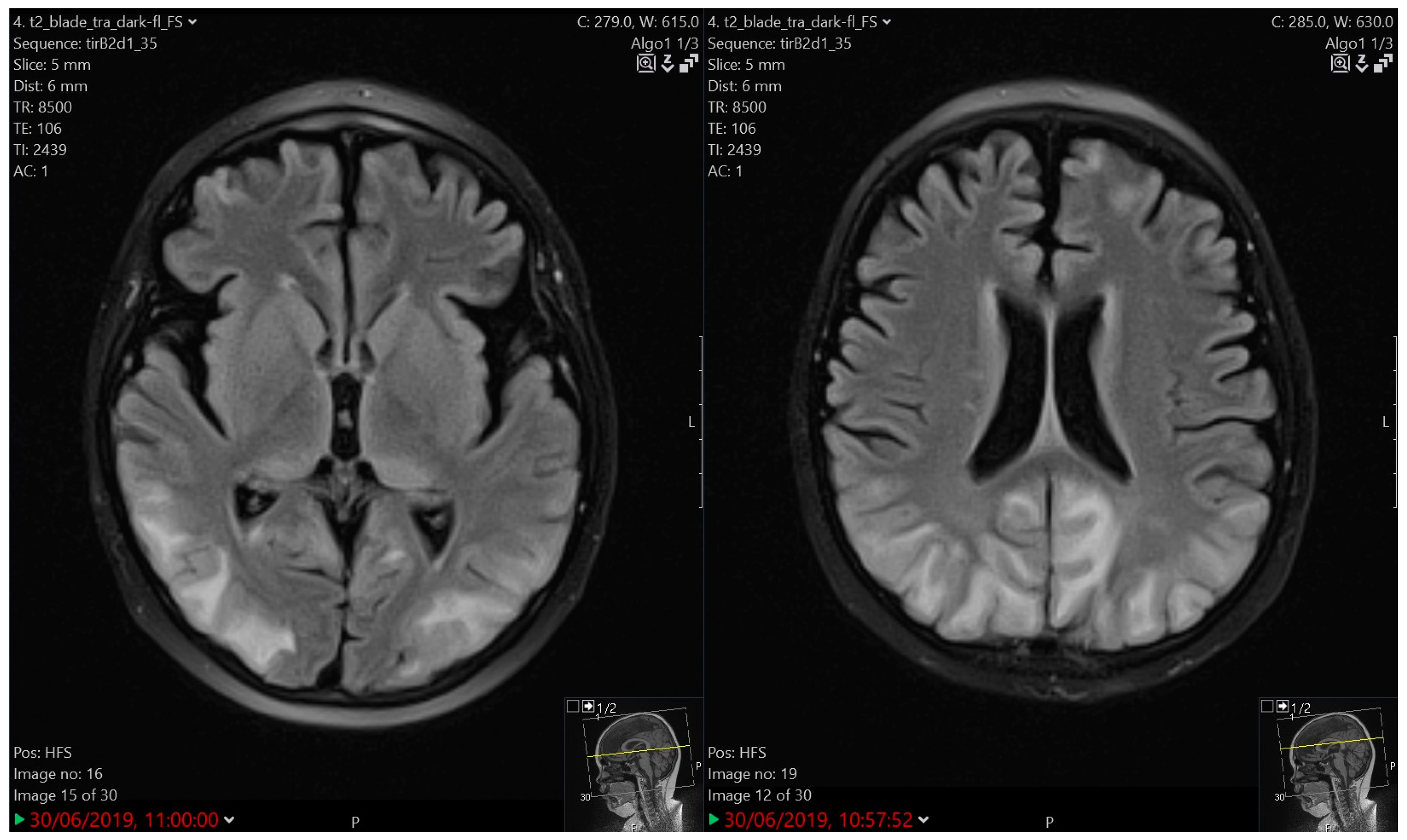
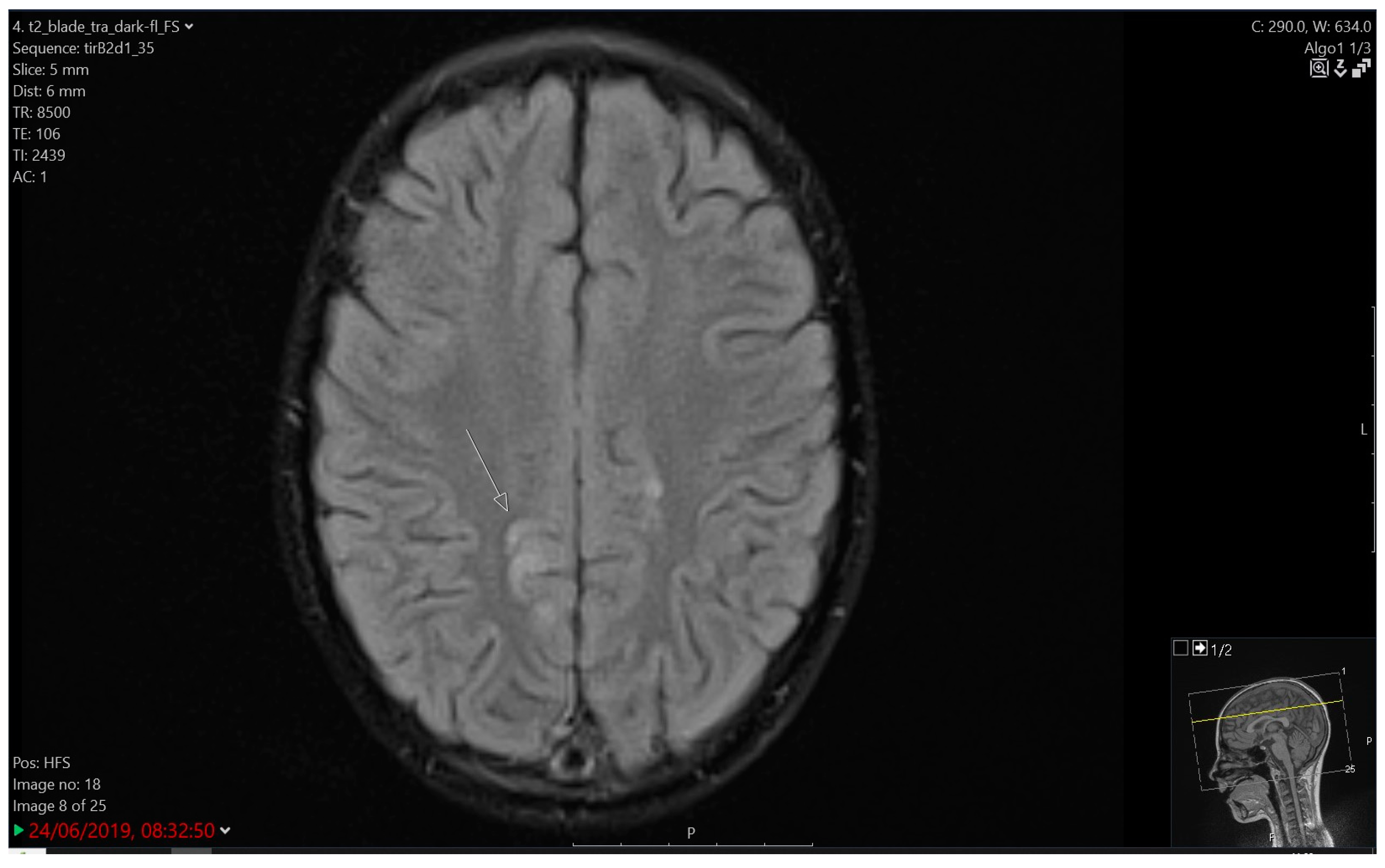
3.4.2. Imaging
3.5. Treatment and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maloney, K.W.; Devidas, M.; Wang, C.; Mattano, L.A.; Friedmann, A.M.; Buckley, P.; Borowitz, M.J.; Carroll, A.J.; Gastier-Foster, J.M.; Heerema, N.A.; et al. Outcome in Children with Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children’s Oncology Group Trial AALL0331. J. Clin. Oncol. 2020, 38, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; de Groot-Kruseman, H.; Van der Velden, V.; Fiocco, M.; van den Berg, H.; De Bont, E.; Egeler, R.M.; Hoogerbrugge, P.; Kaspers, G.; Van der Schoot, E.; et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J. Clin. Oncol. 2016, 34, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Krishnan, A.V.; Lin, C.S.; Goldstein, D.; Friedlander, M.; Kiernan, M.C. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr. Med. Chem. 2008, 15, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Soussain, C.; Ricard, D.; Fike, J.R.; Mazeron, J.J.; Psimaras, D.; Delattre, J.Y. CNS complications of radiotherapy and chemotherapy. Lancet 2009, 374, 1639–1651. [Google Scholar] [CrossRef]
- Zukas, A.M.; Schiff, D. Neurological complications of new chemotherapy agents. Neuro-Oncology 2018, 20, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Sul, J.K.; Deangelis, L.M. Neurologic complications of cancer chemotherapy. Semin. Oncol. 2006, 33, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Kembhavi, S.A.; Somvanshi, S.; Banavali, S.; Kurkure, P.; Arora, B. Pictorial essay: Acute neurological complications in children with acute lymphoblastic leukemia. Indian J. Radiol. Imaging 2012, 22, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Dropcho, E.J. Neurotoxicity of cancer chemotherapy. Semin. Neurol. 2010, 30, 273–286. [Google Scholar] [CrossRef]
- Millan, N.C.; Pastrana, A.; Guitter, M.R.; Zubizarreta, P.A.; Monges, M.S.; Felice, M.S. Acute and sub-acute neurological toxicity in children treated for acute lymphoblastic leukemia. Leuk. Res. 2018, 65, 86–93. [Google Scholar] [CrossRef]
- Gutierrez, A.; Silverman, L.B. Acute Lymphoblastic Leukemia. In Nathan and Oski’s Hematology and Oncology of Infancy and Childhood; Orkin, S.H., Fisher, D.E., Ginsburg, D., Look, A.T., Lux, S.E., Nathan, D.G., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; p. 1536. [Google Scholar]
