Cardiac Implications of Adenotonsillar Hypertrophy and Obstructive Sleep Apnea in Pediatric Patients: A Comprehensive Systematic Review
Abstract
1. Introduction
2. Materials and Methods
Evaluation of the Risk of Publication Quality Distortion
3. Results
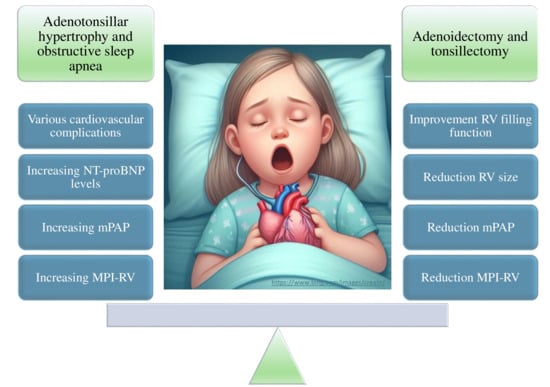
3.1. Association between ATH-Related Apnea and Cardiac Markers
3.2. Prevalence of Pulmonary Hypertension and Associated Risk Factors in Children with OSA
3.3. Changes in Biomarkers of Cardiac Stress and OSA
3.4. Effects of A&T on Heart Function
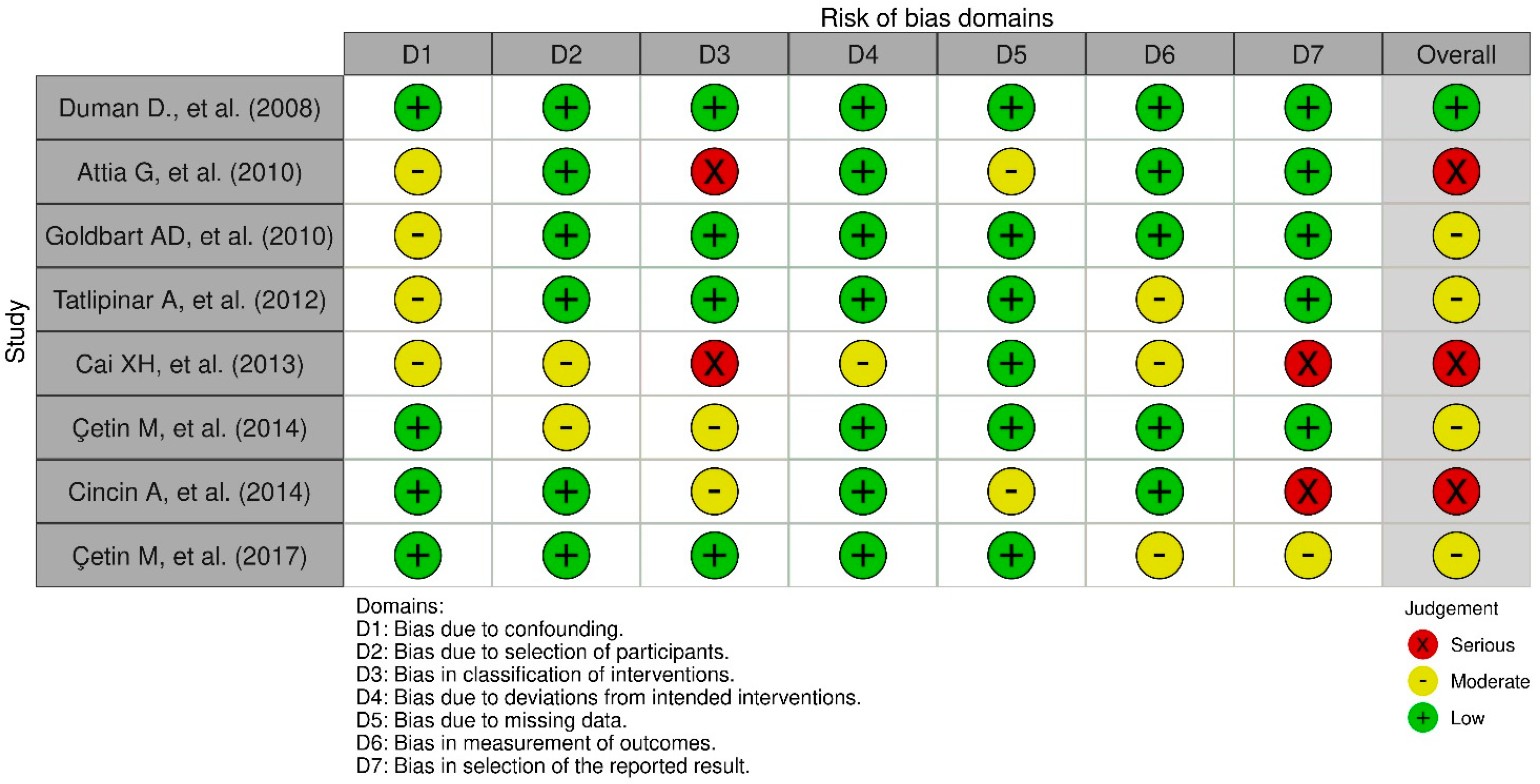
3.5. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gipson, K.; Lu, M.; Kinane, T.B. Sleep-Disordered Breathing in Children. Pediatr. Rev. 2019, 40, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Tagetti, A.; Bonafini, S.; Zaffanello, M.; Benetti, M.V.; Vedove, F.D.; Gasperi, E.; Cavarzere, P.; Gaudino, R.; Piacentini, G.; Minuz, P.; et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J. Hypertens. 2017, 35, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Storari, M.; Scano, A.; Piras, V.; Taibi, R.; Viscuso, D. Obstructive Sleep Apnea, oxidative stress, inflammation and endothelial dysfunction-An overview of predictive laboratory biomarkers. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6939–6948. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Amin, R.S. OSA and Cardiovascular Risk in Pediatrics. Chest 2019, 156, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Baker-Smith, C.M.; Isaiah, A.; Melendres, M.C.; Mahgerefteh, J.; Lasso-Pirot, A.; Mayo, S.; Gooding, H.; Zachariah, J. Sleep-Disordered Breathing and Cardiovascular Disease in Children and Adolescents: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. 2021, 10, e022427. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.H.; Hsu, J.F.; Wang, C.Y.; Chuang, L.P.; Chen, M.C.; Chen, N.H.; Huang, Y.S.; Li, H.Y.; Lee, L.A. Hypertension in Children with Obstructive Sleep Apnea Syndrome-Age, Weight Status, and Disease Severity. Int. J. Environ. Res. Public Health 2021, 18, 9602. [Google Scholar] [CrossRef]
- Widlitz, A.; Barst, R.J. Pulmonary arterial hypertension in children. Eur. Respir. J. 2003, 21, 155–176. [Google Scholar] [CrossRef]
- Abman, S.H.; Hansmann, G.; Archer, S.L.; Ivy, D.D.; Adatia, I.; Chung, W.K.; Hanna, B.D.; Rosenzweig, E.B.; Raj, J.U.; Cornfield, D.; et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation 2015, 132, 2037–2099. [Google Scholar] [CrossRef]
- Rosenzweig, E.B.; Abman, S.H.; Adatia, I.; Beghetti, M.; Bonnet, D.; Haworth, S.; Ivy, D.D.; Berger, R.M.F. Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019, 53, 1801916. [Google Scholar] [CrossRef]
- Ivy, D. Pulmonary Hypertension in Children. Cardiol. Clin. 2016, 34, 451–472. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. JMLA 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Bero, L.; Chartres, N.; Diong, J.; Fabbri, A.; Ghersi, D.; Lam, J.; Lau, A.; McDonald, S.; Mintzes, B.; Sutton, P.; et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: Concerns arising from application to observational studies of exposures. Syst. Rev. 2018, 7, 242. [Google Scholar] [CrossRef]
- Duman, D.; Naiboglu, B.; Esen, H.S.; Toros, S.Z.; Demirtunc, R. Impaired right ventricular function in adenotonsillar hypertrophy. Int. J. Cardiovasc. Imaging 2008, 24, 261–267. [Google Scholar] [CrossRef]
