Potential Predictors of Long COVID in Italian Children: A Cross-Sectional Survey
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
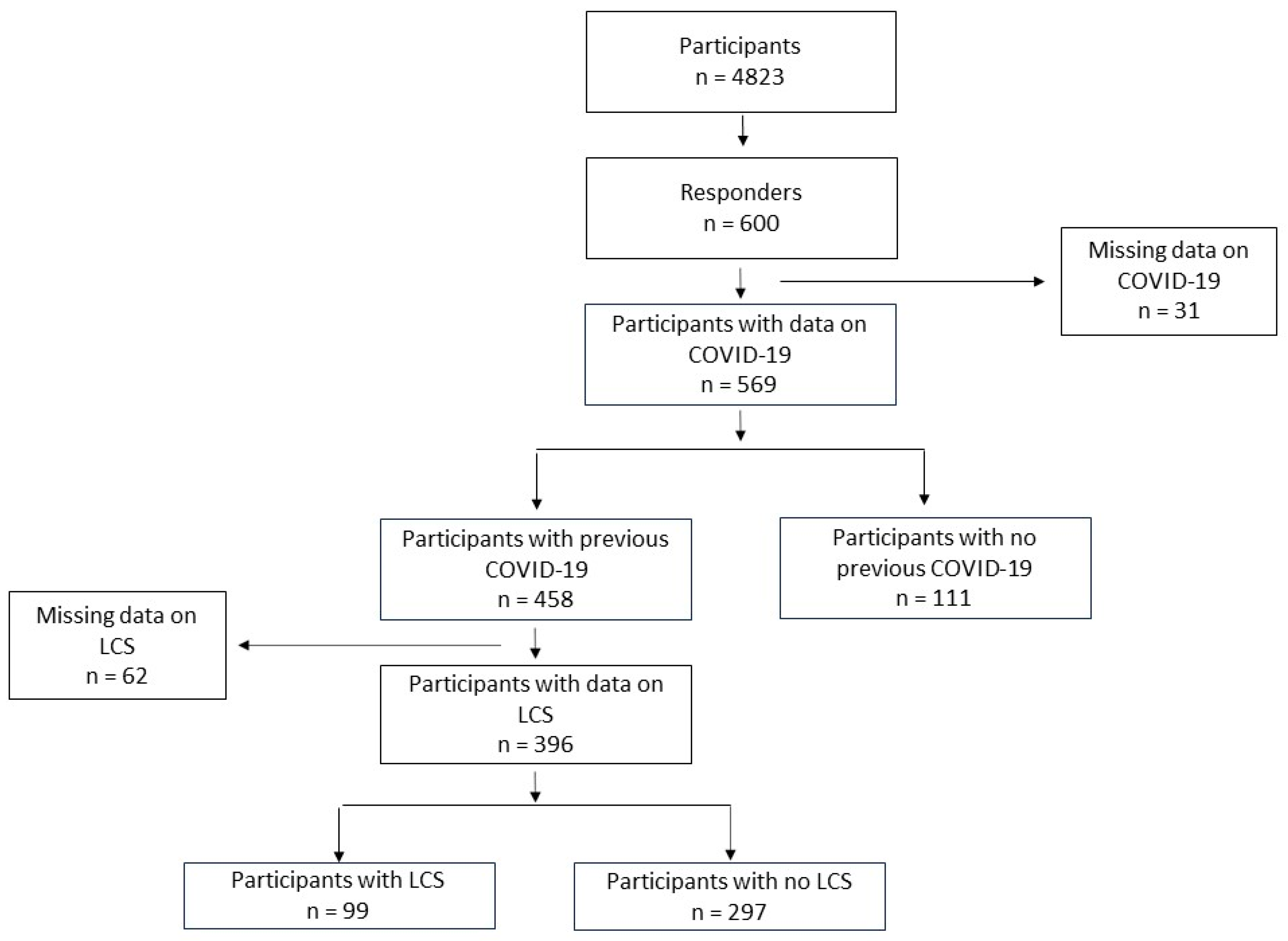
3. Results
3.1. General Characteristics of the Study Population
3.2. Associations of Demographic Data, Comorbidities, and COVID-19 Symptoms with LCS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-De-Las-Peñas, C. Long COVID: Current definition. Infection 2021, 50, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence (NICE): London, UK, 2020.
