Laryngomalacia and Obstructive Sleep Apnea in Children: From Diagnosis to Treatment
Abstract
1. Introduction
2. Methods
3. Results and Discussion
3.1. Epidemiologic Features
3.2. Etiologic Theories and Risk Factors
3.3. Diagnosis
| Authors | Previous PSG | DISE | N | Male | Mean Age | Genetic Syndrome | R | SAF | BA | M |
|---|---|---|---|---|---|---|---|---|---|---|
| Love 2020 [66] | Yes | Yes: propofol + Sevoflorane | 41 | 64.10% | 11 mo. | 22.00% | NA | |||
| Bhushan 2019 [23] | Yes | Yes | 41 | 53.60% | 1.3 yrs. | NA | ||||
| Digoy 2012 [26] | Yes | Yes: laryngoscopy/flexible nasendoscopy via light general anesthesia (sleep endoscopy) sevoflurane (8%) in 100% oxygen | 36 | 56 mo. | 25.6% | NA | ||||
| Mase 2015 [20] | Yes | Yes: flexible nasendoscopy under total intravenous general anesthesia (propofol) | 9 | 55.50% | 17 mo. | NA | ||||
| Boudewyns 2017 [14] | Yes | Yes (no details) | 28 | 60.7%% | 1.5 yrs. | NA | ||||
| Chan 2012 [3] | Yes | Yes: flexible fiber-optic sleep endoscopy | 22 | 73.00% | 7.4 yrs. | 27.00% | ||||
| Revell 2010 [4] | Yes | Yes: direct laryngoscopy under intravenous anesthesia (spontaneous ventilating) | 51 | 50.90% | 7.2 yrs. | |||||
| Garritano 2014 [45] | No | No: direct laryngoscopia previous supraglottoplasty surgery | 17 | 64.70% | 33.7 mo. | 11.80% | 94.10% | 94.10% | 29.40% | |
| Powitzky 2011 [19] | Yes | No: flexible laryngoscopy while inhaling Sevoflorane | 20 | 3.9 mo. | 15.00% | |||||
| O’Connor 2009 [18] | Yes | No: fiberoptic nasopharyngoscopy | 10 | 70.00% | 2.6 mo. | 20.00% | 40.00% | 100.00% | 90.00% | |