- Cincin, A.; Sakalli, E.; Bakirci, E.M.; Dizman, R. Relationship between obstructive sleep apnea-specific symptoms and cardiac function before and after adenotonsillectomy in children with adenotonsillar hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.T.; Hansen, S.L.; Turner, Z.S.; Aden, J.K.; Black, A.B.; Hsu, D.P. Prevalence of Pulmonary Hypertension in Pediatric Patients With Obstructive Sleep Apnea and a Cardiology Evaluation: A Retrospective Analysis. J. Clin. Sleep Med. 2019, 15, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Bitners, A.C.; Arens, R.; Mahgerefteh, J.; Sutton, N.J.; Silver, E.J.; Sin, S.; Khan, M.A.; Yang, C.J. Prevalence of elevated right ventricular pressure in children with obstructive sleep apnea syndrome undergoing pulmonary hypertension screening. J. Clin. Sleep Med. 2021, 17, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Clements, A.C.; Walsh, J.M.; Dai, X.; Skinner, M.L.; Sterni, L.M.; Tunkel, D.E.; Boss, E.F.; Ryan, M.A. Cardiopulmonary Testing before Pediatric Adenotonsillectomy for Severe and Very Severe Obstructive Sleep Apnea Syndrome. Laryngoscope 2021, 131, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, A.D.; Levitas, A.; Greenberg-Dotan, S.; Ben Shimol, S.; Broides, A.; Puterman, M.; Tal, A. B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest 2010, 138, 528–535. [Google Scholar] [CrossRef]
- Granzotto, E.H.; Aquino, F.V.; Flores, J.A.; Lubianca Neto, J.F. Tonsil size as a predictor of cardiac complications in children with sleep-disordered breathing. Laryngoscope 2010, 120, 1246–1251. [Google Scholar] [CrossRef]
- Tatlıpınar, A.; Biteker, M.; Meriç, K.; Bayraktar, G.; Tekkeşin, A.; Gökçeer, T. Adenotonsillar hypertrophy: Correlation between obstruction types and cardiopulmonary complications. Laryngoscope 2012, 122, 676–680. [Google Scholar] [CrossRef]
- Marangu, D.; Jowi, C.; Aswani, J.; Wambani, S.; Nduati, R. Prevalence and associated factors of pulmonary hypertension in Kenyan children with adenoid or adenotonsillar hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1381–1386. [Google Scholar] [CrossRef]
- Koc, S.; Aytekin, M.; Kalay, N.; Ozcetin, M.; Burucu, T.; Ozbek, K.; Celik, A.; Kadi, H.; Gulturk, S.; Koc, F. The effect of adenotonsillectomy on right ventricle function and pulmonary artery pressure in children with adenotonsillar hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 45–48. [Google Scholar] [CrossRef]
- Cai, X.H.; Li, X.C.; Hu, Q.Q.; Yu, C.Y.; Zhou, Y.H.; Su, M.S.; Zhao, Y.P.; Hu, Y.L.; Wang, L.X. Multiple system morbidities associated with children with snore symptom. Pediatr. Pulmonol. 2013, 48, 381–389. [Google Scholar] [CrossRef]
- Abd El-Moneim, E.S.; Badawy, B.S.; Atya, M. The effect of adenoidectomy on right ventricular performance in children. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1584–1588. [Google Scholar] [CrossRef]
- Attia, G.; Ahmad, M.A.; Saleh, A.B.; Elsharkawy, A. Impact of obstructive sleep apnea on global myocardial performance in children assessed by tissue Doppler imaging. Pediatr. Cardiol. 2010, 31, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Çetin, M.; Yılmaz, M.; Özen, S.; Bozan, N.; Coşkun, Ş. Assessment of pulmonary artery pressure and right ventricular function in children with adenotonsillar hypertrophy using different parameters. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Çetin, M.; Bozan, N. The effects of adenotonsillar hypertrophy corrective surgery on left ventricular functions and pulmonary artery pressure in children. Int. J. Pediatr. Otorhinolaryngol. 2017, 101, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Ko, K.O.; Lim, J.W.; Yoon, J.M.; Song, Y.H.; Cheon, E.J. The improvement of right ventricular function after adenotonsillectomy in children with obstructive sleep apnea. Korean J. Pediatr. 2018, 61, 392–396. [Google Scholar] [CrossRef]
