Reliability of Two Recently Developed Procedures Assessing Biological Maturity by Ultrasound Imaging—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Measurements
2.4. Grading
2.5. Statistical Analysis
3. Results
3.1. Stages
3.1.1. Interrater Reliability
3.1.2. Dependence of the Interrater Reliability from the Stage
3.1.3. Intrarater Reliability
3.2. Ossification Ratio
4. Discussion
4.1. Interrater Reliability (Maturity Stages)
4.2. Intrarater Reliability (Maturity Stages)
4.3. Interrater Reliability (OssR)
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engebretsen, L.; Steffen, K.; Bahr, R.; Broderick, C.; Dvorak, J.; Janarv, P.-M.; Johnson, A.; Leglise, M.; Mamisch, T.C.; McKay, D.; et al. The International Olympic Committee Consensus Statement on age determination in high-level young athletes. Br. J. Sports Med. 2010, 44, 476. [Google Scholar] [CrossRef]
- Tanner, J.M. Issues and advances in adolescent growth and development. J. Adolesc. Health Care 1987, 8, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Mellits, E.D.; Dorst, J.P.; Cheek, D.B. Bone age: Its contribution to the prediction of maturational or biological age. Am. J. Phys. Anthr. 1971, 35, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.A. The biology of bone maturation and ageing. Acta Paediatr. 1997, 423, 107–108. [Google Scholar] [CrossRef]
- Malina, R.M.; Peña Reyes, M.E.; Figueiredo, A.J.; Coelho, E.S.M.J.; Horta, L.; Miller, R.; Chamorro, M.; Serratosa, L.; Morate, F. Skeletal age in youth soccer players: Implication for age verification. Clin. J. Sport Med. 2010, 20, 469–474. [Google Scholar] [CrossRef]
- Cadenas-Sanchez, C.; Intemann, T.; Labayen, I.; Peinado, A.B.; Vidal-Conti, J.; Sanchis-Moysi, J.; Moliner-Urdiales, D.; Rodriguez Perez, M.A.; Cañete Garcia-Prieto, J.; Fernández-Santos, J.d.R.; et al. Physical fitness reference standards for preschool children: The PREFIT project. J. Sci. Med. Sport 2019, 22, 430–437. [Google Scholar] [CrossRef]
- Drenowatz, C.; Greier, K. Association of biological maturation with the development of motor competence in Austrian middle school students—A 3-year observational study. Transl. Pediatr. 2019, 8, 402–411. [Google Scholar] [CrossRef]
- Malina, R.M.; Eisenmann, J.C.; Cumming, S.P.; Ribeiro, B.; Aroso, J. Maturity-associated variation in the growth and functional capacities of youth football (soccer) players 13–15 years. Eur. J. Appl. Physiol. 2004, 91, 555–562. [Google Scholar] [CrossRef]