- Ingram, L.C.; Fairclough, D.L.; Furman, W.L.; Sandlund, J.T.; Kun, L.E.; Rivera, G.K.; Pui, C. Cranial nerve palsy in childhood acute lymphoblastic leukemia and non-Hodgkin’s lymphoma. Cancer 1991, 67, 2262–2268. [Google Scholar] [CrossRef]
- Krishnamurthy, S.N.; Weinstock, A.L.; Smith, S.H.; Duffner, P.K. Facial palsy, an unusual presenting feature of childhood leukemia. Pediatr. Neurol. 2002, 27, 68–70. [Google Scholar] [CrossRef]
- Rinne, M.L.; Lee, E.Q.; Wen, P.Y. Central nervous system complications of cancer therapy. J. Support. Oncol. 2012, 10, 133–141. [Google Scholar] [CrossRef]
- Vagace, J.M.; de la Maya, M.D.; Caceres-Marzal, C.; Gonzalez de Murillo, S.; Gervasini, G. Central nervous system chemotoxicity during treatment of pediatric acute lymphoblastic leukemia/lymphoma. Crit. Rev. Oncol. Hematol. 2012, 84, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Pochedly, C. Neurotoxicity due to CNS therapy for leukemia. Med. Pediatr. Oncol. 1977, 3, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, W.A. Neurologic sequelae of methotrexate and ionizing radiation: A new classification. Cancer Treat. Rep. 1981, 65 (Suppl. 1), 89–98. [Google Scholar] [PubMed]
- Taillibert, S.; Le Rhun, E.; Chamberlain, M.C. Chemotherapy-Related Neurotoxicity. Curr. Neurol. Neurosci. Rep. 2016, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Ayalon, I.; Friedman, S.; Binenbaum, Y.; Oppenheimer, N.; Shiran, S.; Grisaru-Soen, G.; Uliel-Sibony, S.; Glatstein, M.; Kaplan, J.M.; Sadot, E. A Case of Methotrexate Neurotoxicity Presented as Status Epilepticus, Encephalopathy, and High Fever. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619862311. [Google Scholar] [CrossRef] [PubMed]
- González-Otárula, K.A.; Álvarez, B.M.; Dubeau, F. Drug-resistant epilepsy after treatment for childhood acute lymphocytic leukaemia: From focal epilepsy to Lennox-Gastaut syndrome. Epileptic Disord. 2016, 18, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.L.; Lu, X.; Mitchell, H.R.; Sung, L.; Devidas, M.; Mattano, L.A., Jr.; Carroll, W.L.; Winick, N.; Hunger, S.P.; Maloney, K.W.; et al. Anxiety, pain, and nausea during the treatment of standard-risk childhood acute lymphoblastic leukemia: A prospective, longitudinal study from the Children’s Oncology Group. Cancer 2016, 122, 1116–1125. [Google Scholar] [CrossRef]