- Bahgat, A.; Bahgat, Y.; Abdelmohaymen, A.; Elwany, M. The effect of adenotonsillectomy on pulmonary hypertension in pediatric obstructive sleep apnea. Egypt. J. Otolaryngol. 2022, 38, 172. [Google Scholar] [CrossRef]
- Sameema, V.V.; Soni, K.; Deora, S.; Sharma, J.B.; Choudhury, B.; Kaushal, D.; Chhabra, S.; Goyal, A. Assessment of preoperative and postoperative cardiac function in children with adenotonsillar hypertrophy: A prospective cohort study. Eur. Arch. Otorhinolaryngol. 2022, 279, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Omer, K.A.; Mlauzi, R.; Basera, W.; McGuire, J.; Meyer, H.; Lawrenson, J.; Peer, S.; Singh, Y.; Zampoli, M. Low incidence of pulmonary hypertension in children with suspected obstructive sleep apnea: A prospective observational study. Int. J. Pediatr. Otorhinolaryngol. 2023, 171, 111648. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Aghajankhah, M.; Banan, R.; Haddadi, S.; Mehri, M.; Aghsaghloo, V.; Leili, E.K. The effects of adeno/tonsillectomy on cardiopulmonary function based on echocardiography indices in children with primary snoring and mild obstructive sleep apnea. Am. J. Otolaryngol. 2022, 43, 103317. [Google Scholar] [CrossRef]
- Maloney, M.A.; Ward, S.L.D.; Su, J.A.; Durazo-Arvizu, R.A.; Breunig, J.M.; Okpara, D.U.; Gillett, E.S. Prevalence of pulmonary hypertension on echocardiogram in children with severe obstructive sleep apnea. J. Clin. Sleep Med. 2022, 18, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.G.; Singh, A.V.; Ehsan, Z.; Birnbaum, B.F. Obstructive Sleep Apnea and Pulmonary Hypertension in Children. Paediatr. Respir. Rev. 2017, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, Z.; Ishman, S.L.; Kimball, T.R.; Zhang, N.; Zou, Y.; Amin, R.S. Longitudinal Cardiovascular Outcomes of Sleep Disordered Breathing in Children: A Meta-Analysis and Systematic Review. Sleep 2017, 40, zsx015. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.J.; Lestrud, S.O.; Hauck, A. Current understanding of the role of sleep-disordered breathing in pediatric pulmonary hypertension. Prog. Pediatr. Cardiol. 2023, 68, 101609. [Google Scholar] [CrossRef]
- Sun, Y.L.; Yuan, B.; Kong, F.; Li, X.M. Effects of adenoidectomy or adenotonsillectomy on the cardiovascular system in children: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2022, 279, 1147–1156. [Google Scholar] [CrossRef]
- Adegunsoye, A.; Ramachandran, S. Etiopathogenetic mechanisms of pulmonary hypertension in sleep-related breathing disorders. Pulm. Med. 2012, 2012, 273591. [Google Scholar] [CrossRef]
- Louise, B.J.; Carys, F.; Philip, M. Narrative review of sleep and pulmonary hypertension. J. Thorac. Dis. 2020, 12, S191–S201. [Google Scholar] [CrossRef] [PubMed]
- Minic, M.; Granton, J.T.; Ryan, C.M. Sleep disordered breathing in group 1 pulmonary arterial hypertension. J. Clin. Sleep Med. 2014, 10, 277–283. [Google Scholar] [CrossRef]
- Kang, K.T.; Hsu, W.C. Efficacy of adenotonsillectomy on pediatric obstructive sleep apnea and related outcomes: A narrative review of current evidence. J. Formos. Med. Assoc. 2023. [Google Scholar] [CrossRef]
- Farhood, Z.; Isley, J.W.; Ong, A.A.; Nguyen, S.A.; Camilon, T.J.; LaRosa, A.C.; White, D.R. Adenotonsillectomy outcomes in patients with Down syndrome and obstructive sleep apnea. Laryngoscope 2017, 127, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Pietrobelli, A.; Piacentini, G.; Guzzo, A.; Antoniazzi, F. The Impact of Growth Hormone Therapy on Sleep-Related Health Outcomes in Children with Prader-Willi Syndrome: A Review and Clinical Analysis. J. Clin. Med. 2023, 12, 5504. [Google Scholar] [CrossRef]