- Stephenson, T.; Allin, B.; Nugawela, M.D.; Rojas, N.; Dalrymple, E.; Pinto Pereira, S.; Soni, M.; Knight, M.; Cheung, E.Y.; Heyman, I.; et al. Long COVID (post-COVID-19 condition) in children: A modified Delphi process. Arch. Dis. Child. 2022, 107, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. “Long-COVID”. Available online: https://www.iss.it/long-covid-cosa-sappiamo (accessed on 2 September 2022).
- Sansone, F.; Pellegrino, G.M.; Caronni, A.; Bonazza, F.; Vegni, E.; Lué, A.; Bocci, T.; Pipolo, C.; Giusti, G.; Di Filippo, P.; et al. Long COVID in Children: A Multidisciplinary Review. Diagnostics 2023, 13, 1990. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Foer, D.; MacPhaul, E.; Lo, Y.-C.; Bates, D.W.; Zhou, L. PASCLex: A comprehensive post-acute sequelae of COVID-19 (PASC) symptom lexicon derived from electronic health record clinical notes. J. Biomed. Inform. 2021, 125, 103951. [Google Scholar] [CrossRef] [PubMed]
- Soprano, C.M.; Ngo, R.; Konys, C.A.; Bazier, A.; Salamon, K.S. Post-Acute Sequelae of COVID-19 (PASC) in Pediatrics: Factors That Impact Symptom Severity and Referral to Treatment. Children 2023, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef]
- Kikkenborg Berg, S.; Dam Nielsen, S.; Nygaard, U.; Bundgaard, H.; Palm, P.; Rotvig, C.; Vinggaard Christensen, A. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 240–248. [Google Scholar] [CrossRef]
- Di Filippo, P.; Attanasi, M.; Dodi, G.; Porreca, A.; Raso, M.; Di Pillo, S.; Chiarelli, F. Evaluation of sleep quality and anxiety in Italian pediatric healthcare workers during the first wave of COVID-19 pandemic. BMC Res. Notes 2021, 14, 219. [Google Scholar] [CrossRef]
- Wang, C.; Ramasamy, A.; Verduzco-Gutierrez, M.; Brode, W.M.; Melamed, E. Acute and post-acute sequelae of SARS-CoV-2 infection: A review of risk factors and social determinants. Virol. J. 2023, 20, 124. [Google Scholar] [CrossRef]
- De Lima, J.B.; Salazar, L.; Fernandes, A.; Teixeira, C.; Marques, L.; Afonso, C. Long COVID in Children and Adolescents: A Retrospective Study in a Pediatric Cohort. Pediatr. Infect. Dis. J. 2023, 42, e109–e111. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Center for Health Statistics. Long COVID—Household Pulse Survey. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed on 20 December 2022).
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, K.M.; Vasarmidi, E.; Russell, A.-M.; Andrejak, C.; Crestani, B.; Delcroix, M.; Dinh-Xuan, A.T.; Poletti, V.; Sverzellati, N.; Vitacca, M.; et al. European Respiratory Society statement on long COVID follow-up. Eur. Respir. J. 2022, 60, 2102174. [Google Scholar] [CrossRef] [PubMed]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2021, 59, 2101341. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in children and adolescents. World J. Pediatr. 2021, 17, 495–499. [Google Scholar] [CrossRef] [PubMed]
- ISARIC. 2021. Available online: https://isaric.org/document/covid-19-long-term-follow-up-study/ (accessed on 6 February 2022).