- Buizer, A.I.; de Sonneville, L.M.; Veerman, A.J. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: A critical review of the literature. Pediatr. Blood Cancer 2009, 52, 447–454. [Google Scholar] [CrossRef]
- Dietrich, J.; Prust, M.; Kaiser, J. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience 2015, 309, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Krull, K.R.; Okcu, M.F.; Potter, B.; Jain, N.; Dreyer, Z.; Kamdar, K.; Brouwers, P. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J. Clin. Oncol. 2008, 26, 4138–4143. [Google Scholar] [CrossRef] [PubMed]
- Krull, K.R.; Khan, R.B.; Ness, K.K.; Ledet, D.; Zhu, L.; Pui, C.H.; Howard, S.C.; Srivastava, D.K.; Sabin, N.D.; Hudson, M.M.; et al. Symptoms of attention-deficit/hyperactivity disorder in long-term survivors of childhood leukemia. Pediatr. Blood Cancer 2011, 57, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Ziereisen, F.; Dan, B.; Azzi, N.; Ferster, A.; Damry, N.; Christophe, C. Reversible acute methotrexate leukoencephalopathy: Atypical brain MR imaging features. Pediatr. Radiol. 2006, 36, 205–212. [Google Scholar] [CrossRef]
- Lo Nigro, L.; Di Cataldo, A.; Schiliro, G. Acute neurotoxicity in children with B-lineage acute lymphoblastic leukemia (B-ALL) treated with intermediate risk protocols. Med. Pediatr. Oncol. 2000, 35, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Parasole, R.; Petruzziello, F.; Menna, G.; Mangione, A.; Cianciulli, E.; Buffardi, S.; Marchese, L.; Nastro, A.; Misuraca, A.; Poggi, V. Central nervous system complications during treatment of acute lymphoblastic leukemia in a single pediatric institution. Leuk. Lymphoma 2010, 51, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Kuskonmaz, B.; Unal, S.; Gumruk, F.; Cetin, M.; Tuncer, A.M.; Gurgey, A. The neurologic complications in pediatric acute lymphoblastic leukemia patients excluding leukemic infiltration. Leuk. Res. 2006, 30, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Rahiman, E.A.; Rajendran, A.; Sankhyan, N.; Singh, P.; Muralidharan, J.; Bansal, D.; Trehan, A. Acute neurological complications during acute lymphoblastic leukemia therapy: A single-center experience over 10 years. Indian J. Cancer 2021, 58, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Baytan, B.; Evim, M.S.; Güler, S.; Güneş, A.M.; Okan, M. Acute Central Nervous System Complications in Pediatric Acute Lymphoblastic Leukemia. Pediatr. Neurol. 2015, 53, 312–318. [Google Scholar] [CrossRef]