- Booth, K.L.; Levy, D.A.; White, D.R.; Meier, J.D.; Pecha, P.P. Management of obstructive sleep apnea in children with achondroplasia: Outcomes of surgical interventions. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110332. [Google Scholar] [CrossRef]
- Zaffanello, M.; Antoniazzi, F.; Tenero, L.; Nosetti, L.; Piazza, M.; Piacentini, G. Sleep-disordered breathing in paediatric setting: Existing and upcoming of the genetic disorders. Ann. Transl. Med. 2018, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.S.C.; Sakthiakumaran, A.; Bassam, A.; Thacker, J.; Walter, L.M.; Davey, M.J.; Nixon, G.M. Children with Down syndrome and sleep disordered breathing have altered cardiovascular control. Pediatr. Res. 2021, 90, 819–825. [Google Scholar] [CrossRef]
- Brito, L.C.; Queiroga, T.; Franco, R.R.; Passone, C.G.B.; Lopes, M.C.; Shea, S.A.; Bueno, C.; Soster, L. Cardiac autonomic control during non-REM and REM sleep stages in paediatric patients with Prader-Willi syndrome. J. Sleep Res. 2021, 30, e13165. [Google Scholar] [CrossRef]
- Oros, M.; Baranga, L.; Plaiasu, V.; Cozma, S.R.; Neagos, A.; Paduraru, L.; Necula, V.; Martu, C.; Dima-Cozma, L.C.; Gheorghe, D.C. Obstructing Sleep Apnea in Children with Genetic Disorders-A Special Need for Early Multidisciplinary Diagnosis and Treatment. J. Clin. Med. 2021, 10, 2156. [Google Scholar] [CrossRef]
| First Author | Type of Study | Purpose | Cases (Number, Age) | Controls (Number, Age) | Methods | Length of Disease before Diagnosis | OSA Severity Indices at Diagnosis | Age at Follow-up | OSA Severity Index at Follow-up | Follow-up Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| OBSERVATIONAL | ||||||||||
| Duman D. et al. (2008) [16] | Observational study with a control group | MPI-RV in ATH, OSA, and PH. Effects of A&T on MPI. | 21 children with grade 3 and 4 ATH (13 males, 7.3 ± 1.8 years). | 21 healthy children matched by age and sex (14 males, 7.2 ± 2.2 years). | Doppler echocardiography before and after A&T. OSA-18 questionnaire. | ≥6 months (ATH duration not known) | OSA-18 score = 83 ± 27 | Not reported | OSA-18 score = 36 ± 12 | 7.3 ± 2.0 months |
| Cincin A. et al. (2014) [17] | Observational study | Subclinical cardiac dysfunction in patients with symptoms due to ATH and the echocardiographic impact of A&T. | 30 children with grade 3 or 4 ATH (age 7.86 ± 3.83 years) | 30 control children, matched for age and sex (age 8 ± 2.77 years) | Echocardiographic and Doppler parameters; tissue Doppler parameters of RV and LV myocardial performance. OSA-18 questionnaire. Repeat echocardiographic examination after A&T. | Not reported | OSA-18 questionnaire | Not reported | Not reported | Not reported |
| RETROSPECTIVE | ||||||||||
| Burns A.T. et al. (2019) [18] | Retrospective analysis | The occurrence of PH in children with OSA and the potential predictors of an elevated PH risk. | 163 children (age 7.7 ± 4.8 years), AHI 5.5 events/h (IQR 2.4–12.1 events/h) | Not available | PSG. PH in children is defined as a mean pulmonary arterial pressure ≥ 25 mmHg, right heart catheterization. | Not reported | PSG, AHI 5.5 events/hour, IQR 2.4–12.1 events/hour | Not reported | Not reported | Not reported |
| Bitners A.C. et al. (2021) [19] | Retrospective review | Prevalence of elevated RV pressure as a marker of PH in children with OSAS. | 620 children with OSA, age 8.9 (5.5–13.1) years | Not available | PSG. Echocardiogram: PH screening within 6 months of PSG. Pulmonary vascular resistance elevated above right atrium pressure or elevated pulmonary vascular resistance. | Not reported | PSG (mild, moderate, severe) | Not reported | Not reported | Not reported |
