The Role of Endoscopic Assistance in Surgery for Pediatric Cholesteatoma in Reducing Residual and Recurrent Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontes Lima, A.; Carvalho Moreira, F.; Sousa Menezes, A.; Esteves Costa, I.; Azevedo, C.; Sá Breda, M.; Dias, L. Is pediatric cholesteatoma more aggressive in children than in adults? A comparative study using the EAONO/JOS classification. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110170. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Dass, A.; Singhal, S.K.; Gupta, N. Comparative study of cholesteatoma in paediatric and adult patients. J. Laryngol. Otol. 2022, 136, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Piras, G.; Sykopetrites, V.; Taibah, A.; Russo, A.; Caruso, A.; Grinblat, G.; Sanna, M. Long term outcomes of canal wall up and canal wall down tympanomastoidectomies in pediatric cholesteatoma. Int. J. Pediatr. Otorhinolaryngol. 2021, 150, 110887. [Google Scholar] [CrossRef] [PubMed]
- Bujía, J.; Holly, A.; Antolí-Candela, F.; Tapia, M.G.; Kastenbauer, E. Immunobiological peculiarities of cholesteatoma in children: Quantification of epithelial proliferation by MIB1. Laryngoscope 1996, 106, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Dodson, E.E.; Hashisaki, G.T.; Hobgood, T.C.; Lambert, P.R. Intact canal wall mastoidectomy with tympanoplasty for cholesteatoma in children. Laryngoscope 1998, 108, 977–983. [Google Scholar] [CrossRef]
- Solis-Pazmino, P.; Siepmann, T.; Scheffler, P.; Ali, N.E.; Lincango-Naranjo, E.; Valdez, T.A.; Prokop, L.J.; Min-Woo Illigens, B.; Ponce, O.J.; Ahmad, I.N. Canal wall up versus canal wall down mastoidectomy techniques in the pediatric population with cholesteatoma: A systematic review and meta-analysis of comparative studies. Int. J. Pediatr. Otorhinolaryngol. 2023, 173, 111658. [Google Scholar] [CrossRef] [PubMed]
- Redaelli de Zinis, L.O.; Tonni, D.; Barezzani, M.G. Single-stage canal wall-down tympanoplasty: Long-term results and prognostic factors. Ann. Otol. Rhinol. Laryngol. 2010, 119, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kroon, V.J.; Mes, S.W.; Borggreven, P.A.; van de Langenberg, R.; Colnot, D.R.; Quak, J.J. Cholesteatoma surgery in the pediatric population: Remaining challenges in the era of mastoid obliteration. Eur. Arch. Otorhinolaryngol. 2023, 280, 1713–1722. [Google Scholar] [CrossRef]