- Funk, A.L.; Kuppermann, N.; Florin, T.A.; Tancredi, D.J.; Xie, J.; Kim, K.; Finkelstein, Y.; Neuman, M.I.; Salvadori, M.I.; Yock-Corrales, A.; et al. Post–COVID-19 Conditions among Children 90 Days After SARS-CoV-2 Infection. JAMA Netw. Open 2022, 5, e2223253. [Google Scholar] [CrossRef] [PubMed]
- Wille, N.; Badia, X.; Bonsel, G.; Burström, K.; Cavrini, G.; Devlin, N.; Egmar, A.-C.; Greiner, W.; Gusi, N.; Herdman, M.; et al. Development of the EQ-5D-Y: A child-friendly version of the EQ-5D. Qual. Life Res. 2010, 19, 875–886. [Google Scholar] [CrossRef]
- Quaranta, V.N.; Portacci, A.; Dragonieri, S.; Locorotondo, C.; Buonamico, E.; Diaferia, F.; Iorillo, I.; Quaranta, S.; Carpagnano, G.E. The Predictors of Long COVID in Southeastern Italy. J. Clin. Med. 2023, 12, 6303. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. RETRACTED: 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Zheng, Y.-B.; Zeng, N.; Yuan, K.; Tian, S.-S.; Yang, Y.-B.; Gao, N.; Chen, X.; Zhang, A.-Y.; Kondratiuk, A.L.; Shi, P.-P.; et al. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. J. Infect. Public Health 2023, 16, 660–672. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2021, 39, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Munblit, D.; Pazukhina, E.; Ricchiuto, A.; Sinatti, D.; Zona, M.; De Matteis, A.; D’ilario, F.; Gentili, C.; Lanni, R.; et al. Post-COVID Condition in Adults and Children Living in the Same Household in Italy: A Prospective Cohort Study Using the ISARIC Global Follow-Up Protocol. Front. Pediatr. 2022, 10, 834875. [Google Scholar] [CrossRef] [PubMed]
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. Eur. J. Pediatr. 2022, 181, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Morello, R.; Mariani, F.; Mastrantoni, L.; De Rose, C.; Zampino, G.; Munblit, D.; Sigfrid, L.; Valentini, P.; Buonsenso, D. Risk factors for post-COVID-19 condition (Long COVID) in children: A prospective cohort study. EClinicalMedicine 2023, 59, 101961. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2021, 28, 611.e9–611.e16. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef]
- Ashkenazi-Hoffnung, L.; Shmueli, E.; Ehrlich, S.; Ziv, A.; Bar-On, O.; Birk, E.; Lowenthal, A.; Prais, D. Long COVID in Children. Pediatr. Infect. Dis. J. 2021, 40, e509–e511. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Klein, N.P.; Stockwell, M.S.; Demarco, M.; Gaglani, M.; Kharbanda, A.B.; Irving, S.A.; Rao, S.; Grannis, S.J.; Dascomb, K.; Murthy, K.; et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA Vaccination in Preventing COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Nonimmunocompromised Children and Adolescents Aged 5–17 Years—VISION Network, 10 States, April 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, P.; David, D.; Attanasi, M.; Rossi, N.; Chiarelli, F. Case report: Increased troponin level in 125 children during COVID-19. Front. Pediatr. 2023, 11, 1059685. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis. Front. Immunol. 2022, 13, 941009. [Google Scholar] [CrossRef]
