Abstract
(1) Background: Bullying is one of the most common forms of aggressive behavior during childhood and adolescence. Some decades ago, researchers began exploring the basis of peer victimization from a biological perspective. Specifically, the Hypothalamic-Pituitary-Adrenal (HPA) and Hypothalamic-Pituitary-Gonadal (HPG) axes have been studied in relation to status-relevant behaviors, such as bullying. (2) Methods: We conducted a systematic review following the PRISMA guide and registered the review protocol at PROSPERO (CRD42023494738). We searched for relevant studies in PubMed, Psycinfo, Scopus, and Web of Science, and assessed them using the Robins E-tool. (3) Results: Our search yielded 152 studies, of which 33 were included in the review. These studies explored the association between testosterone and cortisol levels with bullying behavior, finding diverse results. Most of the studies were rated as having a low risk of bias. (4) Conclusions: This study not only enhances our understanding of bullying, but also provides guidance for the development of prevention and management programs for it. In the future, researchers should continue exploring the joint effects of different hormones on the HPA and HPG axis, using a broader set of biomarkers.
1. Introduction
Bullying is a common form of aggressive behavior during childhood and adolescence, and it has been identified as one of the main sources of stress during these periods [1,2]. This behavior is defined as a type of aggressive behavior that occurs in the school environment and it is characterized by three main aspects: intentionality, repeatability, and power imbalance [3]. Several studies have examined this phenomenon and concluded that there are diverse types of bullying, including physical, verbal, social, psychological, or cyberbullying [4,5].
Recent evidence indicates that one in three students are involved in bullying worldwide [1]. Some years ago, The World Health Organization (WHO) estimated that between 2 and 32% of students were victims of bullying, while between 1 and 36% were bullies [6]. Typically, bullying starts between the ages of 7 and 8 years and reaches its peak between 11 and 14 years [7], after which it decreases and remains stable [8,9,10]. Gender differences have also been observed, with boys being more likely to be involved as victims, bullies, or bullies/victims [8,11,12]. However, it has also been concluded that there are differences in bullying prevalence based on gender. For instance, girls are more likely to be defenders [3]. In addition, a recent systematic review carried out in 2021, found that the prevalence varied by gender depending on the type of bullying. They concluded that although boys were generally more likely to be victims of bullying, girls were more involved in social bullying [12].
Bullying behavior can have negative impact on children’s and adolescents´physical and psychosocial lives. Due to its consequences and high prevalence, bullying is now considered a public health problem [13,14,15,16]. Some decades ago, researchers began studying the physiology behind peer victimization from a biological perspective, expanding their efforts beyond previous behavioral psychological and social models. Studies showed that epigenetic alterations, inflammatory markers, and neuroendocrine factors were associated with bullying behavior [4,17]. Hormone levels have also been studied in relation to bullying, as some hormones may affect behavior. Additionally, certain behaviors can also alter hormone levels.
The neuroendocrine system comprises the hypothalamus and pituitary gland, which are responsible for controlling the main hormonal axes in the body. Of particular interest in relation to status-relevant behaviors like bullying are the Hypothalamic-Pituitary-Adrenal (HPA) and Hypothalamic-Pituitary-Gonadal (HPG) axes [18]. According to the dual hormone hypothesis, basal cortisol and testosterone levels, which are products of the HPA and HPG axes, respectively, affect behavioral systems implicated in dominance and aggression. Several studies have described an association between high testosterone levels and high aggression when cortisol levels are low [19]. However, it is still unclear what happens during childhood and adolescence, which are crucial developmental stages for brain and cognitive maturation.
On the one hand, the HPA axis is responsible for the body’s stress response with cortisol as the final product. The association between cortisol levels and bullying behavior can be bidirectional. First, considering bullying as one of the main stressful events during childhood and adolescence, it can be thought that it influences HPA activity. According to the dual hormone hypothesis, we would also expect an association between testosterone and cortisol, resulting in aggressive behavior. Recently, a systematic review explored the association between cortisol levels and bullying behavior. They concluded that bullying was consistently associated mainly with blunted cortisol reactivity and diurnal cortisol slope. However, although being a statistically significant association, the direction of this relationship is still unclear [20].
On the other hand, the HPG axis, which controls the reproductive system, has been widely studied in relation to aggression. The central nervous system (CNS) is affected by hormones in the body, especially at two developmental stages: the prenatal and pubertal periods [21,22,23]. During this developmental stage, sex hormones can influence various brain structures [23], organizing and activating neuroendocrine circuits that control behavior [24,25]. Although some review studies and meta-analyses have found a statistically positive significant association between prenatal and pubertal sexual hormones and aggressive behavior [26,27,28], only a few studies have analyzed the association between sex hormone levels and bullying behavior, they have found mixed results [29,30,31].
Overall, it is still unclear in which direction cortisol is associated with bullying, and few studies have explored testosterone´s role in bullying. Therefore, this study aims to examine the relationship between sex hormones and cortisol levels with bullying behavior. The study will explore not only the direct association between cortisol and bullying but also the moderating role of cortisol.
2. Materials and Methods
The method used in this systematic review is described in a protocol registered on PROSPERO (reference CRD42023494738 available from https://www.crd.york.ac.uk/prospero/ accessed on 11 February 2024). This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [32]. A completed PRISMA checklist is provided in the Supplementary Materials.
2.1. Study Question
The main objective of this systematic review was to investigate the association between Hypothalamic-Pituitary-Adrenal (HPA) and Hypothalamic-Pituitary-Gonadal (HPG) axis-dependent hormones and bullying behavior in children and adolescents based on previous evidence. The research question was developed around the study’s main objective: Is there any relationship between HPA and HPG axis-dependent hormones and bullying behavior in 6–18-year-old children and adolescents?
2.2. Search Strategy
Two reviewers (IB and AA) conducted the literature search in four electronic databases: PubMed, Psycinfo, Scopus, and Web of Science between April 2023 and July 2023.