- Han, S.Y.; Lee, D.Y.; Chung, J.; Kim, Y.H. Comparison of endoscopic and microscopic ear surgery in pediatric patients: A meta-analysis. Laryngoscope 2019, 129, 1444–1452. [Google Scholar] [CrossRef]
- Basonbul, R.A.; Ronner, E.A.; Kozin, E.D.; Lee, D.J.; Cohen, M.S. Systematic review of endoscopic ear surgery outcomes for pediatric cholesteatoma. Otol. Neurotol. 2021, 42, 108–115. [Google Scholar] [CrossRef]
- Amoodi, H.; Mofti, A.; Fatani, N.H.; Alhatem, H.; Zabidi, A.; Ibrahim, M. Non-echo planar diffusion-weighted imaging in the detection of recurrent or residual cholesteatoma: A systematic review and meta-analysis of diagnostic studies. Cureus 2022, 14, e32127. [Google Scholar] [CrossRef] [PubMed]
- Merkus, P.; Ten Tije, F.A.; Stam, M.; Tan, F.M.L.; Pauw, R.J. Implementation of the “EAONO/JOS definitions and classification of middle ear cholesteatoma”–From STAM to STAMCO. J. Int. Adv. Otol. 2017, 13, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Linder, T.E.; Shah, S.; Martha, A.S.; Röösli, C.; Emmett, S.D. Introducing the “ChOLE” classification and its comparison to the EAONO/JOS consensus classification for cholesteatoma staging. Otol. Neurotol. 2019, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.R.; Pedersen, C.N.; Grosfjeld, L.R.; Faber, C.E.; Djurhuus, B.D. Recurrence of cholesteatoma—A retrospective study including 1.;006 patients for more than 33 years. Int. Arch. Otorhinolaryngol. 2020, 24, e18–e23. [Google Scholar] [PubMed]
- Stangerup, S.E.; Drozdziewicz, D.; Tos, M. Cholesteatoma in children.; predictors and calculation of recurrence rates. Int. J. Pediatr. Otorhinolaryngol. 1999, 49 (Suppl. S1), S69–S73. [Google Scholar] [CrossRef] [PubMed]
- James, A.L.; Cushing, S.; Papsin, B.C. Residual cholesteatoma after endoscope- guided surgery in children. Otol. Neurotol. 2016, 37, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, L.; Wang, M.; Wang, Y.; Zou, J. Endoscopic versus microscopic surgery for treatment of middle ear cholesteatoma: A systematic review and meta-analysis. Am. J. Otolaryngol. 2021, 42, 102451. [Google Scholar] [CrossRef]