- Gil, S.M.; Badiola, A.; Bidaurrazaga-Letona, I.; Zabala-Lili, J.; Gravina, L.; Santos-Concejero, J.; Lekue, J.A.; Granados, C. Relationship between the relative age effect and anthropometry, maturity and performance in young soccer players. J. Sports Sci. 2014, 32, 479–486. [Google Scholar] [CrossRef]
- Huertas, F.; Ballester, R.; Gines, H.J.; Hamidi, A.K.; Moratal, C.; Lupiáñez, J. Relative Age Effect in the Sport Environment. Role of Physical Fitness and Cognitive Function in Youth Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 2837. [Google Scholar] [CrossRef]
- Malina, R.M.; Reyes, M.E.P.; Eisenmann, J.C.; Horta, L.; Rodrigues, J.; Miller, R. Height, mass and skeletal maturity of elite Portuguese soccer players aged 11–16 years. J. Sports Sci. 2000, 18, 685–693. [Google Scholar] [CrossRef]
- Sherar, L.; Baxter-Jones, A.; Faulkner, R.; Russell, K. Do physical maturity and birth date predict talent in mal youth ice hockey players? J. Sports Sci. 2007, 25, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Romann, M.; Rössler, R.; Marie, J.; Faude, O. Relative age effects in Swiss talent development—A nationwide analysis of all sports. J. Sports Sci. 2018, 36, 2025–2031. [Google Scholar] [CrossRef]
- Pearson, D.T.; Naughton, G.A.; Torode, M. Predictability of physiological testing and the role of maturation in talent identification for adolescent team sports. J. Sci. Med. Sport 2006, 9, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Vaeyens, R.; Lenoir, M.; Williams, A.M.; Philippaerts, R.M. Talent Identification and Development Programmes in Sport. Sports Med. 2008, 38, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Rüeger, E.; Hutmacher, N.; Eichelberger, P.; Löcherbach, C.; Albrecht, S.; Romann, M. Ultrasound Imaging-Based Methods for Assessing Biological Maturity during Adolescence and Possible Application in Youth Sport: A Scoping Review. Children 2022, 9, 1985. [Google Scholar] [CrossRef]
- Cossio-Bolaños, M.; Campos, R.G.; Andruske, C.L.; Flores, A.V.; Luarte-Rocha, C.; Olivares, P.R.; Garcia-Rubio, J.; de Arruda, M. Physical Growth, Biological Age, and Nutritional Transitions of Adolescents Living at Moderate Altitudes in Peru. Int. J. Environ. Res. Public Health 2015, 12, 12082–12094. [Google Scholar] [CrossRef]
- Ke, D.; Lu, D.; Cai, G.; Wang, X.; Zhang, J.; Suzuki, K. Chronological and Skeletal Age in Relation to Physical Fitness Performance in Preschool Children. Front. Pediatr. 2021, 9, 641353. [Google Scholar] [CrossRef]