| Weinstein 2016 [67] | Yes | Unknown: fiberoptic nasopharyngoscopy AND direct laryngoscopy | 23 | 69.50% | 7.1 mo. | |||||
| Ching 2017 [21] | Yes | No: fiberoptic nasopharyngoscopy | 8 | 62.50% | 13.1 mo. | |||||
| Cortes 2019 [22] | Yes | No: fiberoptic nasopharyngoscopy OR direct laryngoscopy | 9 | 55.50% | 5.5 mo. | 77.70% | 88.80% | 100.00% | ||
| Fard 2020 [36] | Yes | No | 108 | |||||||
| Vberkest 2020 [24] | Yes | No: fiberoptic nasopharyngoscopy AND direct laryngoscopy | 44 | 54.50% | 25.00% | 2.00% | 19.00% | 25.00% | 50.00% | |
| Ratanakorn 2021 [68] | Yes | No: fiberoptic nasopharyngoscopy | 57 | 47.30% | 3.6 mo. | |||||
| Zafereo 2008 [17] | Yes | No: fiberoptic nasopharyngoscopy | 10 | 4 mo. | ||||||
| Valera 2006 [16] | No: fiberoptic nasopharyngoscopy AND direct laryngoscopy | 7 | 57.10% | 6.8 mo. | 100.00% | 100.00% | 100.00% |
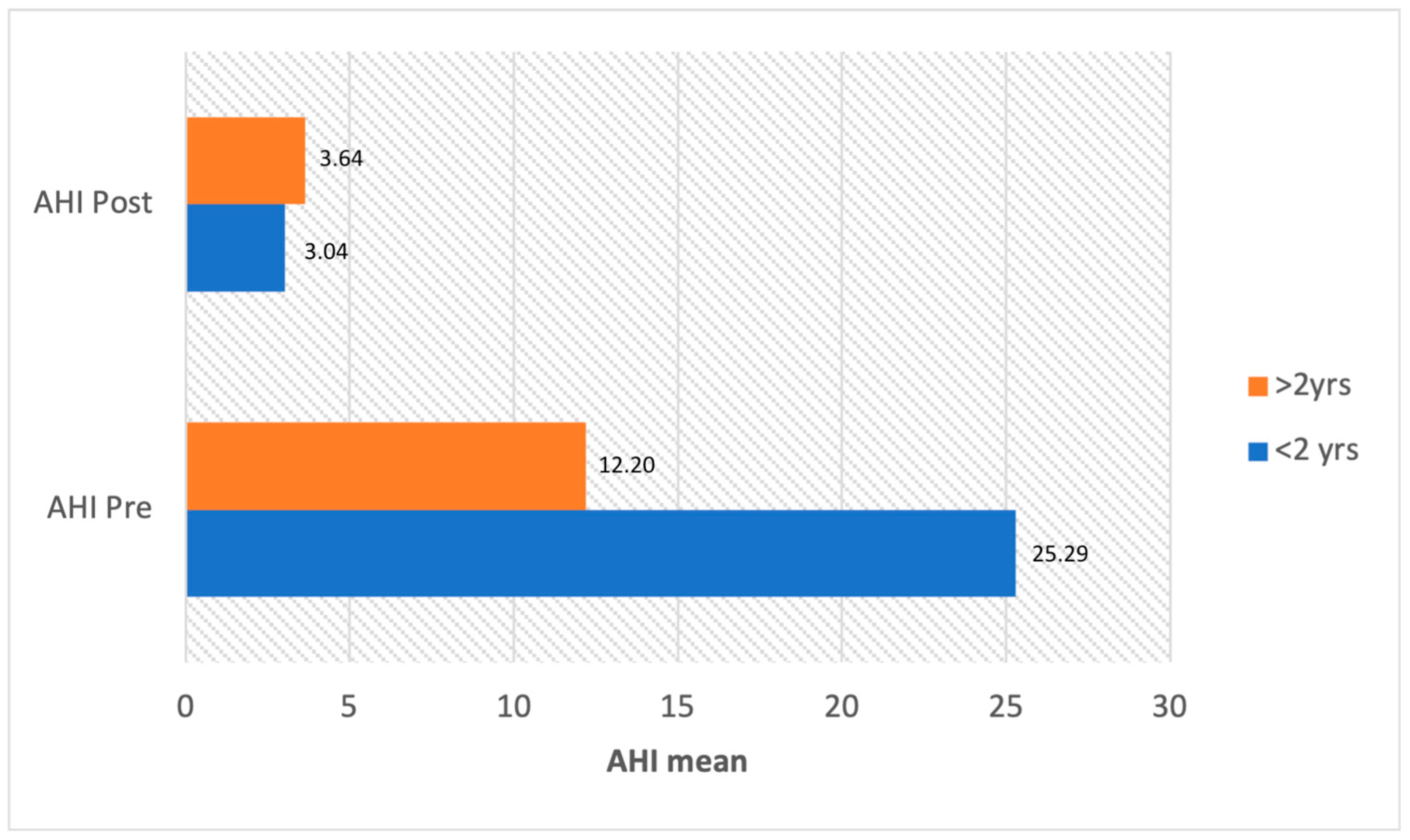
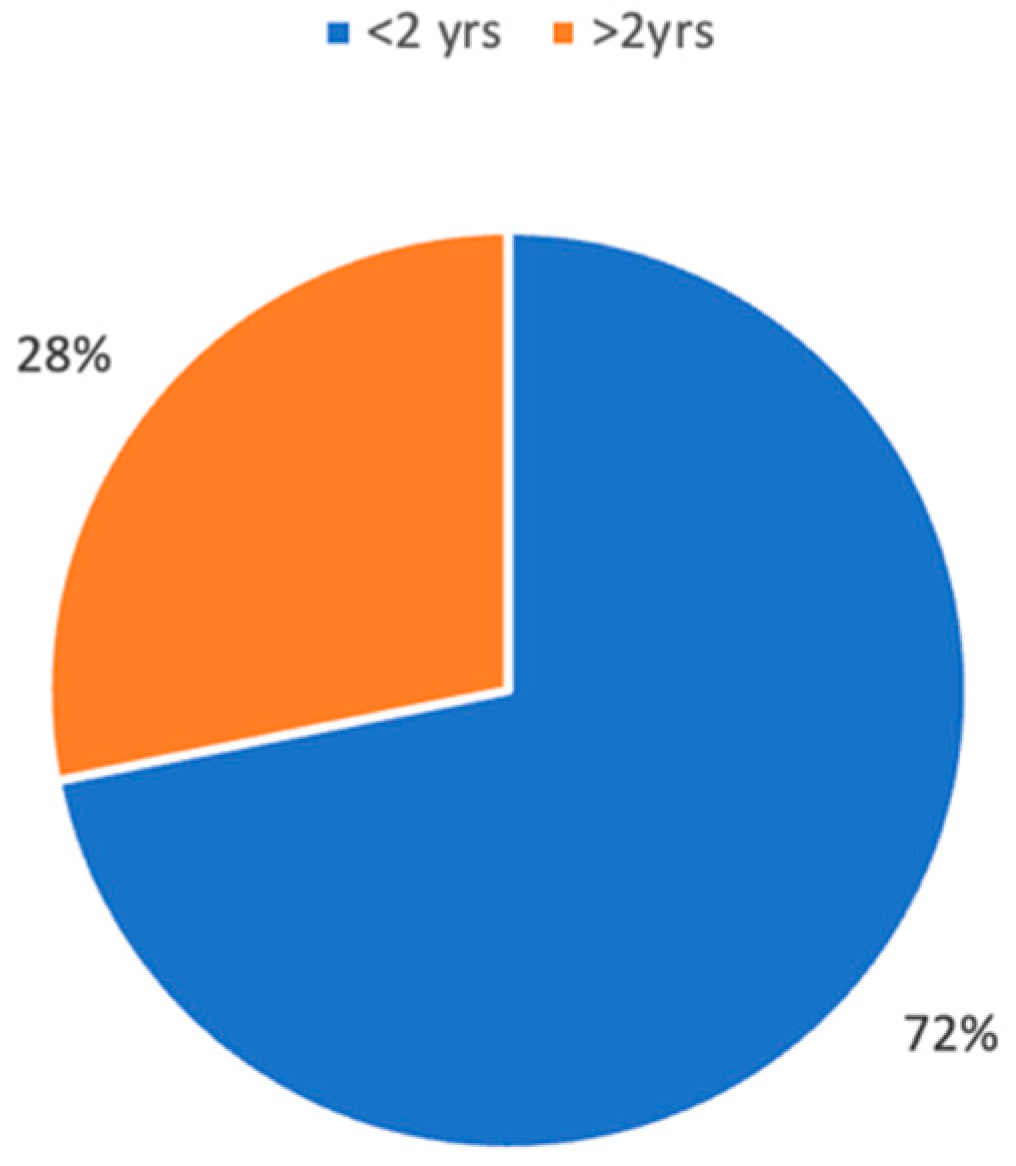
3.4. Treatment and Outcomes
3.4.1. Population under 2 Years
3.4.2. Population over 2 Years
3.4.3. Complications and Follow-Up
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandra, R.K.; Gerber, M.E.; Holinger, L.D. Histological insight into the pathogenesis of severe laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2001, 61, 31–38. [Google Scholar] [CrossRef]
- Landry, A.M.; Thompson, D.M. Laryngomalacia: Disease presentation, spectrum, and management. Int. J. Pediatr. 2012, 2012, 753526. [Google Scholar] [CrossRef]