Different terms were used related to exposure (hormone, testosterone, estradiol, cortisol, dehydroepiandrosterone, HPA axis, HPG axis, 2D:4D ratio), outcome (bullying, peer victimization, peer aggression, school violence), and population (child, adolescent). After formulating the search strategy, it was adapted for each database. Subsequently, language (English and Spanish) and source-type (journal article) restrictions were applied.
As an example, the complete research string used for PubMed was: ((“hormon*”[Title/Abstract] OR “testosterone”[Title/Abstract] OR “estradiol”[Title/Abstract] OR “cortisol”[Title/Abstract] OR “dhea”[Title/Abstract] OR “dehydroepiandrosterone”[MeSH Terms] OR “hpa”[Title/Abstract] OR “hpg”[Title/Abstract] OR “2d:4d ratio”[Title/Abstract]) AND (“bullying”[MeSH Terms] OR “bully*”[Title/Abstract] OR “peer victimization”[Title/Abstract] OR “school violence”[Title/Abstract] OR “peer aggression”[Title/Abstract]) AND (“child*”[Title/Abstract] OR “child”[MeSH Terms] OR “adolescent*”[Title/Abstract] OR “adolescent”[MeSH Terms] OR “adolescent”[MeSH Terms] NOT “adult”[MeSH Terms] OR “adult”[Title/Abstract])).
2.3. Eligibility Criteria
The eligibility criteria are described in Table 1.

Table 1.
Inclusion and exclusion criteria.
2.4. Study Selection and Data Extraction
To ensure consistency, two reviewers (IB and AA) defined the criteria and underwent training before beginning the search, screening, and data extraction process. The search yielded 152 results, out of which 88 were duplicated. Based on the eligibility criteria, the two reviewers independently screened titles, abstracts, and full texts. After the screening process, 33 articles were included in the study. Any disagreement between reviewers was solved by consensus discussion with a third expert (JI).
The data from selected articles were extracted independently by IB and AA, and then confirmed by a third researcher (NL). The following data were extracted from the articles: (1) study characteristics (author, year, design), (2) population characteristics (geographical location, sample size, age at the assessment of the exposure and outcome), (3) information about hormonal biomarkers (samples used and analysis method), (4) information about bullying assessment (instrument or scale used), (5) information about other variables of interest in the study (mediators, moderators, other variables), (6) main results of the study, and (7) information for assessing research quality.
2.5. Quality of Studies (Risk of Bias)
To assess the quality of each study, the two reviewers used the Robins E-tool, which is designed to assess observational epidemiologic studies mainly in the context of systematic reviews [33]. The tool assesses the quality of the studies on 7 domains including confounding, selection of participants in the study, classification of exposures, departures from intended exposures, missing data, measurement of outcomes, and selection of the reported result. As confounding factors, gender and age were considered, which are related to hormone levels and bullying. Based on the score, the risk of bias in the studies can be classified as low risk of bias, some concern about bias, high risk of bias, or very high risk of bias. High risk rates indicate that the study has serious methodological errors in analyzed domains.
3. Results
3.1. Literature Search and Study Selection
The search initially yielded 152 records, but after removing duplicates, 64 records remained. Upon screening the titles and abstracts, 37 papers were identified for full text review. After a detailed reading of these articles, four of them were excluded based on the inclusion and exclusion criteria. Finally, a total of 33 studies were included in this systematic review. The process is summarized in Figure 1.
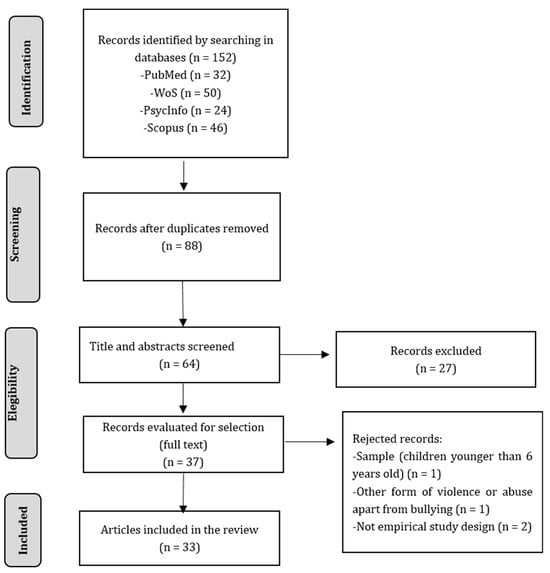
Figure 1.
Flowchart of the systematic review.
3.2. Description of Studies Included
Table 2 presents the general characteristics of the participants in the studies. The publication dates ranged from 2006 to 2023, with over a third (35%; n = 12) of the studies published during the last five years. One third of the studies were conducted in Europe (n = 11; 33%), another third was published in the USA (n = 11; 33%), eight in Canada (n = 8; 24%), two in China (n = 2; 6%), and one in Brazil (n = 1; 3%). Regarding study design, 18 were cross-sectional (n = 18; 55%) and 15 were longitudinal (n = 16; 45%). The studies used a variety of sample sizes, ranging from 31 participants [34] to 659 participants [35].

Table 2.
Main characteristics of the included studies.
3.2.1. Bullying Assessment
Bullying was assessed using various methods in the reviewed papers. A single study used peer nomination to identify the children and adolescents involved in bullying situations [53], while three relied on interviews conducted with participants’ mothers [48,49,64]. However, the majority of studies used self-reported questionnaires to identify participants´ bullying involvement. Although there is no single questionnaire used to assess bullying behavior, the most commonly used scales in the reviewed papers were “The Social Experiences Questionnaire” [38,44,58,61,62] and questionnaires based on the “Olweus Bully Victim Questionnaire” [29,31,35,54,59,65].