- Moneir, W.; Hemdan, A.; El-Kholy, N.A.; El-Kotb, M.; El-Okda, M. Endoscopic transcanal attico-antrostomy versus endoscopic-assisted canal wall up mastoidectomy in management of localized cholesteatoma: A randomized clinical trial. Eur. Arch. Otorhinolaryngol. 2022, 279, 4371–4378. [Google Scholar] [CrossRef]
- Iannella, G.; Pace, A.; Greco, A.; Polimeni, A.; Maniaci, A.; Mucchino, A.; Lechien, J.R.; Saibene, A.M.; Mat, Q.; Gargula, S.; et al. Endaural microscopic approach versus endoscopic transcanal approach in treatment of attic cholesteatomas. Am. J. Otolaryngol. 2023, 44, 103860. [Google Scholar] [CrossRef]
- Salem, J.; Bakundukize, J.; Milinis, K.; Sharma, S.D. Mastoid obliteration versus canal wall down or canal wall up mastoidectomy for cholesteatoma: Systematic review and meta-analysis. Am. J. Otolaryngol. 2023, 44, 103751. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Liu, W.; Chen, M.; Shao, J.; Zhang, X.; Ma, N.; Li, Y.; Peng, Y.; Zhang, J. Comparison of the EAONO/JOS.; STAMCO and ChOLE cholesteatoma staging systems in the prognostic evaluation of acquired middle ear cholesteatoma in children. Eur. Arch. Otorhinolaryngol. 2022, 279, 5583–5590. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, D.; Soloperto, D.; Rubini, A.; Villari, D.; Genovese, E.; Artioli, F.; Presutti, L. Endoscopic exclusive transcanal approach to the tympanic cavity cholesteatoma in pediatric patients: Our experience. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.B.; Zuniga, M.G.; Sweeney, A.D.; Bertrand, N.M.; Wanna, G.B.; Haynes, D.S.; Wootten, C.T.; Rivas, A. Pediatric endoscopic cholesteatoma surgery. Otolaryngol. Head Neck Surg. 2016, 154, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Sarcu, D.; Isaacson, G. Long-term results of endoscopically assisted pediatric cholesteatoma surgery. Otolaryngol. Head Neck Surg. 2016, 154, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Basonbul, R.A.; Kozin, E.D.; Lee, D.J. Residual cholesteatoma during second-look procedures following primary pediatric endoscopic ear surgery. Otolaryngol. Head Neck Surg. 2017, 157, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Ghadersohi, S.; Carter, J.M.; Hoff, S.R. Endoscopic