- Cavallo, F.; Mohn, A.; Chiarelli, F.; Giannini, C. Evaluation of Bone Age in Children: A Mini-Review. Front. Pediatr. 2021, 9, 580314. [Google Scholar] [CrossRef]
- Doyle, E.; Márquez-Grant, N.; Field, L.; Holmes, T.; Arthurs, O.; Rijn, R.; Hackman, L.; Kasper, K.; Lewis, J.; Loomis, P.; et al. Guidelines for best practice: Imaging for Age Estimation in the Living. J. Forensic Radiol. Imaging 2019, 16, 38–49. [Google Scholar] [CrossRef]
- Chaumoitre, K.; Saliba-Serre, B.; Adalian, P.; Signoli, M.; Leonetti, G.; Panuel, M. Forensic use of the Greulich and Pyle atlas: Prediction intervals and relevance. Eur. Radiol. 2017, 27, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Manzoor Mughal, A.; Hassan, N.; Ahmed, A. Bone age assessment methods: A critical review. Pak. J. Med. Sci. 2014, 30, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Fred, A.; Mettler, J.; Huda, W.; Yoshizumi, T.T.; Mahesh, M. Effective Doses in Radiology and Diagnostic Nuclear Medicine: A Catalog. Radiology 2008, 248, 254–263. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, Y.; Feng, Q.; Zhang, C. Summation of Ossification Ratios of Radius, Ulna and Femur: A New Parameter to Evaluate Bone Age by Ultrasound. Ultrasound Med. Biol. 2020, 46, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.A.; Diedrich, V.; Schmitt, O. Determination of skeletal maturity by ultrasound: A preliminary report. Skelet. Radiol. 1995, 24, 417–420. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, C. Tanner-Whitehouse skeletal maturity score derived from ultrasound images to evaluate bone age. Eur. Radiol. 2023, 33, 2399–2406. [Google Scholar] [CrossRef]
- Utczas, K.; Muzsnai, A.; Cameron, N.; Zsakai, A.; Bodzsar, E.B. A comparison of skeletal maturity assessed by radiological and ultrasonic methods. Am. J. Hum. Biol. 2017, 29. [Google Scholar] [CrossRef]
- Torenek Ağırman, K.; Bilge, O.M.; Miloğlu, Ö. Ultrasonography in determining pubertal growth and bone age. Dentomaxillofacial Radiol. 2018, 47, 20170398. [Google Scholar] [CrossRef]
- Bilgili, Y.; Hizel, S.; Kara, S.A.; Sanli, C.; Erdal, H.H.; Altinok, D. Accuracy of Skeletal Age Assessment in Children From Birth to 6 Years of Age With the Ultrasonographic Version of the Greulich-Pyle Atlas. J. Ultrasound Med. 2003, 22, 683–690. [Google Scholar] [CrossRef]