- Chan, D.K.; Truong, M.T.; Koltai, P.J. Supraglottoplasty for occult laryngomalacia to improve obstructive sleep apnea syndrome. Arch. Otolaryngol. Head. Neck Surg. 2012, 138, 50–54. [Google Scholar] [CrossRef]
- Revell, S.M.; Clark, W.D. Late-onset laryngomalacia: A cause of pediatric obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 231–238. [Google Scholar] [CrossRef]
- Roger, G.; Denoyelle, F.; Triglia, J.M.; Garabedian, E.N. Severe laryngomalacia: Surgical indications and results in 115 patients. Laryngoscope 1995, 105, 1111–1117. [Google Scholar] [CrossRef]
- Thompson, D.M. Laryngomalacia: Factors that influence disease severity and outcomes of management. Curr. Opin. Otolaryngol. Head. Neck Surg. 2010, 18, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Jain, S. Management of stridor in severe laryngomalacia: A review article. Cureus 2022, 14, e29585. [Google Scholar] [CrossRef]
- Leonard, J.A.; Reilly, B.K. Laryngomalacia in the Premature Neonate. Neoreviews 2021, 22, e653–e659. [Google Scholar] [CrossRef]
- Tawfik, K.O.; Sedaghat, A.R.; Ishman, S.L. Trends in Inpatient Pediatric Polysomnography for Laryngomalacia and Craniofacial Anomalies. Ann. Otol. Rhinol. Laryngol. 2016, 125, 82–89. [Google Scholar] [CrossRef]
- Archer, S.M. Acquired flaccid larynx: A case report supporting the neurologic theory of laryngomalacia. Arch. Otolaryngol. Head. Neck Surg. 1992, 118, 654–657. [Google Scholar] [CrossRef]