| Clements A.C. et al. (2021) [20] | Retrospective review | OSA severity level and cardiopulmonary comorbidities that could be identified via preoperative testing. | 358 children (age 5.9 ± 3.6 years; range 1.1–21.8 years) with severe OSAS undergoing A&T (genetic syndromes, prematurity, congenital heart disease, and pulmonary comorbidities were included). | Not available | PSG and preoperative testing. oAHI, hypoxia and hypercapnia, severity of OSAS. | Not reported | PSG = 30.3 (23.8) | Not reported | Not reported | Not reported |
| CROSS-SECTIONAL | ||||||||||
| Goldbart AD et al. (2010) [21] | Cross-sectional and longitudinal | Relationship between NT-proBNP and cardiovascular function in children with OSA. | 90 children with OSA (age 20 ± 7 months). | 45 healthy children. | Children undergoing A&T for OSA, PSG, echocardiography, and CRP and NT-proBNP assay. Three months after A&T, 72 children were re-examined for NT-proBNP and CRP assay. | 3 months | AHI 16.9 ± 16 events/hour | Not reported | Not reported | 3 months |
| Granzotto E.H. et al. (2010) [22] | Cross-sectional study | Association of palatine tonsil size (T/P, radiography) and pulmonary artery pressure measured by Doppler echocardiography in children with an indication for A&T. | 45 children (age 72.0 ± 32.3 months). | Not available. | Brodsky scale; OSA-18 questionnaire; Palatine tonsil size according to Shintani; Doppler ecocardiogram. Children with an indication for A&T. | 24.7 ± 27.8 (2–168) months | OSA-18 = 86.20 ± 20.60 (31–126) | Not reported | Not reported | Not reported |
| Tatlipinar A et al. (2012) [23] | Cross-sectional study | Association between upper airway obstruction and cardiopulmonary complications. | 95 children with OSA and ATH; 4 groups: only hypertrophic adenoids (n. 40, age 6.96 ± 2.11 years); only hypertrophy of the tonsils (n.6, age 7.00 ± 1.54 years); hypertrophic adenoids and tonsils (n.35, 6.69 ± 1.68 years) | 14 children (age 7.21 ± 2.08 years) | Brodsky score and adenoids-to-nasopharynx ratio. OSA-18 and Brouilette’s questionnaire. Transthoracic two-dimensional echocardiography. | Not reported | OSA-18, Brouilette classification | Not reported | Not reported | Not reported |
| Marangu D. et al. (2014) [24] | Cross sectional hospital-based survey | Prevalence and associated PH factors in children with ATH. | 123 children aged 2.5 (IQR 1.4–3.5) years with adenoid hypertrophy and OSA | Not available | Brodsky classification and Friedman classification. Doppler echocardiography to determine PH. | Median 14 (IQR 2–51) months | Clinical symptoms | Not reported | Not reported | Not reported |
| COMPARATIVE | ||||||||||
| Koc S. et al. (2012) [25] | Comparative study | RV function and mean pulmonary artery pressure in children with ATH undergoing A&T. | 27 children (age 8 ± 2 years) with only ATH. | Not available. | Grades 3 or 4 hypertrophy of the tonsils. A&T and echocardiogram. | Not reported | Brodsky scale | Not reported | Not reported | 3 months |
| Cai X.H. et al. (2013) [26] | Comparative observational study | Relationship between snoring and morbidity in children. | 152 snoring children: 63 primary snorers (age 6.02 ± 2.79 years), 89 with OSA (age 5.57 ± 2.55 years) | 60 controls (age 6.00 ± 2.48 years) | PSG. Maxillofacial malformations, echocardiogram. | Not reported | AHI = 15.6 events/hour (5.1–85.7) | 8.50 ± 2.17 years | Not reported | Approximately 3 years |
| PROSPECTIVE | ||||||||||