- Anastasopoulou, S.; Nielsen, R.L.; Als-Nielsen, B.; Banerjee, J.; Eriksson, M.A.; Helenius, M.; Heyman, M.M.; Johannsdottir, I.M.; Jonsson, O.G.; MacGregor, S.; et al. Acute central nervous system toxicity during treatment of pediatric acute lymphoblastic leukemia: Phenotypes, risk factors and genotypes. Haematologica 2022, 107, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, L.; McGowan, S.; Hough, R.; Vora, A.; Moorman, A.V.; Halsey, C. Acute neurotoxicity during ALL therapy is associated with treatment intensity, age and female sex—An analysis of SAE reports from the UKALL 2003 Trial. Blood 2018, 132 (Suppl. 1), 1379. [Google Scholar] [CrossRef]
- Cruz-Chávez, D.A.; López-Pérez, B.J.; Solórzano-Gómez, E.; Venta-Sobero, J.A.; Flores-Villegas, L.V.; Toledo-Lozano, C.G.; Castro-Loza, G.V.; Sandoval-Pacheco, R.; Torres-Vallejo, A.; Marmol-Realpe, K.S.F.; et al. Neurological Involvement in Pediatric Patients with Acute Leukemia: A Retrospective Cohort. Children 2022, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.A.; Brown, A.L.; Brackett, J.; Dreyer, Z.E.; Moore, I.K.; Mitby, P.; Hooke, M.C.; Hockenberry, M.J.; Lupo, P.J.; Scheurer, M.E. Disparities in Neurotoxicity Risk and Outcomes among Pediatric Acute Lymphoblastic Leukemia Patients. Clin. Cancer Res. 2018, 24, 5012–5017. [Google Scholar] [CrossRef]
- Harris, R.D.; Bernhardt, M.B.; Zobeck, M.C.; Taylor, O.A.; Gramatges, M.M.; Schafer, E.S.; Lupo, P.J.; Rabin, K.R.; Scheurer, M.E.; Brown, A.L. Ethnic-specific predictors of neurotoxicity among patients with pediatric acute lymphoblastic leukemia after high-dose methotrexate. Cancer 2023, 129, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Robison, L.L. Epidemiology of leukemia in childhood. In Nathan and Oski’s Hematology and Oncology of Infancy and Childhood; Orkin, S.H., Fisher, D.E., Ginsburg, D., Look, A.T., Lux, S.E., Nathan, D.G., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 1239–1256. [Google Scholar]
- Śliwa-Tytko, P.; Kaczmarska, A.; Lejman, M.; Zawitkowska, J. Neurotoxicity Associated with Treatment of Acute Lymphoblastic Leukemia Chemotherapy and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 5515. [Google Scholar] [CrossRef]
- Pavlovsky, S.; Eppinger-Helft, M.; Sackmann Muriel, F. Factors that influence the appearance of central nervous system leukemia. Blood 1973, 42, 935–938. [Google Scholar] [CrossRef]