- Prokop-Piotrkowska, M.; Marszałek-Dziuba, K.; Moszczyńska, E.; Szalecki, M.; Jurkiewicz, E. Traditional and New Methods of Bone Age Assessment-An Overview. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 251–262. [Google Scholar] [CrossRef]
- Khan, K.M.; Miller, B.S.; Hoggard, E.; Somani, A.; Sarafoglou, K. Application of ultrasound for bone age estimation in clinical practice. J. Pediatr. 2009, 154, 243–247. [Google Scholar] [CrossRef]
- Herrmann, J.; Säring, D.; Auf der Mauer, M.; Groth, M.; Jopp-van Well, E. Forensic age assessment of the knee: Proposal of a new classification system using two-dimensional ultrasound volumes and comparison to MRI. Eur. Radiol. 2021, 31, 3237–3247. [Google Scholar] [CrossRef]
- Wirtz, M.; Kutschmann, M. Analyzing interrater agreement for categorical data using Cohen’s kappa and alternative coefficients. Die Rehabil. 2007, 46, 370–377. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Applying the right statistics: Analyses of measurement studies. Ultrasound Obstet. Gynecol. 2003, 22, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Weir, P.L.; Till, K.; Romann, M.; Cobley, S. Relative Age Effects Across and Within Female Sport Contexts: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 1451–1478. [Google Scholar] [CrossRef] [PubMed]
- Baxter-Jones, A.D.G.; Barbour-Tuck, E.N.; Dale, D.; Sherar, L.B.; Knight, C.J.; Cumming, S.P.; Ferguson, L.J.; Kowalski, K.C.; Humbert, M.L. The role of growth and maturation during adolescence on team-selection and short-term sports participation. Ann. Hum. Biol. 2020, 47, 316–323. [Google Scholar] [CrossRef]
- Cumming, S.P.; Searle, C.; Hemsley, J.K.; Haswell, F.; Edwards, H.; Scott, S.; Gross, A.; Ryan, D.; Lewis, J.; White, P.; et al. Biological maturation, relative age and self-regulation in male professional academy soccer players: A test of the underdog hypothesis. Psychol. Sport Exerc. 2018, 39, 147–153. [Google Scholar] [CrossRef]
- Windschall, D.; Collado, P.; Vojinovic, J.; Magni-Manzoni, S.; Balint, P.; Bruyn, G.A.W.; Hernandez-Diaz, C.; Nieto, J.C.; Ravagnani, V.; Tzaribachev, N.; et al. Age-Related Vascularization and Ossification of Joints in Children: An International Pilot Study to Test Multiobserver Ultrasound Reliability. Arthritis Care Res. 2020, 72, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.; Rocha, L.P.B.; Mathur, S.; Santana, L.; Melo, P.F.; Silva, V.; Durigan, J.L.Q.; Cipriano, G., Jr. Reliability of skeletal muscle ultrasound in critically ill trauma patients. Rev. Bras. Ter. Intensiv. 2019, 31, 464–473. [Google Scholar] [CrossRef]