| Abd El-Moneim E.S. et al. (2009) [27] | Prospective crossover observational study | Changes in RV performance parameters after adenoidectomy in children with adenoid hypertrophy. | 30 children with adenoidal hypertrophy (median age 5 years, range 2.5 and 12 years). | Not available. | Follow-up echocardiographic examination. Brouilette’s questionnaire. Echocardiogram and cardiac Doppler examination one day before and at the follow-up visit. | Duration of obstructive apnea symptoms 2.2 (1.2–9) years | Brouilette score (>3.5) | Not reported | Not reported | 36 (30–52) days |
| Attia G. et al. (2010) [28] | Prospective study | Impact of OSA on myocardial performance using tissue Doppler, echocardiography, and after A&T. | 42 children with OSA (5 ± 3.14 years) | 45 healthy children matched by age and gender. | PSG (AHI), echocardiography; tissue Doppler ultrasound. A&T, re-evaluated by PSG and echocardiography. | Not specified | PSG, AHI 11.74 ± 2.6 events/hour | Not reported | Not reported | 6–8 months |
| Çetin M. et al. (2014) [29] | [Prospective study] | RV function before and after A&T in children with ATH. | 41 children (age 6.0 ± 2.5 years): 15 adenoidectomies, 26 tonsillectomies | 40 control children (age 6.0 ± 2.4 years). | Tissue Doppler, pulse echocardiogram, and conventional echocardiography preoperatively and at follow-up. | Not reported | Questionnaire of symptoms | Not specified | Not reported | 6 months |
| Çetin M. et al. (2017) [30] | Prospective study | LV function in children with ATH; effects of A&T on LV function by comparing pre- and post-operative data. | 30 children (age 5.9 ± 2.1 years) with upper airway obstruction, who underwent adenoidectomy/T&A. | 30 healthy children (age 5.9 ± 2.1 years). | Tissue Doppler echocardiography, conventional echocardiography, before and after A&T. Sinus radiographs and Brodsky scale. | Not reported | Questionnaire | Not reported | Not reported | 6 months |
| Kim D.Y. et al. (2018) [31] | Prospective cohort study | To assess the impact of A&T on RV function in children with OSA caused by ATH. | 37 children (7.72 ± 2.22 years) underwent T&A. | Not available | Cohen and Konak method and Brodsky scale, STOP questionnaire, transthoracic echocardiography before and after A&T. | Not reported | STOP Questionare | Not reported | Not reported | 12 months |
| Bahgat A. et al. (2022) [32] | Prospective study | To establish pulmonary arterial systolic pressure in children with OSA with ATH. To evaluate whether A&T has any effect on pulmonary blood pressure. | 50 children (age 8.34 ± 3.57 years) with loud snoring and OSA due to ATH. | Not available | Brodsky scale, OSA-18 questionnaire, lateral X-ray of the nasopharynx, echocardiography. A&T: 3 months follow-up after A&T. | Not reported | OSA-18 questionnaire | Not reported | Not reported | 3 months |
| Sameema V.V. et al. (2022) [33] | Prospective study | Parameters of cardiac function via echocardiography before and after A&T in children with ATH. | 23 children (age 7.43 ± 2.19 years; range 4–12 years) with ATH. | Not available | Echocardiographic examination prior to A&T surgery. Follow-up with echocardiographic examination. | 2.22 ± 1.47 years | Clinical criteria | Not reported | Not reported | 3 months |
| Omer K.A. et al. (2023) [34] | Prospective observational study | Incidence of PH in children with suspected OSA and association between PH and OSA. | 170 children (age 3.8 years, IQR 2.7–6.4 years). Children with comorbidities are excluded. | Not available | MOS score: MOS 1–2 (mild-moderate) and MOS 3–4 (severe). PH = mean pressure in the pulmonary artery on echocardiography. | Not reported | Overnight oximetry (McGill Oximetry Score, ODI) | Not reported | Not reported | Not reported |
| CLINICAL TRIAL | ||||||||||