- Ranieri, J.; Guerra, F.; Cilli, E.; Martelli, A.; Capuani, A.; Di Giacomo, D. Psychological Distress and Negative Emotions in Post-COVID Infection: A Comparative Study of the COVID and NO-COVID Young Patients. Psychol. Rep. 2023. [Google Scholar] [CrossRef]
- Akgül, S.; Akdemir, D.; Nalbant, K.; Derman, O.; Ersöz Alan, B.; Tüzün, Z.; Kanbur, N. The effects of the COVID-19 lockdown on adolescents with an eating disorder and identifying factors predicting disordered eating behavior. Early Interv. Psychiatry 2021, 16, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Pusica, Y.; Sohaei, D.; Prassas, I.; Diamandis, E.P. An overview of mental health during the COVID-19 pandemic. Diagnosis 2021, 8, 403–412. [Google Scholar] [CrossRef] [PubMed]
- DesRoches, C.M.; Campbell, E.G.; Rao, S.R.; Donelan, K.; Ferris, T.G.; Jha, A.; Kaushal, R.; Levy, D.E.; Rosenbaum, S.; Shields, A.E.; et al. Electronic Health Records in Ambulatory Care—A National Survey of Physicians. N. Engl. J. Med. 2008, 359, 50–60. [Google Scholar] [CrossRef]
- Heiervang, E.; Goodman, R. Advantages and limitations of web-based surveys: Evidence from a child mental health survey. Chest 2009, 46, 69–76. [Google Scholar] [CrossRef]
| All Participants | Non LCS Participants | LCS Participants | OR (CI 95%) | p Value | |
|---|---|---|---|---|---|
| n = 396 | n = 297 | n = 99 | |||
| Gender (%) | 0.909 | ||||
| Male | 194 (49.0) | 146 (49.2) | 48 (48.5) | Ref. | |
| Female | 202 (51.0) | 151 (50.8) | 51 (51.5) | 1.03 (0.65, 1.62) | |
| Age (%) | 0.294 | ||||
| 6–12 years | 348 (87.9) | 264 (88.9) | 84 (84.8) | Ref. | |
| >12 years | 48 (12.1) | 33 (11.1) | 15 (15.2) | 1.43 (0.72, 2.74) | |
| Ethnicity (%) | 0.383 | ||||
| Non-Caucasian | 35 (11.8) | 28 (12.8) | 7 (9.0) | Ref. | |
| Caucasian | 262 (88.2) | 191 (87.2) | 71 (91.0) | 1.46 (0.64, 3.82) | |
| COVID-19 vaccine | 0.130 | ||||
| No | 109 (44.1) | 72 (48.0) | 37 (38.1) | Ref. | |
| Yes | 138 (55.9) | 78 (52.0) | 60 (61.9) | 1.49 (0.89, 2.53) | |
| Doses of COVID-19 vaccine | 1.98 (0.5) | 1.97 (0.5) | 1.98 (0.4) | 1.04 (0.50, 2.18) | 0.914 |
| 1 | |||||
| 2 | |||||
| Concurrent comorbidities | |||||
| Neurological diseases | 0.312 | ||||
| No | 391 (99.0) | 294 (99.3) | 97 (98.0) | Ref. | |
| Yes | 4 (1.0) | 2 (0.7) | 2 (2.0) | 3.02 (0.31, 29.4) | |
| Gastrointestinal diseases | <0.001 | ||||
| No | 381 (96.5) | 292 (98.6) | 89 (89.9) | Ref. | |
| Yes | 14 (3.5) | 4 (1.4) | 10 (10.1) | 7.97 (2.55, 30.6] | |
| Heart diseases | 0.172 | ||||