3.2.2. Hormones Assessment
This study aimed to analyze the association between bullying behavior and HPA and HPG axis-dependent hormone levels. All but one study explored the role of cortisol in bullying. Most studies determined cortisol levels in saliva, except for four studies that determined cortisol levels in hair [35,36,51,52]. Saliva samples (as well as blood samples) refer to a time point cortisol levels or acute stress, whereas hair samples are used to collect information about cumulative cortisol levels and are used as an indicator of chronic stress levels.
With regard to saliva samples, cortisol reactivity, the cortisol-awakening response (CAR), diurnal cortisol curves, or total cortisol levels were studied, depending on the time of collection and the number of samples. To understand this, it is essential to know that cortisol levels follow a daily cycle that maintains healthy physiological functioning. Cortisol levels sharply increase in the morning, approximately 30 min after waking (known as the cortisol-awakening response). Subsequently, cortisol levels decline throughout the day, reaching their lowest points after sleep begins. Some articles rely on multiple saliva cortisol samples to study the cortisol secreted during specific hours by means of the cortisol curve or by measuring the area under the curve. Furthermore, as long as the HPA axis functions properly, the stress response and cortisol secretion follow predictable patterns and react by activating the HPA axis to stressful situations. However, this can change under chronic stress conditions.
Once the samples were collected, different analysis techniques were used to determine cortisol levels. More than 60% (n = 18) of the articles used enzyme immunoassays as hormone analysis techniques. In addition to this technique, studies analyzed hormones using several other techniques, such as luminescence or competitive radioimmunoassays.
Only three studies [29,30,31] have analyzed the role of HPG axis-dependent hormones in bullying behavior. All three studies measured testosterone levels in saliva, and one of them measured the participants’ 2D:4D ratio as an indicator of prenatal sex hormones [29].
3.3. Risk of Bias in Individual Studies
In our assessment, two studies were rated with some concerns of risk of bias (n = 2; 6%), three studies were deemed to have a very high risk of bias (n = 3; 9%), while the remaining (n = 28; 85%) were rated as having a low risk of bias. The overall risk of bias ratings has been detailed in Table 2, and we have summarized the rating for individual studies across seven domains: confounding, selection of participants into the study, classification of exposures, departures from intended exposures, missing data, measurement of outcomes and selection of the reported result measurement exposure (Appendix A).
Measurement of the exposure was the domain with a higher risk of bias rating since the methods used to measure exposure bullying were not validated and used only a few questions to identify participants as having bullying involvement. As a result, the rest of the domains were not evaluated, which was considered a very high bias risk. Those articles with “some concerns” are due to the selection of participants, since their characteristics may have affected the results.
3.4. Association between Bullying and Hormones
The association between bullying and hormone levels can occur in several ways. Previous evidence showed that hormones could be integrated in the study of bullying following two mechanisms. On the one hand, hormones could act as predictors of bullying. The dual hormone hypothesis states that high testosterone levels influence behaviors where social dominance is involved, as could be the case with bullying, and that elevated testosterone levels predict such behaviors when cortisol levels are low [19]. On the other hand, bullying has been identified as a stressful situation [2] and therefore it is understood that, as with other stressful events, it could alter the activity of the HPA axis.
To better understand the results, a distinction was made in depending not only on the hormones analyzed but also on the role (outcome, moderator, or predictor) played in this association between bullying and hormones.
3.4.1. HPA-Dependent Hormones and Bullying
A single study focused on exploring the predictive role of cortisol and testosterone in bullying behavior [29]. Another ten studies explored the mediating role of cortisol (n = 10; 30%) [30,34,38,56,57,59,60,61,62]. The remainder (n = 21; 64%) studied the influence of bullying on cortisol levels.
Cortisol as a predictor of bullying behavior
In our previous study we analyzed the effects of testosterone and cortisol jointly with contextual factors on bullying behavior, exploring three different roles (victim, bully, bully/victim). Results showed that lower cortisol levels, together with a worse perceived school environment and less peer and social support, were associated with bullying involvement as a bully [29].
Cortisol as an outcome of bullying behavior
The association between cortisol levels and bullying behavior was examined in previous studies using different cortisol samples. When cortisol was analyzed in hair samples, mixed results were found. One study found that cumulative victimization was non-linearly related to hair cortisol levels, although peer victimization was not directly associated with HPA activity [52]. Another study showed that this association was nonlinear and sex-dependent. Boys who experienced moderate victimization had lower levels of hair cortisol, whereas boys who experienced higher victimization showed higher hair cortisol concentrations [51]. A third study concluded that, although polyvictimized (i.e., conventional crime, maltreatment, peer and sibling victimization, sexual victimization, and witnessing and indirect victimization) youth showed higher hair cortisol levels, peer and sibling victimization was not significantly related to hair cortisol levels [36]. Finally, in a previous study, we explored not only the associations between victimization by peers but also the implications participants have as bullies or bullies and victims. Results showed a trend association between being involved in bullying as a bully/victim and higher hair cortisol concentration (HCC). However, involvement as bullies or victims was not associated with higher HCC [35].
While some studies used hair samples, most of the analyzed studies used saliva samples. Regarding cortisol reactivity measures in saliva after a stressful situation, most studies concluded that victims showed lower cortisol reactivity than their counterparts [37,39,45,46,48,64]. Nevertheless, two studies found that bullied children and adolescents had elevated cortisol reactivity [41,43]. Finally, another two studies found no significant association between cortisol reactivity and peer victimization [44,63].
Other studies have explored the association between bullying behavior and total cortisol levels. Most of them found that bullying or peer rejection was associated with higher total cortisol levels [41,42,53]. Likewise, in 2017, Gonzalez-Cabrera et al. found that, in addition to cybervictims, cyberbullies/victims also had higher total cortisol levels [42]. However, another study did not observe a direct association between cortisol levels and victimization [40].
Moreover, some studies have analyzed the cortisol awakening response (CAR). According to two previous studies, bullied students had a lower cortisol awakening response [46,47], but other studies have not confirmed this association [38,42,59]. Most studies found that students who were bullied had flatter daily cortisol slopes [38,42,46,53]. However, two studies found no relationship between victimization and cortisol slopes or patterns [34,55,59].