| Nemati S. et al. (2022) [35] | Quasi-experimental clinical trial study | To evaluate the A&T effects on cardiac function in children with snoring and OSA (AHI: 12.2 ± 7.02 events/hour) due to ATH. | 42 children (age 7–11 years) with snoring and ATH (grades 3 and 4), A&T candidates. | Not available | Brodsky classification, lateral neck X-ray, PSG. Echocardiography performed one week before and after A&T. | Not reported | PSG, AHI 12.24 ± 7.02 events/hour | Not reported | Not reported | 3–6 months |
| First Author | Interpretation of Cardiology Findings | Authors’ Conclusions |
|---|---|---|
| OBSERVATIONAL | ||
| Duman D. et al. (2008) [16] | MPI-RV initially higher in children with Grade 3 and 4 ATH than controls. MPI-RV improved following A&T similar to the controls. | ATH increases the MPI-RV and subclinical RV dysfunction. A&T can reverse these changes. |
| Cincin A. et al. (2014) [17] | Before surgery: Patients with symptoms of OSA due to ATH had higher mPAP and impaired RV function. After surgery: Patients with symptoms of OSA from ATH had significant effects on both LV and RV function. | Before surgery patients with ATH showed higher mPAP and after surgery they showed significant improvement. |
| RETROSPECTIVE | ||
| Burns A.T. et al. (2019) [18] | Low prevalence of PH in pediatric patients with suspected OSA. None of the patients with PH had severe OSA. | PH in pediatric OSA is relatively low. |
| Bitners A.C. et al. (2021) [19] | High RV pressure was present in a low percentage of children (4%). High RV pressure did not appear related to OSA severity or low oxygen levels during sleep. | Prevalence of elevated RV pressure in children with OSA is low. Severe disease and obesity are risk factors for PH development in children with OSA. |
| Clements A.C. et al. (2021) [20] | Children with very severe OSA (oAHI ≥ 60 events/hour) underwent more pre-operative cardiopulmonary tests. OSA severity did not predict abnormal findings. | Severity of OSA is not predictive of pre-A&T cardiopulmonary abnormalities. |
| CROSS-SECTIONAL | ||
| Goldbart A.D. et al. (2010) [21] | OSA was associated with high NT-proBNP levels (increased cardiac stress). Surgical treatment reduced NT-proBNP. Inflammation (increased CRP) was related to alterations in tricuspid flow rate. | NT-proBNP levels increase in children with OSA and decrease following A&T. Echocardiographic parameters suggest an increase in pulmonary pressure in children with OSA that decreases after treatment. |
| Granzotto E.H. et al. (2009) [22] | The T/P ratio help to assess systolic pulmonary blood pressure and identify patients with PH. | Good correlation between T/P and mPAP in children with ATH and surgical indications for SDB. |
| Tatlipinar A. et al. (2012) [23] | Correlation between mPAP and cardiac function indicators (including tricuspid annular plane systolic excursion, MPI-RV, and adenoidal–nasopharyngeal ratio). | Patients with ATH are at increased risk of cardiopulmonary complications and associated with more severe OSA symptoms. |
| Marangu D. et al. (2014) [24] | One fifth of children with ATH had PH. Nasal obstruction (3-fold) and adenoidal-to-nasopharyngeal ratio >0.825 (5-fold) increased the risk. | Nasal blockage and adenoidal hypertrophy are risk factors for PH. |
| COMPARATIVE | ||
| Koc S. et al. (2012) [25] | A&T led to improvements in cardiac function. Enhancements in tricuspid valve function decreased MPI-RV and reduced mPAP. | A&T improves MPI-RV and reduces mPAP. |
| Cai X.H. et al. (2013) [26] | Children with OSA and with primary snoring had greater alterations in cardiac parameters than controls. | Children with OSA have higher mPAP. |
| PROSPECTIVE | ||