transcanal approach to the middle ear for management of pediatric cholesteatoma. Laryngoscope 2017, 127, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Le Nobel, G.J.; Cushing, S.L.; Papsin, B.C.; James, A.L. Intraoperative bleeding and the risk of residual cholesteatoma: A multivariate analysis. Otol. Neurotol. 2017, 38, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Glikson, E.; Feinmesser, G.; Sagiv, D.; Wolf, M.; Migirov, L.; Shapira, Y. Trans-canal endoscopic ear surgery and canal wall-up tympano-mastoidectomy for pediatric middle ear cholesteatoma. Eur. Arch. Otorhinolaryngol. 2019, 276, 3021–3026. [Google Scholar] [CrossRef]
- Yaniv, D.; Tzelnick, S.; Ulanovski, D.; Hilly, O.; Raveh, E. Effect of endoscope assistance in tympanomastoidectomy for lowering the rate of residual cholesteatoma: Results from 91 paediatric patients. Clin. Otolaryngol. 2019, 44, 1105–1108. [Google Scholar] [CrossRef]
- Dixon, P.R.; James, A.L. Evaluation of residual disease following transcanal totally endoscopic vs postauricular surgery among children with middle ear and attic cholesteatoma. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 408–413. [Google Scholar] [CrossRef]
- Curran, J.F.; Coleman, H.; Tikka, T.; Iyer, A. Comparison of outcomes of endoscopic ear surgery with microsurgery for cholesteatoma: A prospective study of 91 cases with three-year follow-up. Clin. Otolaryngol. 2022, 47, 197–202. [Google Scholar] [CrossRef]
- Manzoor, N.F.; Totten, D.J.; McLeod, M.E.; Sherry, A.D.; Perkins, E.L.; Haynes, D.S.; Rivas, A. Comparative analysis of recidivism after endoscopic and microscopic-based cholesteatoma resection. Otol. Neurotol. 2022, 43, 466–471. [Google Scholar] [CrossRef]
- Hu, X.; Chen, M.; Dai, W.; Zhang, C.; Li, S. Efficiency of intraoperative endoscopic inspection in reducing residuals in canal-wall-up surgery for pediatric cholesteatoma involving the mastoid. Eur. Arch. Otorhinolaryngol. 2023, 280, 3593–3600. [Google Scholar] [CrossRef]

| Variable | (No of Patients) | Overall Relapse Estimate (%) | Time (Years) | p Value | Residual Disease Estimate (%) | Time (Years) | p Value | Recurrent Disease Estimate (%) | Time (Years) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Site of origin | pars flaccida (32) | 51 ± 10 | 10 | 0.8 | 28 ± 9 | 12 | 0.9 | 30 ± 9 | 12 | 0.7 |