Finally, according to some researchers, the relationship between victimization and cortisol levels may be sex-dependent. One studyfound that verbally bullied girls had lower cortisol levels than boys [54]. Additionally, some years later, another study concluded that bullied students had lower cortisol levels and flattened cortisol responses, but these associations were only statistically significant for boys [47].
Cortisol as a moderator in the association of bullying and other mental health outcomes
As mentioned above, seven of the studies reviewed examined the moderating or mediating role of cortisol levels (n = 7; 21%). The vast majority of the studies have focused on exploring the moderating role of cortisol levels in the association between bullying victimization and other psychological or mental health problems.
Three studies explored the moderating role of cortisol in the association between bullying victimization and depressive symptoms. These studies found that higher cortisol levels increase the association between peer victimization and depressive symptoms [56,58,60]. Moreover, three other studies have explored the moderating role of cortisol in the association between bullying victimization and aggression. One study showed that increased cortisol levels (AUC) buffered the link between victimization and next-day aggression only in boys [57]. Another study concluded that both, adolescents with high testosterone and high cortisol levels, and those with low testosterone and low cortisol levels responded more aggressively when victimized by peers [30]. Finally, one study observed that, at high levels of victimization, children with high cortisol levels presented not only higher levels of aggressiveness, but also higher levels of frustration. When victimization levels were low, children with lower cortisol levels also presented with higher levels of frustration.
In addition, a recent study found that blunted cortisol reactivity accounted for some of the effects of relational victimization on externalizing and internalizing problems, but this was only observed in boys [63]. Finally, one study analyzed the moderating role of cortisol in the effect of victimization on structural brain changes. They found that, in boys with a low daily cortisol output (assessed as the area under the curve [AUC]), high victimization was associated with a smaller right vlPFC (Ventrolateral Prefrontal Cortex) surface area. However, in the case of boys with a high AUC, high victimization was associated with a larger right vlPFC surface area. In addition, in boys with a steeper diurnal slope, it was concluded that high victimization was associated with a smaller right vlPFC surface area, whereas boys with a flatter diurnal slope showed that high victimization was associated with a larger right vlPFC surface area [59].
3.4.2. HPG-Dependent Hormones and Bullying
Only three studies have investigated the association between HPG-axis-dependent hormone levels and bullying. First, in 2009 Vaillancourt et al. explored whether testosterone influenced peer victimization on bullying behavior. They concluded that the association between the two variables varied according to sex. Specifically, girls who were verbally bullied had lower testosterone levels than their counterparts, whereas among boys, those who were verbally bullied showed higher testosterone levels [31]. Testosterone levels have also been analyzed as a predictor of bullying through the analysis of the 2D:4D ratio, an indicator of prenatal sex hormone exposure. In this case, however, none of the studied variables were related to bullying behavior [29]. Finally, a recent study explored the moderating effects of testosterone and cortisol on the association between bullying and aggressive behavior. Results showed that adolescents with high testosterone and cortisol levels or with low testosterone and cortisol levels responded more aggressively when victimized or provoked [30].
4. Discussion
The main objective of this systematic review was to summarize the observational evidence assessing the association between HPA and HPG hormones and bullying behavior. Based on our selection criteria, 33 studies were included in the systematic review. To facilitate the interpretation of how these hormones and bullying behavior are related, we discuss the main findings separately for HPA hormones and HPG hormones, respectively.
Concerning the association between cortisol and bullying, a single study explored this relationship with cortisol as a predictor [29]. Seven studies examined the moderating role of cortisol in the association between bullying and other mental or psychological disorders and all found it to play an important role [30,56,57,58,59,60,63]. Finally, the association between cortisol levels and bullying behavior was examined using different cortisol samples analyzed in saliva and hair samples, and mixed results were found, as was concluded in a systematic review carried out in 2019 whichexplored the association between bullying behavior and cortisol levels determined using saliva [20]. Specifically, the HPA is both hyper-and hypo-responsive to social stressors. The reason for these confounding responses may depend not only on methodological concerns but also on the type of stressor and the time of occurrence. The HPA axis exhibits hyperactivated responses when the stressor is recent. Stress, however, can be detrimental as well when the stressor occurs distantly in time or is absent. These responses are consistent with the chronic stress hypothesis, which argues that the axis is activated when the stressor is initiated and that its continued activity causes a decrease in cortisol release below the normal levels and suggests dysregulation on the HPA axis [66].
Moreover, those that analyzed the effect of bullying behavior on chronic stress, using hair samples, also found mixed results. One recent systematic review concluded that the evidence exploring the associations between indicators of social adversity and hair cortisol in children provides inconsistent and limited results. They posit that this may be due to the existence of possible factors moderating the associations between adversity and physiological stress, such as the relationship with their caregiver, regulatory physiological processes, genetic factors that could influence the perception or response of stress, or environmental factors affecting hair cortisol levels [67].
Regarding the association between HPG hormones and bullying behavior, only one previous study that explored the association between prenatal androgen levels and bullying behavior found no statistically significant result. The main reason why this study did not find a correlation between aggressive behavior and the 2D:4D index, may be the sample size [29]. Additionally, in contrast to previous studies, the participants in this paper were younger than participants in other studies, which established an association between prenatal androgen levels and aggressive behavior. Among HPG hormone levels, pubertal testosterone was studied in relation to bullying involvement, specifically in three studies. One found no association between testosterone and bullying behavior [29]. The second concluded that the association between the two variables varied according to sex. Girls who were verbally bullied had lower testosterone levels than their counterparts, whereas boys who were verbally bullied showed higher testosterone levels [31]. Finally, Calvete and Orue in their recent study showed that adolescents with high testosterone and cortisol levels or with low testosterone and cortisol levels responded more aggressively when victimized or provoked [30]. The reason why one of the studies did not find a statistically significant association between the variables may be because the participants in this study were younger than the participants in the other two. As testosterone levels increase at puberty and participants in our study were in prepubertal stages, their testosterone levels may be very low and homogeneous.