| No | 392 (99.2) | 295 (99.7) | 97 (98.0) | Ref. | |
| Yes | 3 (0.8) | 1 (0.3) | 2 (2.0) | 5.69 (0.46, 180.0) | |
| Respiratory diseases | 0.014 | ||||
| No | 388 (98.2) | 294 (99.3) | 94 (94.9) | Ref. | |
| Yes | 7 (1.8) | 2 (0.7) | 5 (5.1) | 7.45 (1.51, 58.70) | |
| Asthma | 0.212 | ||||
| No | 386 (97.7) | 291 (98.3) | 95 (96.0) | Ref. | |
| Yes | 9 (2.3) | 5 (1.7) | 4 (4.0) | 2.46 (0.57, 9.84) | |
| Allergic Rhinitis | |||||
| No | 353 (89.4) | 279 (94.3) | 74 (74.7) | Ref. | <0.001 |
| Yes | 42 (10.6) | 17 (5.7) | 25 (25.3) | 5.50 (2.83, 10.90) | |
| Atopic Dermatitis | 0.001 | ||||
| No | 372 (94.2) | 286 (96.6) | 86 (86.9) | Ref. | |
| Yes | 23 (5.8) | 10 (3.4) | 13 (13.1) | 4.29 (1.81, 10.50) | |
| Food Allergy | 0.008 | ||||
| No | 379 (95.9) | 289 (97.6) | 90 (90.9) | Ref. | |
| Yes | 16 (4.05) | 7 (2.4) | 9 (9.1) | 4.09 (1.40, 12.00) | |
| Rheumatologic diseases | 0.043 | ||||
| No | 392 (99.2) | 296 (100) | 96 (97.0) | Ref. | |
| Yes | 3 (0.8) | 0 (0.0) | 3 (3.0) | 21.51 (1.10, 420.12) | |
| Hematological diseases | 0.995 | ||||
| No | 394 (99.7) | 295 (99.7) | 99 (100) | Ref. | |
| Yes | 1 (0.3) | 1 (0.3) | 0 (0.0) | 0.99 (0.04, 24.49) | |
| Immunological diseases | 0.080 | ||||
| No | 393 (99.5) | 296 (100) | 97 (98.0) | Ref. | |
| Yes | 2 (0.5) | 0 (0.0) | 2 (2.0) | 15.21 (0.73, 319.50) | |
| Diabetes | 0.178 | ||||
| No | 394 (99.7) | 296 (100) | 98 (99.0) | Ref. | |
| Yes | 1 (0.3) | 0 (0.0) | 1 (1.0) | 9.03 (0.37, 223.49) | |
| Endocrinological diseases | 0.178 | ||||
| No | 394 (99.7) | 296 (100) | 98 (99.0) | Ref. | |
| Yes | 1 (0.3) | 0 (0.0) | 1 (1.0) | 9.03 (0.37, 223.49) | |
| Obesity | 0.313 | ||||
| No | 388 (98.2) | 292 (98.6) | 96 (97.0) | Ref. | |
| Yes | 7 (1.8) | 4 (1.4) | 3 (3.0) | 2.30 (0.42, 11.20) | |
| Anxiety | 0.054 | ||||
| No | 391 (99.0) | 295 (99.7) | 96 (97.0) | Ref. | |
| Yes | 4 (1.0) | 1 (0.3) | 3 (3.0) | 8.41 (0.96, 243.00) |
| All Participants | Non LCS Participants | LCS Participants | OR (CI 95%) | p Value | |
|---|---|---|---|---|---|
| n = 396 | n = 297 | n = 99 | |||
| Fever (%) | 0.050 | ||||
| No | 198 (50.0) | 157 (52.9) | 41 (41.4) | Ref. | |
| Yes | 198 (50.0) | 140 (47.1) | 58 (58.6) | 1.58 (1.00, 2.52) | |
| Headache (%) | 0.223 | ||||
| No | 221 (55.8) | 171 (57.6) | 50 (50.5) | Ref. | |
| Yes | 175 (44.2) | 126 (42.4) | 49 (49.5) | 1.33 (0.84, 2.10) | |
| Fatigue (%) | <0.001 | ||||
| No | 328 (82.8) | 258 (86.9) | 70 (70.7) | Ref. | |
| Yes | 68 (17.2) | 39 (13.1) | 29 (29.3) | 2.73 (1.57, 4.74) | |
| Muscle pain (%) | 0.009 | ||||
| No | 307 (77.5) | 240 (80.8) | 67 (67.7) | Ref. | |
| Yes | 89 (22.5) | 57 (19.2) | 32 (32.3) | 2.01 (1.20, 3.34) | |
| Runny nose | |||||
| No | 273 (68.9) | 212 (71.4) | 61 (61.6) | Ref. | 0.074 |
| Yes | 123 (31.1) | 85 (28.6) | 38 (38.4) | 1.55 (0.96, 2.50) | |