- Lenk, L.; Alsadeq, A.; Schewe, D.M. Involvement of the central nervous system in acute lymphoblastic leukemia: Opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 2020, 39, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Howard, S.C. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008, 9, 257–268. [Google Scholar] [CrossRef]
- Anastasopoulou, S.; Heyman, M.; Eriksson, M.A.; Niinimäki, R.; Taskinen, M.; Mikkel, S.; Vaitkeviciene, G.E.; Johannsdottir, I.M.; Myrberg, I.H.; Jonsson, O.G.; et al. Seizures during treatment of childhood acute lymphoblastic leukemia: A population-based cohort study. Eur. J. Paediatr. Neurol. 2020, 27, 72–77. [Google Scholar] [CrossRef]
- Bhojwani, D.; Sabin, N.D.; Pei, D.; Yang, J.J.; Khan, R.B.; Panetta, J.C.; Krull, K.R.; Inaba, H.; Rubnitz, J.E.; Metzger, M.L.; et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2014, 32, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.K.; Marshall, G.M.; Barbaro, P.M.; Quinn, M.C.; George, C.; Mayoh, C.; Sutton, R.; Revesz, T.; Giles, J.E.; Barbaric, D.; et al. Methotrexate-related central neurotoxicity: Clinical characteristics, risk factors and genome-wide association study in children treated for acute lymphoblastic leukemia. Haematologica 2022, 107, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Khan, R.B.; Laningham, F.H.; Crews, K.R.; Pui, C.H.; Daw, N.C. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann. Oncol. 2008, 19, 178–184. [Google Scholar] [CrossRef]
- Vezmar, S.; Becker, A.; Bode, U.; Jaehde, U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy 2003, 49, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.T.; Kamen, B.A. A biochemical perspective of methotrexate neurotoxicity with insight on nonfolate rescue modalities. J. Investig. Med. 1996, 44, 522–530. [Google Scholar] [PubMed]
- Weiss, H.D.; Walker, M.D.; Wiernik, P.H. Neurotoxicity of commonly used antineoplastic agents (first of two parts). N. Engl. J. Med. 1974, 291, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.S.; Wiernik, P.H. Neurotoxicity of antineoplastic drugs. Semin. Oncol. 1982, 9, 103–130. [Google Scholar]
- Colosimo, C.; Di Lella, G.M.; Petrone, A.M.; Mastrangelo, S.; Riccardi, R. CNS radiochemoprophylaxis in children with acute lymphoblastic leukemia. Neurotoxicity and diagnostic imaging. Rays 1994, 19, 511–526. [Google Scholar]