- Zaidman, C.M.; Wu, J.S.; Wilder, S.; Darras, B.T.; Rutkove, S.B. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve 2014, 50, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zhang, K.; Peng, Z.; Cui, J.H.; Hu, N.; Deng, Z.H. Forensic age estimation of living persons from the knee: Comparison of MRI with radiographs. Forensic Sci. Int. 2016, 268, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kvist, O.; Luiza Dallora, A.; Nilsson, O.; Anderberg, P.; Sanmartin Berglund, J.; Flodmark, C.E.; Diaz, S. A cross-sectional magnetic resonance imaging study of factors influencing growth plate closure in adolescents and young adults. Acta Paediatr. 2021, 110, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.; Muñoz, A.; Beltrán, I.; Labajo, E.; Perea, B.; Sánchez, J.A. Assessment of adulthood in the living Spanish population based on ossification of the medial clavicle epiphysis using ultrasound methods. Forensic Sci. Int. 2018, 284, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.B.; Codinha, S.; García, A.M.; Sánchez, J.A.S. Estimating legal age based on fusion of The proximal humeral epiphysis. Int. J. Leg. Med. 2017, 131, 1133–1140. [Google Scholar] [CrossRef]
- Torlak, G.; Kiter, E.; Oto, M.; Akman, A. Ultrasonographic evaluation of the Risser sign. Is it a reliable and reproducible method? Spine 2012, 37, 316–320. [Google Scholar] [CrossRef]
- Pitlović, V.; Sarić, G.; Pitlović, H.; Jovanović, S.; Jurisić, D. A correlation of peak height velocity and olecranon apophysis ossification assessed by ultrasound. Coll. Antropol. 2013, 37, 1285–1289. [Google Scholar] [PubMed]



| Mean | SD | Median | Min | Max | |
|---|---|---|---|---|---|
| Age (in years) | 13.6 | 2.3 | 13.5 | 10 | 17 |
| Body height (in cm) | 161.5 | 10.1 | 163.5 | 142 | 175 |
| Body weight (in kg) | 52.6 | 11.3 | 57.5 | 32 | 68 |
| Definition of Stages 1–3 | |
|---|---|
| Stage 1 | The growth plate is open and there is a large gap (2–3 mm) between epiphysis and metaphysis. From the outer cortex into the physis, there is a right-angle step-off (metaphyseal zone of calcification) (Figure 1a). |
| Stage 2 | The growth plate has a small diameter. There is only a shallow notch between epiphysis and metaphysis (Figure 1b). |
| Stage 3 | The growth plate is closed. No gap can be seen between epiphysis and metaphysis (Figure 1c). |
| Cohen’s Kappa [95% CI] | ||