| Sore throat | 0.110 | ||||
| No | 270 (68.2) | 209 (70.4%) | 61 (61.6%) | Ref. | |
| Yes | 126 (31.8) | 88 (29.6) | 38 (38.4) | 1.48 (0.91, 2.38) | |
| Cough | 0.108 | ||||
| No | 289 (73.0) | 223 (75.1) | 66 (66.7) | Ref. | |
| Yes | 107 (27.0) | 74 (24.9) | 33 (33.3) | 1.51 (0.91, 2.46) | |
| Dyspnea | 0.868 | ||||
| No | 391 (98.7) | 293 (98.7) | 98 (99.0) | Ref. | |
| Yes | 5 (1.3) | 4 (1.4) | 1 (1.0) | 0.82 (0.03, 6.03) | |
| Pain during the respiration | 0.997 | ||||
| No | 395 (99.7) | 296 (99.7) | 99 (100) | Ref. | |
| Yes | 1 (0.3) | 1 (0.3) | 0 (0.0) | 0.99 (0.04, 25.58) | |
| Chest pain | 0.799 | ||||
| No | 389 (98.2) | 292 (98.3) | 97 (98.0) | Ref. | |
| Yes | 7 (1.77) | 5 (1.68) | 2 (2.02) | 1.26 (0.16, 6.21) | |
| Abdominal pain | 0.045 | ||||
| No | 382 (96.5) | 290 (97.6) | 92 (92.9) | Ref. | |
| Yes | 14 (3.6) | 7 (2.4) | 7 (7.1) | 3.14 (1.03, 9.59) | |
| Vomiting | 0.235 | ||||
| No | 367 (92.7) | 278 (93.6) | 89 (89.9) | Ref. | |
| Yes | 29 (7.3) | 19 (6.4) | 10 (10.1) | 1.65 (0.71, 3.64) | |
| Diarrhea | 0.235 | ||||
| No | 367 (92.7) | 278 (93.6) | 89 (89.9) | Ref. | |
| Yes | 29 (7.3) | 19 (6.4) | 10 (10.1) | 1.65 (0.71, 3.64) | |
| Loss of taste | 0.818 | ||||
| No | 378 (95.5) | 283 (95.3) | 95 (96.0) | Ref. | |
| Yes | 18 (4.6) | 14 (4.7) | 4 (4.0) | 0.87 (0.24, 2.54) | |
| Hearing loss | 0.250 | ||||
| No | 395 (99.7) | 297 (100) | 98 (99.0) | Ref. | |
| Yes | 1 (0.3) | 0 (0.0) | 1 (1.0) | 9.06 (0.37, 224.24) | |
| Confusion | 0.311 | ||||
| No | 389 (98.2) | 293 (98.7) | 96 (97.0) | Ref. | |
| Yes | 7 (1.8) | 4 (1.4) | 3 (3.03) | 2.31 (0.42, 11.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavo, M.; Di Filippo, P.; Porreca, A.; Prezioso, G.; Orlandi, G.; Rossi, N.; Chiarelli, F.; Attanasi, M. Potential Predictors of Long COVID in Italian Children: A Cross-Sectional Survey. Children 2024, 11, 221. https://doi.org/10.3390/children11020221
Schiavo M, Di Filippo P, Porreca A, Prezioso G, Orlandi G, Rossi N, Chiarelli F, Attanasi M. Potential Predictors of Long COVID in Italian Children: A Cross-Sectional Survey. Children. 2024; 11(2):221. https://doi.org/10.3390/children11020221
Chicago/Turabian StyleSchiavo, Marco, Paola Di Filippo, Annamaria Porreca, Giovanni Prezioso, Greta Orlandi, Nadia Rossi, Francesco Chiarelli, and Marina Attanasi. 2024. "Potential Predictors of Long COVID in Italian Children: A Cross-Sectional Survey" Children 11, no. 2: 221. https://doi.org/10.3390/children11020221
APA StyleSchiavo, M., Di Filippo, P., Porreca, A., Prezioso, G., Orlandi, G., Rossi, N., Chiarelli, F., & Attanasi, M. (2024). Potential Predictors of Long COVID in Italian Children: A Cross-Sectional Survey. Children, 11(2), 221. https://doi.org/10.3390/children11020221