- Ochs, J.; Mulhern, R.; Fairclough, D.; Parvey, L.; Whitaker, J.; Ch’ien, L.; Mauer, A.; Simone, J. Comparison of neuropsychologic functioning and clinical indicators of neurotoxicity in long-term survivors of childhood leukemia given cranial radiation or parenteral methotrexate: A prospective study. J. Clin. Oncol. 1991, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Pääkkö, E.; Harila-Saari, A.; Vanionpää, L.; Himanen, S.; Pyhtinen, J.; Lanning, M. White matter changes on MRI during treatment in children with acute lymphoblastic leukemia: Correlation with neuropsychological findings. Med. Pediatr. Oncol. 2000, 35, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Marini, A.M.; Finiels-Marlier, F.; Martin, B.; Paul, S.M. MK-801 and memantine protect cultured neurons from glutamate toxicity induced by glutamate carboxypeptidase-mediated cleavage of methotrexate. Eur. J. Pharmacol. 1993, 248, 303–312. [Google Scholar] [CrossRef]
- Gregorios, J.B.; Gregorios, A.B.; Mora, J.; Marcillo, A.; Fojaco, R.M.; Green, B. Morphologic alterations in rat brain following systemic and intraventricular methotrexate injection: Light and electron microscopic studies. J. Neuropathol. Exp. Neurol. 1989, 48, 33–47. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Harding, B.L.; Grossman, S.A. Methotrexate neurotoxicity: In vitro studies using cerebellar explants from rats. Cancer Res. 1989, 49, 2502–2505. [Google Scholar] [PubMed]
- Bruce-Gregorios, J.H.; Agarwal, R.P.; Oracion, A.; Ramirez, A.; Lin, L. Effects of methotrexate on RNA and purine synthesis of astrocytes in primary culture. J. Neuropathol. Exp. Neurol. 1991, 50, 770–778. [Google Scholar] [CrossRef]
- Pavlovic, S.; Kotur, N.; Stankovic, B.; Zukic, B.; Gasic, V.; Dokmanovic, L. Pharmacogenomic and Pharmacotranscriptomic Profiling of Childhood Acute Lymphoblastic Leukemia: Paving the Way to Personalized Treatment. Genes 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, L.A.; Goldman, R.D.; Zivin, L.S.; Fuchs, P.C. Cerebellar toxicity following high-dose cytosine arabinoside. J. Clin. Oncol. 1985, 3, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, M.D.; Hines, J.D. Cerebellar degeneration caused by high-dose cytosine arabinoside: A clinicopathological study. Ann. Neurol. 1983, 14, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Jaing, T.H.; Lin, J.L.; Lin, Y.P.; Yang, S.H.; Lin, J.J.; Hsia, S.H. Hyperammonemic encephalopathy after induction chemotherapy for acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2009, 31, 955–956. [Google Scholar] [CrossRef] [PubMed]