| pars tensa/sinus (39) | 44 ± 8 | 12 | 26 ± 10 | 12 | 27 ± 7 | 12 | ||||
| Sex | female (21) | 49 ± 11 | 10 | 0.9 | 24 ± 9 | 11 | 0.8 | 31 ± 11 | 12 | 0.9 |
| male (50) | 47 ± 7 | 12 | 27 ± 7 | 12 | 27 ± 7 | 12 | ||||
| Side | right (31) | 41 ± 9 | 12 | 0.4 | 23 ± 8 | 12 | 0.7 | 27 ± 8 | 12 | 0.9 |
| left (40) | 52 ± 8 | 12 | 29 ± 7 | 12 | 29 ± 8 | 12 | ||||
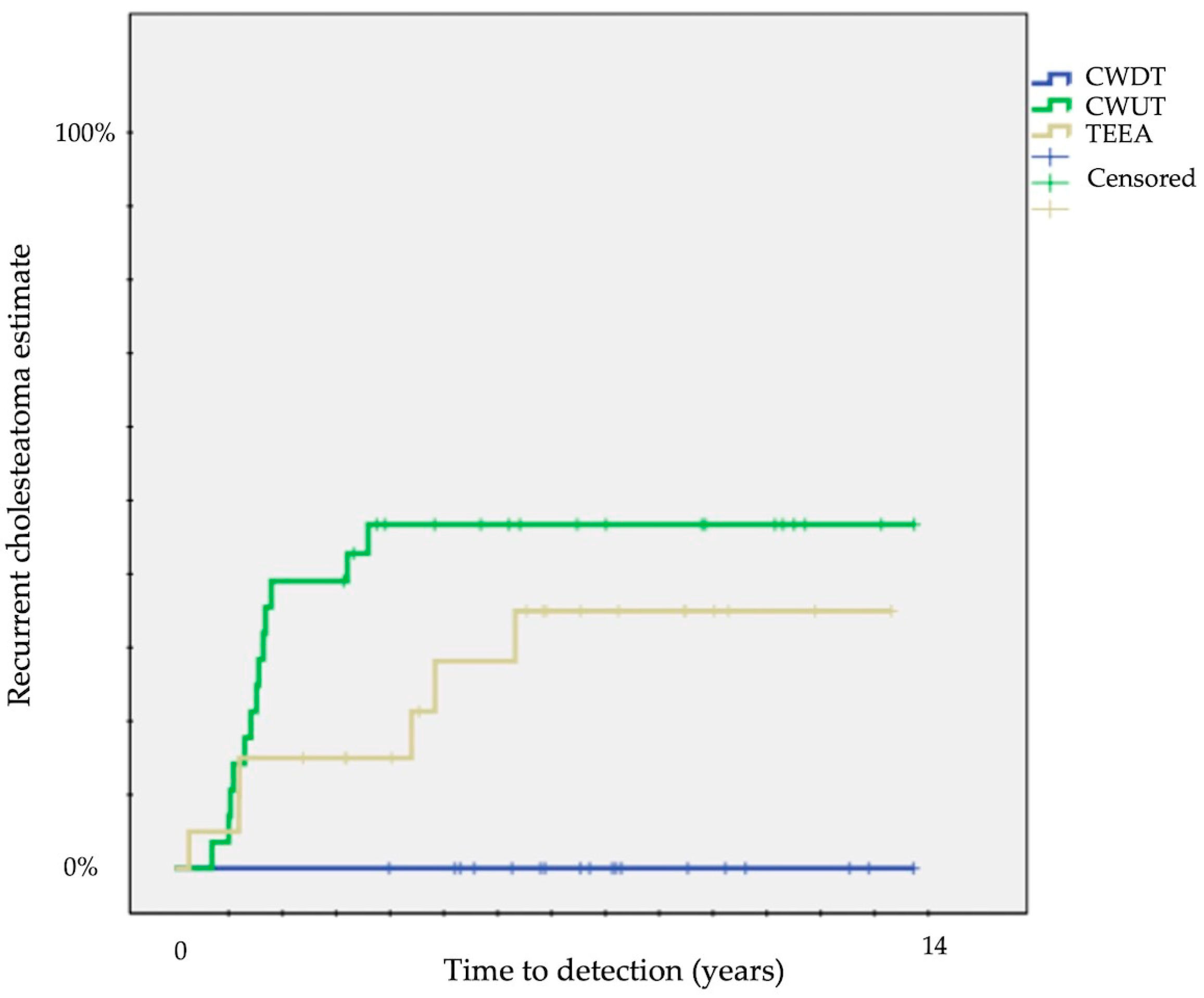
| Surgical approach | CWD (18) | 33 ± 11 | 12 | 0.04 | 33 ± 11 | 12 | 0.1 | 0 | 12 | 0.006 |
| CWU (31) | 60 ± 9 | 12 | 34 ± 9 | 12 | 43 ± 9 | 12 | ||||
| endoscopic (22) | 40 ± 11 | 12 | 9 ± 6 | 12 | 32 ± 11 | 12 | ||||
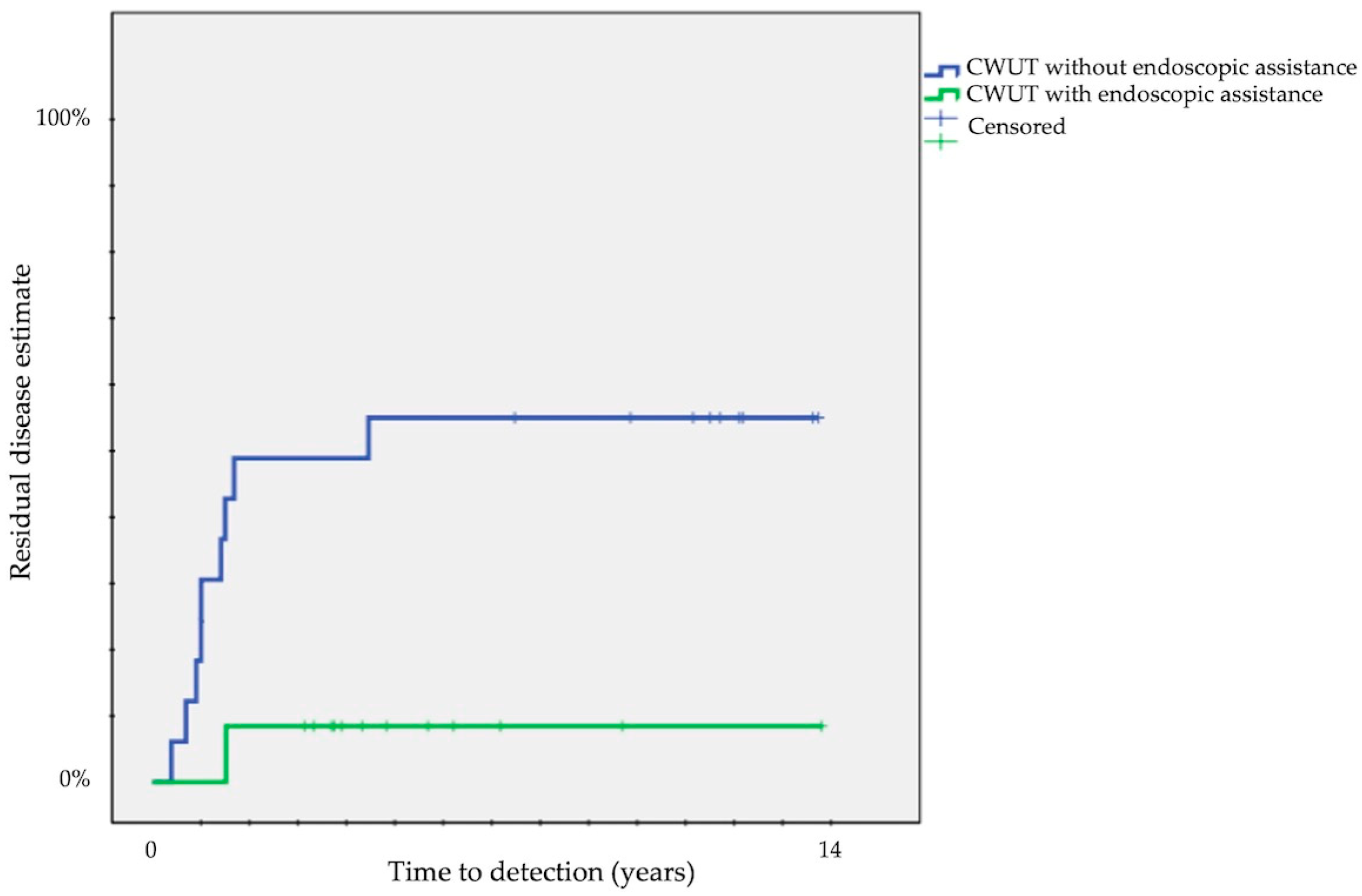
| Endoscopic assistance | no (36) | 50 ± 8 | 12 | 0.6 | 42 ± 8 | 12 | 0.003 | 19 ± 7 | 12 | 0.1 |
| yes (35) | 46 ± 10 | 12 | 9 ± 5 | 12 | 40 ± 10 | 12 | ||||
| Difficult sites (STAMCO) | no (26) | 51 ± 11 | 12 | 0.6 | 30 ± 10 | 12 | 0.9 | 29 ± 9 | 12 | 0.7 |
| supratubal recess (12) | 43 ± 15 | 11 | 21 ± 13 | 11 | 17 ± 11 | 11 | ||||
| sinus tympani (24) | 38 ± 10 | 12 | 21 ± 8 | 12 | 26 ± 9 | 12 | ||||
| both sites (9) | 67 ± 16 | 12 | 33 ± 16 | 12 | 44 ± 17 | 12 | ||||
| Tympanic involvement (STAMCO) | no (7) | 33 ± 19 | 9 | 0.3 | 17 ± 15 | 9 | 0.4 | 17 ± 15 | 9 | 0.4 |
| yes (64) | 49 ± 7 | 12 | 27 ± 6 | 12 | 30 ± 6 | 12 | ||||
| Attic involvement (STAMCO) | no (9) | 48 ± 18 | 11 | 0.7 | 22 ± 14 | 11 | 0.8 | 39 ± 18 | 11 | 0.7 |
| yes (62) | 47 ± 7 | 12 | 27 ± 6 | 12 | 27 ± 6 | 12 | ||||
| Supratubal recess involvement | no (50) | 45 ± 7 | 12 | 0.5 | 25 ± 6 | 12 | 0.8 | 28 ± 7 | 12 | 0.8 |
| yes (21) | 53 ± 11 | 12 | 29 ± 10 | 12 | 29 ± 10 | 12 | ||||
| Sinus tympani involvement | no (38) | 49 ± 9 | 12 | 0.9 | 29 ± 8 | 12 | 0.9 | 25 ± 7 | 12 | 0.7 |
| yes (33) | 46 ± 9 | 12 | 24 ± 8 | 12 | 31 ± 8 | 12 | ||||
| Mastoid involvement (STAMCO) | no (33) | 35 ± 9 | 12 | 0.1 | 18 ± 7 | 12 | 0.2 | 23 ± 8 | 12 | 0.4 |
| yes (38) | 57 ± 8 | 12 | 33 ± 8 | 12 | 32 ± 8 | 12 | ||||
| Ossicular condition (STAMCO) | intact (9) | 11 ± 11 | 9 | 0.03 | 11 ± 11 | 9 | 0.6 | 0 | 9 | 0.2 |