Strengths and Limitations
To the best of our knowledge, this is the first systematic review analyzing jointly the association that HPG- and HPA-dependent hormones have with bullying behavior. We conducted this review following the PRISMA statement guidelines and documented the methods in a protocol registered on PROSPERO before starting the review, strengthening the trustworthiness of the process and results. Additionally, for the search, no limits were placed on publication dates and study types. However, several limitations should be noted too. First, we limited to studies written in either English or Spanish. Furthermore, reviewers were not blinded to the study authors and affiliations during the process.
Regarding the studies, there was considerable methodological heterogeneity between studies; and the use of different instruments to measure bullying or different methods to determine hormone levels may have contributed to these mixed results. In addition, few studies have explored the association of the different roles of bullying behavior.
5. Conclusions
The studies analyzed in our review in general were of high quality, but some gaps have been identified. For future research, as noted in the method section, we recommend exploring the effect of different hormones of the HPA and HPG axes and using a broader set of biomarkers (i.e., estradiol, dehydroepiandrosterone, LH, or FSH hormones). This recommendation would respond to the fact that these axes have been shown to be linked in the previous literature [19,66].
Additionally, certain methodological aspects should also be taken into account when evaluating these hormones. The HPA and HPG axes are not static, and their activity varies at different stages of development. Specifically, during puberty, they undergo an increase in their activity, so that assessing the pubertal stage and controlling for this would be the most appropriate methodologically. Likewise, in terms of bullying assessments, it has also been found that various methods are used, such as questionnaires, interviews, or peer nominations. It would be highly recommended for future studies the use previously validated scales or questionnaires (i.e., OBVQ). Finally, future studies should investigate the relationship between cyberbullying and hormone levels, as there is scarce evidence of this association. Further research is needed to better understand how bullying affects physical and emotional development during childhood and adolescence. Biological measures may not only improve our understanding of aggressive behavior but also guide the development of prevention and management programs for it.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children11020241/s1, Table S1: PRISMA 2020 Checklist.
Author Contributions
Conceptualization, I.B., A.A (Ane Arregi)., A.A (Ainara Andiarena) and J.I.; methodology, I.B., A.A. (Ane Arregi) and N.L.; validation, A.A (Ainara Andiarena), N.L., O.V. and J.I.; investigation, I.B. and A.A.—original draft preparation, I.B.; writing—review and editing, A.A. (Ainara Anadiarena), N.L., O.V. and J.I.; supervision, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
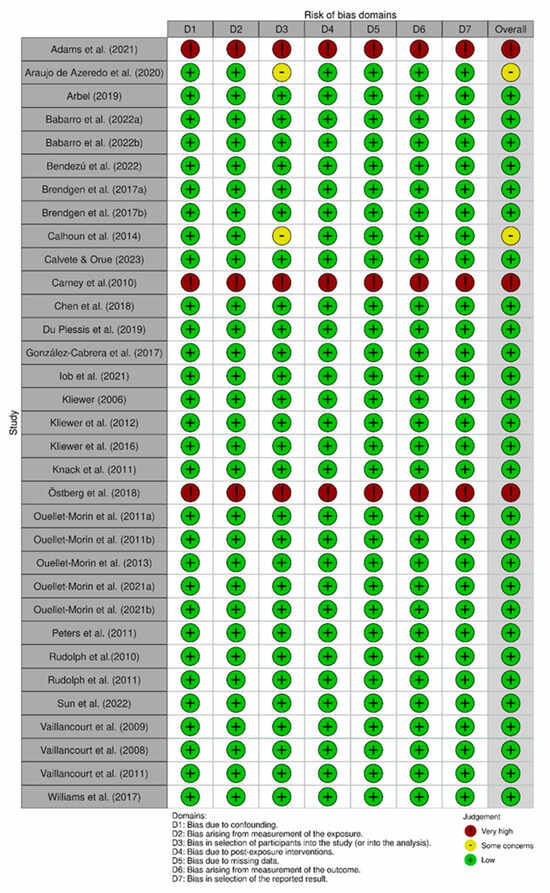
Figure A1.
Traffic-light plot summarizing the risk of bias in the selected studies. Note: Studies analyzed [29,30,31,34,35,36,37,38,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,67].