|---|---|---|
| Interrater Reliability (JB, NH) | Intrarater Reliability (NH, NH2) | |
| Fibula (Fib) | 0.82 [0.60, 1.00] | 1.00 [1.00, 1.00] |
| Lateral tibia (TL) | 0.90 [0.70, 1.00] | 1.00 [1.00, 1.00] |
| Lateral femur (FL) | 0.89 [0.67, 1.00] | 1.00 [1.00, 1.00] |
| Medial tibia (TM) | 0.86 [0.60, 1.00] | 0.70 [0.31, 1.00] |
| Medial femur (FM) | 0.69 [0.37, 1.00] | 0.88 [0.66, 1.00] |
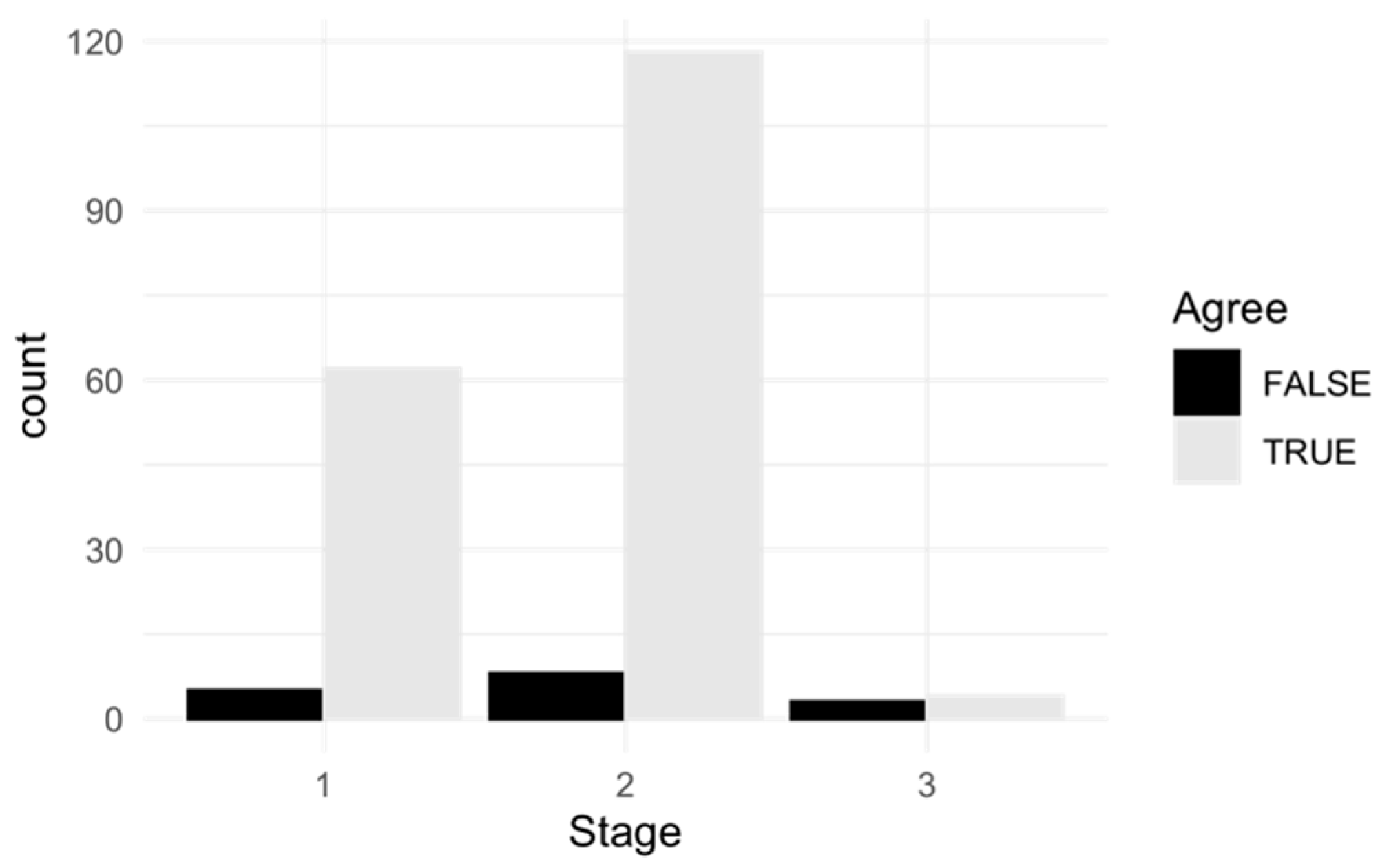
| Agreement | Disagreement | Row Total | ||||
|---|---|---|---|---|---|---|
| Absolute | Relative | Absolute | Relative | Absolute | Relative | |
| Stage 1 | 62 | 31% | 5 | 2.5% | 67 | 33.5% |
| Stage 2 | 118 | 59% | 8 | 4% | 126 | 63% |
| Stage 3 | 4 | 2% | 3 | 1.5% | 7 | 3.5% |
| Column total | 184 | 92% | 16 | 8% | 200 | 100% |
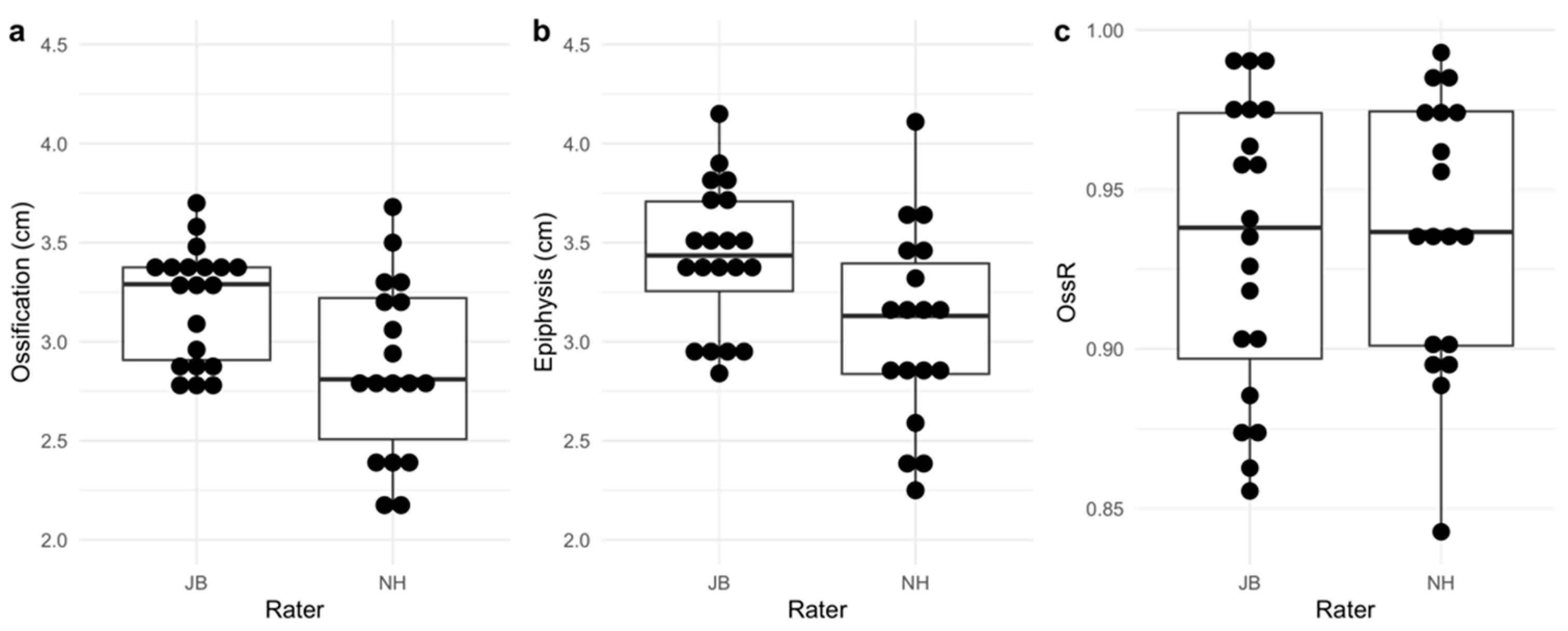
| Bias [95% CI] in cm | Absolute MDC [95% CI] in cm | Relative MDC [95% CI] % | ICC(A,1) [95% CI] | |
|---|---|---|---|---|
| OssR | 0.004 | 0.030 | 3.225 | 0.930 |
| [−0.004, 0.011] | [0.024, 0.036] | [2.550, 3.901] | [0.828, 0.973] | |
| Diameter ossification center | 0.316 | 0.995 | 32.718 | 0.047 |
| [0.064, 0.569] | [0.787, 1.204] | [25.865, 39.571] | [−0.272, 0.429] | |
| Diameter epiphysis | 0.326 | 1.016 | 31.155 | 0.205 |
| [0.068, 0.583] | [0.803, 1.229] | [24.629, 37.680] | [−0.162, 0.569] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutmacher, N.; Busch, J.D.; Rüeger, E.; Romann, M.; Eichelberger, P. Reliability of Two Recently Developed Procedures Assessing Biological Maturity by Ultrasound Imaging—A Pilot Study. Children 2024, 11, 326. https://doi.org/10.3390/children11030326
Hutmacher N, Busch JD, Rüeger E, Romann M, Eichelberger P. Reliability of Two Recently Developed Procedures Assessing Biological Maturity by Ultrasound Imaging—A Pilot Study. Children. 2024; 11(3):326. https://doi.org/10.3390/children11030326
Chicago/Turabian StyleHutmacher, Nicole, Jasmin D. Busch, Eva Rüeger, Michael Romann, and Patric Eichelberger. 2024. "Reliability of Two Recently Developed Procedures Assessing Biological Maturity by Ultrasound Imaging—A Pilot Study" Children 11, no. 3: 326. https://doi.org/10.3390/children11030326
APA StyleHutmacher, N., Busch, J. D., Rüeger, E., Romann, M., & Eichelberger, P. (2024). Reliability of Two Recently Developed Procedures Assessing Biological Maturity by Ultrasound Imaging—A Pilot Study. Children, 11(3), 326. https://doi.org/10.3390/children11030326