| M + S+ (37) | 43 ± 9 | 12 | 25 ± 7 | 12 | 29 ± 8 | 12 | ||||
| M + S− (18) | 66 ± 12 | 12 | 32 ± 12 | 12 | 40 ± 12 | 12 | ||||
| M− (7) | 71 ± 17 | 12 | 43 ± 19 | 12 | 31 ± 19 | 12 | ||||
| Ch (Chole classification) | 1a (3) | 33 ± 8 | 7 | 0.9 | 33 ± 27 | 7 | 0.7 | 33 ± 8 | 7 | 0.8 |
| 1b (3) | 100 | 5 | 0 | 6 | 0 | 5 | ||||
| 2a (5) | 61 ± 10 | 3 | 40 ± 8 | 3 | 40 ± 22 | 7 | ||||
| 2b (25) | 47 ± 13 | 12 | 20 ± 8 | 12 | 20 ± 8 | 12 | ||||
| 3 (35) | 40 ± 22 | 12 | 30 ± 8 | 12 | 30 ± 8 | 12 | ||||
| Mastoid status | well pneumatized (27) | 51 ± 10 | 12 | 0.4 | 19 ± 8 | 12 | 0.5 | 36 ± 10 | 12 | 0.3 |
| partially pneumatized (22) | 50 ± 11 | 12 | 32 ± 10 | 12 | 32 ± 10 | 12 | ||||
| sclerotic (22) | 38 ± 11 | 12 | 28 ± 10 | 12 | 14 ± 7 | 12 |
| Author | Cholesteatoma Type | Surgical Approach (No of Ears) | Residual Cholesteatoma Rate | Recurrent Cholesteatoma Rate | Mean Follow-Up Months (Range) | Notes |
|---|---|---|---|---|---|---|
| Marchioni et al. [21] | Including congenital | MA CWUT (28) | 17% | 34% | 36 (8–88) | Second look in selected cases |
| TEEA (31) | 13% | 19% | ||||
| Hunter et al. [22] | MA CWUT (47) | 9% | 9% | 18.8 (7–48) | Second look in selected cases | |
| MA + EA (21) TEEA (8) | 10% | 10% | ||||
| James et al. [15] | Including congenital, ear canal, and implantation | MA CWUT (108) | 24% | Not analyzed | Median length of maximum follow-up 74 months | Second look in selected cases |
| MA CWUT + EA or TEEA (127) | 15% | Median length of maximum follow-up 38 months | ||||
| Sarcu et al. [23] | Including congenital | MA CWUT + EA (42) | 14% | Not analyzed | 60.2 (12–188) | In 17% of ears, residuals were not detected with microscope but were detected with endoscope during initial surgery; second look in selected cases |
| Cohen et al. [24] | Including congenital | MA CWUT (24) | 25% | Not analyzed | Not reported | Second look in all cases |
| MA CWUT + EA or TEEA (32) | 28% | |||||
| Ghadersohi et al. [25] | Including congenital | MA + EI (7) | 29% | 14% | 31 (9–55) | Second look in selected cases MRI in all cases |
| EA (middle ear) + MA (mastoid) (9) | 7% | 13% | ||||
| TEEA (22) | 0 | 5% | ||||