- Frantzeskaki, F.; Rizos, M.; Papathanassiou, M.; Nikitas, N.; Lerikou, M.; Armaganidis, A.; Dimopoulos, G. L-asparaginase fatal toxic encephalopathy during consolidation treatment in an adult with acute lymphoblastic leukemia. Am. J. Case Rep. 2013, 14, 311–314. [Google Scholar] [CrossRef]
- Sudour, H.; Schmitt, C.; Contet, A.; Chastagner, P.; Feillet, F. Acute metabolic encephalopathy in two patients treated with asparaginase and ondasetron. Am. J. Hematol. 2011, 86, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Pathophysiology of brain dysfunction in hyperammonemic syndromes: The many faces of glutamine. Mol. Genet. Metab. 2014, 113, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, T.; Sakuraba, S.; Kimura, K.; Mizoguchi, A. Sevoflurane in combination with propofol, not thiopental, induces a more robust neuroapoptosis than sevoflurane alone in the neonatal mouse brain. J. Anesth. 2014, 28, 815–820. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.E.; De Graaff, J.C.; Dorris, L.; Disma, N.; Withington, D.; Bell, G.; Grobler, A.; Stargatt, R.; Hunt, R.W.; Sheppard, S.J.; et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. Lancet 2019, 393, 664–677, Erratum in Lancet 2019, 394, 638. [Google Scholar] [CrossRef]
- Sun, L.S.; Li, G.; Miller, T.L.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016, 315, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.O.; Zaccariello, M.J.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Schulte, P.J.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; Sprung, J.; et al. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 2018, 129, 89–105. [Google Scholar] [CrossRef]
- Banerjee, P.; Rossi, M.G.; Anghelescu, D.L.; Liu, W.; Breazeale, A.M.; Reddick, W.E.; Glass, J.O.; Phillips, N.S.; Jacola, L.M.; Sabin, N.D.; et al. Association between Anesthesia Exposure and Neurocognitive and Neuroimaging Outcomes in Long-term Survivors of Childhood Acute Lymphoblastic Leukemia. JAMA Oncol. 2019, 5, 1456–1463. [Google Scholar] [CrossRef]
- Sharma, R.; Ibrahim, D.; Habana, J.; Habana, J.A.; Thibodeau, R.; Knipe, H.; Thakur, A.; Jones, J.; Skandhan, A.K.P.; Gaillard, F. Methotrexate-Related Leukoencephalopathy. Reference Article. Available online: https://radiopaedia.org/articles/methotrexate-related-leukoencephalopathy?lang=us (accessed on 23 October 2024).