References
- UNESCO. Más Allá de Los Números: Poner Fin a La Violencia y El Acoso En El Ámbito Escolar; UNESCO: Paris, France, 2021; ISBN 978-92-3-300148-0. [Google Scholar]
- Vanaelst, B.; De Vriendt, T.; Huybrechts, I.; Rinaldi, S.; De Henauw, S. Epidemiological Approaches to Measure Childhood Stress. Paediatr. Perinat. Epidemiol. 2012, 26, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K. Bullying: Definition, Types, Causes, Consequences and Intervention. Soc. Personal. Psychol. Compass 2016, 9, 519–532. [Google Scholar] [CrossRef]
- Garaigordobil, M.; Oñederra, J.A. Revisión Teórica Del Bullying: Concepto, Teorías y Prevalencia Del Fenómeno. In La Violencia Entre Iguales. Revisión Teórica y Estrategias de Intervención; Pirámide: Madrid, Spain, 2010; pp. 29–178. [Google Scholar]
- García-García, J.; Ortega, E.; De la Fuente, L.; Zaldívar, F.; Gil-Fenoy, M.J. Systematic Review of the Prevalence of School Violence in Spain. Procedia—Soc. Behav. Sci. 2017, 237, 125–129. [Google Scholar] [CrossRef]
- Currie, C.; Zanotti, C.; Morgan, A.; Currie, D.; de Looze, M.; Roberts, C.; Samdal, O.; Smith, O.R.F.; Banekow, V. Social Determinants of Health and Well-Being among Young People. In Health Behaviour in School-Aged Children Study: International Report from the 2009/2010 Survey; WHO: Copenhagen, 2012; ISBN 978 92 890 1423 6. [Google Scholar]
- González-Cabrera, J.; Montiel, I.; Ortega-Barón, J.; Calvete, E.; Orue, I.; Machimbarrena, J.M. Epidemiology of Peer Victimization and Its Impact on Health-Related Quality of Life in Adolescents: A Longitudinal Study. Sch. Ment. Health 2021, 13, 338–346. [Google Scholar] [CrossRef]
- Álvarez-García, D.; García, T.; Núñez, J.C. Predictors of School Bullying Perpetration in Adolescence: A Systematic Review. Aggress. Violent Behav. 2015, 23, 126–136. [Google Scholar] [CrossRef]
- Díaz-Aguado, M.J.; Martínez, R.; Martín, J. El Acoso Entre Adolescentes En España. Prevalencia, Papeles Adoptados Por Todo El Grupo y Características a Las Que Atribuyen La Victimización. Rev. Educ. 2013, 362, 348–379. [Google Scholar] [CrossRef]
- Hymel, S.; Swearer, S.M. Four Decades of Research on School Bullying: An Introduction. Am. Psychol. 2015, 70, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jansen, D.; Veenstra, R.; Ormel, J.; Verhulst, F.C.; Reijneveld, S.A. Early Risk Factors for Being a Bully, Victim, or Bully/Victim in Late Elementary and Early Secondary Education. The Longitudinal TRAILS Study. BMC Public Health 2011, 11, 440. [Google Scholar] [CrossRef]
- Rodrigues Mandira, M.; Stoltz, T. Bullying Risk and Protective Factors among Elementary School Students over Time: A Systematic Review. Int. J. Educ. Res. 2021, 109, 101838. [Google Scholar] [CrossRef]
- Moore, S.E.; Norman, R.E.; Suetani, S.; Thomas, H.J.; Sly, P.D.; Scott, J.G. Consequences of Bullying Victimization in Childhood and Adolescence: A Systematic Review and Meta-Analysis. World J. Psychiatry 2017, 7, 60–76. [Google Scholar] [CrossRef]
- Wolke, D.; Lereya, S.T.; Fisher, H.L.; Lewis, G.; Zammit, S. Bullying in Elementary School and Psychotic Experiences at 18 Years: A Longitudinal, Population-Based Cohort Study. Psychol. Med. 2014, 44, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, R.; Maughan, B.; Arseneault, L. Adult Health Outcomes of Childhood Bullying Victimization: Evidence from a Five-Decade Longitudinal British Birth Cohort. Am. J. Psychiatry 2014, 171, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.; Harel-Fisch, Y.; Fogel-Grinvald, H.; Dostaler, S.; Hetland, J.; Simons-Morton, B.; Molcho, M.; de Matos, M.G.; Overpeck, M.; Due, P.; et al. A Cross-National Profile of Bullying and Victimization among Adolescents in 40 Countries. Int. J. Public Health 2009, 54, 216–224. [Google Scholar] [CrossRef]
- Vaillancourt, T. The Neurobiology of Bullying Victimization. In The Cambridge Handbook of Violent Behavior and Aggression; Cambridge University Press: Cambridge, UK, 2018; pp. 175–186. ISBN 9781316847992. [Google Scholar]
- Gore, A.C. Neuroendocrine Systems. In Fundamental Neuroscience; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123858719. [Google Scholar]
- Mehta, P.H.; Josephs, R.A. Testosterone and Cortisol Jointly Regulate Dominance: Evidence for a Dual-Hormone Hypothesis. Horm. Behav. 2010, 58, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, W.; Sosnowski, D.W.; Noh, H.; McGuire, K.; Wright, A.W. Peer Victimization and Cortisol Production in Children and Adolescents: A Systematic Review. J. Appl. Biobehav. Res. 2019, 24, e12172. [Google Scholar] [CrossRef]
- Schulz, K.M.; Molenda-Figueira, H.A.; Sisk, C.L. Back to the Future: The Organizational-Activational Hypothesis Adapted to Puberty and Adolescence. Horm. Behav. 2009, 55, 597–604. [Google Scholar] [CrossRef]
- Sisk, C.L.; Foster, D.L. The Neural Basis of Puberty and Adolescence. Nat. Neurosci. 2004, 7, 1040–1047. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal Hormones Organize the Adolescent Brain and Behavior. Front. Neuroendocrinol. 2005, 26, 163–174. [Google Scholar] [CrossRef]
- Adolphs, R. Neural Systems for Recognizing Emotion. Curr. Opin. Neurobiol. 2002, 12, 169–177. [Google Scholar] [CrossRef]
- Berenbaum, S.A.; Beltz, A.M. Sexual Differentiation of Human Behavior: Effects of Prenatal and Pubertal Organizational Hormones. Front. Neuroendocrinol. 2011, 32, 183–200. [Google Scholar] [CrossRef]