| Le Nobel 2017 et al. [26] | Exclusive middle ear/attic including congenital | MA atticotomy + EA (79) | 9% | Not analyzed | 52 (12–126) | Second look or MRI in selected cases; residual correlated with intraoperative bleeding |
| TEEA (33) | 12% | |||||
| Glikson et al. [27] | Exclusive middle ear/attic | MA CWUT (19) | 16% | 37% | 37.2 | Clinical and MRI follow-up |
| TEEA (30) | 10% | 7% | 32.6 | |||
| Yaniv et al. [28] | MA CWUT (42) | 38% | 14% | 51 | Clinical and MRI follow-up | |
| MA CWUT + EA (49) | 18% | 33% | 64 | |||
| Dixon et al. [29] | Exclusive middle ear/attic | MA (112) | 11% | Not analyzed | Not reported | Two years’ second look or MRI in selected cases |
| TEEA (65) | 6% | |||||
| Curran et al. [30] | MA (30) or MA + EA (35) | 5% | 2% | (24–60) | Including adults; 18 months’ second look or MRI in all cases | |
| TEEA (26) | 4% | 4% | ||||
| Manzoor et al. [31] | Including congenital | MA (253) | 6% | 4% | Not reported | Including adults; second look in 28% of cases |
| MA + EA (79) or TEEA (43) | 13% | 7% | ||||
| Hu et al. [32] | Extended to the mastoid, including congenital | MA CWUT + EI (32) | 6% | 9% | 24 all patients | In 1 ear, residuals were not detected with microscope but were detected with endoscope during initial surgery; CT +/− MRI to detect residual disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassif, N.; Redaelli de Zinis, L.O. The Role of Endoscopic Assistance in Surgery for Pediatric Cholesteatoma in Reducing Residual and Recurrent Disease. Children 2024, 11, 369. https://doi.org/10.3390/children11030369
Nassif N, Redaelli de Zinis LO. The Role of Endoscopic Assistance in Surgery for Pediatric Cholesteatoma in Reducing Residual and Recurrent Disease. Children. 2024; 11(3):369. https://doi.org/10.3390/children11030369
Chicago/Turabian StyleNassif, Nader, and Luca Oscar Redaelli de Zinis. 2024. "The Role of Endoscopic Assistance in Surgery for Pediatric Cholesteatoma in Reducing Residual and Recurrent Disease" Children 11, no. 3: 369. https://doi.org/10.3390/children11030369
APA StyleNassif, N., & Redaelli de Zinis, L. O. (2024). The Role of Endoscopic Assistance in Surgery for Pediatric Cholesteatoma in Reducing Residual and Recurrent Disease. Children, 11(3), 369. https://doi.org/10.3390/children11030369