- Tamrazi, B.; Almast, J. Your brain on drugs: Imaging of drug-related changes in the central nervous system. Radiographics 2012, 32, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Ghali, M.G.Z.; Davanzo, J.; Leo, M.; Rizk, E. Posterior reversible encephalopathy syndrome in pediatric patients: Pathophysiology, diagnosis, and management. Leuk. Lymphoma 2019, 60, 2365–2372. [Google Scholar] [CrossRef]
- Heckl, S.; Aschoff, A.; Kunze, S. Radiation-induced cavernous hemangiomas of the brain: A late effect predominantly in children. Cancer 2002, 94, 3285–3291. [Google Scholar] [CrossRef]
- Cannon, J.P.; Lee, T.A.; Clark, N.M.; Setlak, P.; Grim, S.A. The risk of seizures among the carbapenems: A meta-analysis. J. Antimicrob. Chemother. 2014, 69, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.; Hough, R.; Moppett, J.; Vora, A.; Mitchell, C.; Goulden, N. ‘Stroke-like syndrome’ caused by intrathecal methotrexate in patients treated during the UKALL 2003 trial. Leukemia 2013, 27, 954–956. [Google Scholar] [CrossRef]


| Patient | Age | Sex | ALL Lineage | Risk | Treatment Phase (AE) | Clinical Presentation (AE) | Duration (AE) | EEG (AE) | MRI (AE) | Grading (CTCAE v. 5.0) | Neurological Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | B | IR | Protocol II | Tonic seizure | 3 min | Regular | Normal | 2 | CR |
| 2 | 8 | F | B | HR | Protocol IB | Tonic-clonic status epilepticus | 30 min | Lower voltage | Discrete atrophic changes | 3 | 2 years of AET |
| 3 | 7.5 | M | B | HR | Protocol II | Tonic-clonic seizure | 5 days | Very slow background activity | PRES | 3 | 1 year of AET, atrophic MRI brain changes |
| 4 | 7.5 | F | T | HR | Protocol IA | Atonic seizure, Subsequent right-sided hemiparesis | 2 days | Slower activity, high voltage slow waves left | Cerebro- vascular ischemia | 3 | Ongoing AET, atrophic MRI brain changes |
| 5 | 3.5 | F | B | HR | Protocol IA | Tonic status epilepticus | 10 min | Slower activity bilaterally | PRES | 4 | CR, atrophic MRI brain changes |
| 6 | 5 | F | B | HR | Consolidation HR-1 block | Tonic-clonic seizure | 10 min | Regular | Normal | 3 | CR |
| 7 | 7.5 | F | B | IR | Protocol II | Tonic-clonic seizure | 30 s | Focal changes over FCT bilaterally with generalisation | LE | 3 | 5 years of AET; CTX modification |
| 8A | 6 | F | B | IR | Protocol II | Tonic seizure | 1 min | Slower activity CTP right | LE | 3 | Selective mutism |
| 8B | 10 | relapse | N/A | Protocol II-IDA | Complex partial seizure with generalisation; status epilepticus | 30 min | Focal changes left FCTO | LE, cortical oedema | 4 | 2 years of AET; ITT modification | |
| 9 | 3 | M | B | HR | Maintenance | Complex partial seizure with generalisation; status epilepticus | 2 h | Focal changes left | LE, pontine cavernoma, mineralising angiopathy | 4 | Two neurosurgical procedures; 5 years of AET |
| 10 | 6 | F | B | SR | Protocol IB | Reduced level of consciousness | 5 min | Focal changes right | Not performed. Brain CT normal | 3 | CR |
| 11 | 4.5 | M | B | SR | Protocol II | Headache, vomiting | unknown | Not performed | LE, Chiari malformation type I | 2 | CR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranjčec, I.; Rajačić, N.; Janjić, T.; Kukuruzović, M.; Jadrijević-Cvrlje, F.; Pavlović, M.; Roganović, J. Acute Neurotoxicity in Children Treated for Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma: A 10-Year Single-Centre Experience. Children 2025, 12, 31. https://doi.org/10.3390/children12010031
Kranjčec I, Rajačić N, Janjić T, Kukuruzović M, Jadrijević-Cvrlje F, Pavlović M, Roganović J. Acute Neurotoxicity in Children Treated for Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma: A 10-Year Single-Centre Experience. Children. 2025; 12(1):31. https://doi.org/10.3390/children12010031
Chicago/Turabian StyleKranjčec, Izabela, Nada Rajačić, Tamara Janjić, Monika Kukuruzović, Filip Jadrijević-Cvrlje, Maja Pavlović, and Jelena Roganović. 2025. "Acute Neurotoxicity in Children Treated for Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma: A 10-Year Single-Centre Experience" Children 12, no. 1: 31. https://doi.org/10.3390/children12010031
APA StyleKranjčec, I., Rajačić, N., Janjić, T., Kukuruzović, M., Jadrijević-Cvrlje, F., Pavlović, M., & Roganović, J. (2025). Acute Neurotoxicity in Children Treated for Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma: A 10-Year Single-Centre Experience. Children, 12(1), 31. https://doi.org/10.3390/children12010031