| Abd El-Moneim E.S. et al. (2009) [27] | Following adenoidectomy, cardiac dynamics improved: increased flow through the tricuspid and pulmonary valves, improved RV filling function, and reduced RV size. | Relief of OSA by adenoidectomy results in improved RV filling and RV output and reduced mPAP. |
| Attia G. et al. (2010) [28] | Cardiac function abnormalities in mPAP are related to OSA severity, and are reversible by surgical treatment. | Cardiac evaluation in children with OSA due to ATH is essential. Surgical treatment significantly improves heart function and PH. |
| Çetin M. et al. (2014) [29] | Surgery positively impacted heart function and mPAP in children with ATH who improved after surgery. | A&T have positive impact on heart function in children with ATH. |
| Çetin M. et al. (2017) [30] | Children with ATH had abnormalities in cardiac parameters (thicker interventricular septum and a higher mPAP). After surgery, these parameters improved. | mPAP in patients with ATH is higher in the preoperative period and improves following A&T. |
| Kim D.Y. et al. (2018) [31] | A&T led to an improvement in RV function (improvement in the MPI-RV in children with OSA). Intervention did not significantly affect the mPAP or maximal velocity of tricuspid regurgitation. | OSA from ATH impaired RV function. |
| Bahgat A. et al. [32] | Surgery positively affected pulmonary arterial systolic pressures, with normalization within 2 months of the operation. | ATH can cause higher pulmonary arterial systolic pressure in children with OSA. A&T is an effective therapeutic measure. |
| Sameema V.V. et al. (2022) [33] | A&T led to a reduction in mPAP and improved RV function. Diastolic RV dysfunction improved in some patients. | ATH can cause reversible subclinical cardiac dysfunction, which improves after A&T. |
| Omer K.A. et al. (2023) [34] | Small percentage of children with OSA developed HP. No substantial disparities in mPAP or other parameters between children with mild-to-moderate OSA and severe OSA. | PH is rare in children with uncomplicated OSA. No association between PH and OSA severity. No differences in clinical severity and OSA in children with and without PH. |
| CLINICAL TRIAL | ||
| Nemati S. et al. (2022) [35] | A&T led to significant improvements in RV function. RV function indices improved after surgery. | A&T improves cardiac function indices in patients with primary snoring, RV function, and reduced pulmonary blood pressure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaffanello, M.; Ersu, R.H.; Nosetti, L.; Beretta, G.; Agosti, M.; Piacentini, G. Cardiac Implications of Adenotonsillar Hypertrophy and Obstructive Sleep Apnea in Pediatric Patients: A Comprehensive Systematic Review. Children 2024, 11, 208. https://doi.org/10.3390/children11020208
Zaffanello M, Ersu RH, Nosetti L, Beretta G, Agosti M, Piacentini G. Cardiac Implications of Adenotonsillar Hypertrophy and Obstructive Sleep Apnea in Pediatric Patients: A Comprehensive Systematic Review. Children. 2024; 11(2):208. https://doi.org/10.3390/children11020208
Chicago/Turabian StyleZaffanello, Marco, Refika Hamutcu Ersu, Luana Nosetti, Giulio Beretta, Massimo Agosti, and Giorgio Piacentini. 2024. "Cardiac Implications of Adenotonsillar Hypertrophy and Obstructive Sleep Apnea in Pediatric Patients: A Comprehensive Systematic Review" Children 11, no. 2: 208. https://doi.org/10.3390/children11020208
APA StyleZaffanello, M., Ersu, R. H., Nosetti, L., Beretta, G., Agosti, M., & Piacentini, G. (2024). Cardiac Implications of Adenotonsillar Hypertrophy and Obstructive Sleep Apnea in Pediatric Patients: A Comprehensive Systematic Review. Children, 11(2), 208. https://doi.org/10.3390/children11020208