- Hönekopp, J.; Watson, S. Meta-Analysis of the Relationship between Digit-Ratio 2D:4D and Aggression. Pers. Individ. Dif. 2011, 51, 381–386. [Google Scholar] [CrossRef]
- Archer, J.; Graham-Kevan, N.; Davies, M. Testosterone and Aggression: A Reanalysis of Book, Starzyk, and Quinsey’s (2001) Study. Aggress. Violent Behav 2005, 10, 241–261. [Google Scholar] [CrossRef]
- Grotzinger, A.D.; Mann, F.D.; Patterson, M.W.; Tackett, J.L.; Tucker-Drob, E.M.; Harden, K.P. Hair and Salivary Testosterone, Hair Cortisol, and Externalizing Behaviors in Adolescents. Psychol. Sci. 2018, 29, 688–699. [Google Scholar] [CrossRef]
- Babarro, I.; Andiarena, A.; Fano, E.; García-Baquero, G.; Lebeña, A.; Arranz-Freijo, E.B.; Ibarluzea, J. Do Prepubertal Hormones, 2D:4D Index and Psychosocial Context Jointly Explain 11-Year-Old Preadolescents’ Involvement in Bullying? Biol. Psychol. 2022, 172, 108379. [Google Scholar] [CrossRef]
- Calvete, E.; Orue, I. Do Testosterone and Cortisol Levels Moderate Aggressive Responses to Peer Victimization in Adolescents? Dev. Psychopathol. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, T.; DeCatanzaro, D.; Duku, E.; Muir, C. Androgen Dynamics in the Context of Children’s Peer Relations: An Examination of the Links between Testosterone and Peer Victimization. Aggress. Behav. 2009, 35, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 1–9. [Google Scholar] [CrossRef]
- Bero, L.; Chartres, N.; Diong, J.; Fabbri, A.; Ghersi, D.; Lam, J.; Lau, A.; McDonald, S.; Mintzes, B.; Sutton, P.; et al. The Risk of Bias in Observational Studies of Exposures (ROBINS-E) Tool: Concerns Arising from Application to Observational Studies of Exposures. Syst. Rev. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.G.; Turner-Henson, A.; Davis, S.; Soistmann, H.C. Relationships Among Perceived Stress, Bullying, Cortisol, and Depressive Symptoms in Ninth-Grade Adolescents: A Pilot Study. Biol. Res. Nurs. 2017, 19, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Babarro, I.; Ibarluzea, J.; Theodorsson, E.; Fano, E.; Guxens, M.; Sunyer, J.; Andiarena, A. Hair Cortisol as a Biomarker of Chronic Stress in Preadolescents: Influence of School Context and Bullying. Child Neuropsychol. 2023, 29, 742–759. [Google Scholar] [CrossRef] [PubMed]
- de Azeredo, L.A.; Viola, T.W.; Rothmann, L.M.; Trentin, R.; Arteche, A.X.; Kristensen, C.H.; Buchweitz, A.; Grassi-Oliveira, R. Hair Cortisol Levels and Mental Health Problems in Children and Adolescents Exposed to Victimization. Stress 2020, 23, 546–555. [Google Scholar] [CrossRef]
- Bendezú, J.J.; Calhoun, C.D.; Vinograd, M.; Patterson, M.W.; Rudolph, K.D.; Giletta, M.; Hastings, P.; Nock, M.K.; Slavich, G.M.; Prinstein, M.J. Exploring Joint HPA–Inflammatory Stress Response Profiles in Adolescent Girls: Implications for Developmental Models of Neuroendocrine Dysregulation. Dev. Psychobiol. 2022, 64, e22247. [Google Scholar] [CrossRef] [PubMed]
- Brendgen, M.; Ouellet-Morin, I.; Lupien, S.J.; Vitaro, F.; Dionne, G.; Boivin, M. Environmental Influence of Problematic Social Relationships on Adolescents’ Daily Cortisol Secretion: A Monozygotic Twin-Difference Study. Psychol. Med. 2017, 47, 460–470. [Google Scholar] [CrossRef]
- Calhoun, C.D.; Helms, S.W.; Heilbron, N.; Rudolph, K.D.; Hastings, P.D.; Prinstein, M.J. Relational Victimization, Friendship, and Adolescents’ Hypothalamic- Pituitary-Adrenal Axis Responses to an in Vivo Social Stressor. Dev. Psychopathol. 2014, 26, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.L.V.; Hazler, R.J.; Oh, I.; Hibel, L.C.; Granger, D.A. The Relations between Bullying Exposures in Middle Childhood, Anxiety, and Adrenocortical Activity. J. Sch. Violence 2010, 9, 194–211. [Google Scholar] [CrossRef]
- Chen, G.; Kong, Y.; Deater-Deckard, K.; Zhang, W. Bullying Victimization Heightens Cortisol Response to Psychosocial Stress in Chinese Children. J. Abnorm. Child Psychol. 2018, 46, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- González-Cabrera, J.; Calvete, E.; León-Mejía, A.; Pérez-Sancho, C.; Peinado, J.M. Relationship between Cyberbullying Roles, Cortisol Secretion and Psychological Stress. Comput. Human Behav. 2017, 70, 153–160. [Google Scholar] [CrossRef]
- Kliewer, W. Violence Exposure and Cortisol Responses in Urban Youth. Int. J. Behav. Med. 2006, 13, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, W.; Dibble, A.E.; Goodman, K.L.; Sullivan, T.N. Physiological Correlates of Peer Victimization and Aggression in African American Urban Adolescents. Dev. Psychopathol. 2012, 24, 637–650. [Google Scholar] [CrossRef]
- Kliewer, W. Victimization and Biological Stress Responses in Urban Adolescents: Emotion Regulation as a Moderator. J. Youth Adolesc. 2016, 45, 1812–1823. [Google Scholar] [CrossRef]
- Knack, J.M.; Jensen-Campbell, L.A.; Baum, A. Worse than Sticks and Stones? Bullying Is Associated with Altered HPA Axis Functioning and Poorer Health. Brain Cogn. 2011, 77, 183–190. [Google Scholar] [CrossRef]
- Östberg, V.; Låftman, S.B.; Modin, B.; Lindfors, P. Bullying as a Stressor in Mid-Adolescent Girls and Boys-Associations with Perceived Stress, Recurrent Pain, and Salivary Cortisol. Int. J. Environ. Res. Public Health 2018, 15, 364. [Google Scholar] [CrossRef]
- Ouellet-Morin, I.; Danese, A.; Bowes, L.; Shakoor, S.; Ambler, A.; Pariante, C.M.; Papadopoulos, A.S.; Caspi, A.; Moffitt, T.E.; Arseneault, L. A Discordant Monozygotic Twin Design Shows Blunted Cortisol Reactivity among Bullied Children. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 574–582.e3. [Google Scholar] [CrossRef]
- Ouellet-Morin, I.; Odgers, C.L.; Danese, A.; Bowes, L.; Shakoor, S.; Papadopoulos, A.S.; Caspi, A.; Moffitt, T.E.; Arseneault, L. Blunted Cortisol Responses to Stress Signal and Social and Behavioral Problems Among Maltreated/Bullied 12-Year-Old Children. Biol. Psychiatry 2011, 70, 1–18. [Google Scholar] [CrossRef]
- Ouellet-Morin, I.; Wong, C.C.Y.; Danese, A.; Pariente, C.M.; Papadopoulos, A.S.; Mill, J.; Arseneault, L. Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: A longitudinal study of discordant monozygotic twins. Psychol Med. 2013, 43, 1813–1823. [Google Scholar] [CrossRef]
- Ouellet-Morin, I.; Cantave, C.; Geoffroy, M.C.; Brendgen, M.; Vitaro, F.; Tremblay, R.; Boivin, M.; Lupien, S.; Sylvana, C. Associations between Developmental Trajectories of Peer Victimization, Hair Cortisol, and Depressive Symptoms: A Longitudinal Study. J. Child Psychol. Psychiatry 2021, 62, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ouellet-Morin, I.; Cantave, C.; Lupien, S.; Geoffroy, M.C.; Brendgen, M.; Vitaro, F.; Tremblay, R.; Boivin, M.; Côté, S. Cumulative Exposure to Socioeconomic and Psychosocial Adversity and Hair Cortisol Concentration: A Longitudinal Study from 5 Months to 17 Years of Age. Psychoneuroendocrinology 2021, 126, 105153. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.; Riksen-Walraven, J.M.; Cillessen, A.H.N.; de Weerth, C. Peer Rejection and HPA Activity in Middle Childhood: Friendship Makes a Difference. Child Dev. 2011, 82, 1906–1920. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, T.; Duku, E.; Decatanzaro, D.; Macmillan, H.; Muir, C.; Schmidt, L.A. Variation in Hypothalamic-Pituitary-Adrenal Axis Activity among Bullied and Non-Bullied Children. Aggress. Behav. 2008, 34, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, T.; Duku, E.; Becker, S.; Schmidt, L.A.; Nicol, J.; Muir, C.; MacMillan, H. Peer Victimization, Depressive Symptoms, and High Salivary Cortisol Predict Poorer Memory in Children. Brain Cogn. 2011, 77, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.E.; Santo, J.B.; Bukowski, W.M. Indirect Effects of HPA Axis Dysregulation in the Association between Peer Victimization and Depressed Affect during Early Adolescence. Psychoneuroendocrinology 2021, 132, 105356. [Google Scholar] [CrossRef]
- Arbel, R.; Schacter, H.L.; Han, S.C.; Timmons, A.C.; Spies Shapiro, L.; Margolin, G. Day-to-Day Friends’ Victimization, Aggression Perpetration, and Morning Cortisol Activity in Late Adolescents. Dev. Psychobiol. 2019, 61, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Brendgen, M.; Ouellet-Morin, I.; Lupien, S.; Vitaro, F.; Dionne, G.; Boivin, M. Does Cortisol Moderate the Environmental Association between Peer Victimization and Depression Symptoms? A Genetically Informed Twin Study. Psychoneuroendocrinology 2017, 84, 42–50. [Google Scholar] [CrossRef]
- Du Plessis, M.R.; Smeekens, S.; Cillessen, A.H.N.; Whittle, S.; Güroglu, B. Bullying the Brain? Longitudinal Links between Childhood Peer Victimization, Cortisol, and Adolescent Brain Structure. Front. Psychol. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Iob, E.; Baldwin, J.R.; Plomin, R.; Steptoe, A. Adverse Childhood Experiences, Daytime Salivary Cortisol, and Depressive Symptoms in Early Adulthood: A Longitudinal Genetically Informed Twin Study. Transl. Psychiatry 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.D.; Troop-Gordon, W.; Granger, D.A. Peer Victimization and Aggression: Moderation by Individual Differences in Salivary Cortiol and Alpha-Amylase. J. Abnorm. Child Psychol. 2010, 38, 843–856. [Google Scholar] [CrossRef]
- Rudolph, K.D.; Troop-Gordon, W.; Granger, D.A. Individual Differences in Biological Stress Responses Moderate the Contribution of Early Peer Victimization to Subsequent Depressive Symptoms. Psychopharmacology 2011, 214, 209–219. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Y.; Wang, X.; Zilioli, S.; Chi, P.; Chen, L.; Xiao, J.; Lin, D. Cortisol Reactivity as a Mediator of Peer Victimization on Child Internalizing and Externalizing Problems: The Role of Gender Differences. Res. Child Adolesc. Psychopathol. 2022, 50, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Bowes, L.; Maughan, B.; Ball, H.; Shakoor, S.; Ouellet-Morin, I.; Caspi, A.; Moffitt, T.E.; Arseneault, L. Chronic Bullying Victimization across School Transitions: The Role of Genetic and Environmental Influences. Dev. Psychopathol. 2013, 25, 333–346. [Google Scholar] [CrossRef]
- Chen, F.R.; Raine, A.; Granger, D.A. Testosterone and Proactive-Reactive Aggression in Youth: The Moderating Role of Harsh Discipline. J. Abnorm. Child Psychol. 2018, 46, 1599–1612. [Google Scholar] [CrossRef]
- Liu, C.H.; Doan, S.N. Innovations in Biological Assessments of Chronic Stress through Hair and Nail Cortisol: Conceptual, Developmental, and Methodological Issues. Dev. Psychobiol. 2019, 61, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Bryson, H.E.; Price, A.M.; Goldfeld, S.; Mensah, F. Associations between Social Adversity and Young Children’s Hair Cortisol: A Systematic Review. Psychoneuroendocrinology 2021, 127, 105176. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).