- Ramgopal, S.; Kothare, S.V.; Rana, M.; Singh, K.; Khatwa, U. Obstructive sleep apnea in infancy: A 7-year experience at a pediatric sleep center. Pediatr. Pulmonol. 2014, 49, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Thevasagayam, M.; Rodger, K.; Cave, D.; Witmans, M.; El-Hakim, H. Prevalence of laryngomalacia in children presenting with sleep-disordered breathing. Laryngoscope 2010, 120, 1662–1666. [Google Scholar] [CrossRef]
- Montgomery-Downs, H.E.; Gozal, D. Snore-associated sleep fragmentation in infancy: Mental development effects and contribution of secondhand cigarette smoke exposure. Pediatrics 2006, 117, e496–e502. [Google Scholar] [CrossRef]
- Boudewyns, A.; Van de Heyning, P.; Verhulst, S. Drug-induced sedation endoscopy in children <2 years with obstructive sleep apnea syndrome: Upper airway findings and treatment outcomes. Eur. Arch. Otorhinolaryngol. 2017, 274, 2319–2325. [Google Scholar]
- Hoff, S.R.; Schroeder, J.W., Jr.; Rastatter, J.C.; Holinger, L.D. Supraglottoplasty Outcomes in relation to age and comorbid conditions. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 245–249. [Google Scholar] [CrossRef]
- Valera, F.C.; Tamashiro, E.; de Araújo, M.M.; Sander, H.H.; Küpper, D.S. Evaluation of the efficacy of supraglottoplasty in obstructive sleep apnea syndrome associated with severe laryngomalacia. Arch. Otolaryngol. Head. Neck Surg. 2006, 132, 489–493. [Google Scholar] [CrossRef]
- Zafereo, M.E.; Taylor, R.J.; Pereira, K.D. Supraglottoplasty for laryngomalacia with obstructive sleep apnea. Laryngoscope 2008, 118, 1873–1877. [Google Scholar] [CrossRef]
- O’Connor, T.E.; Bumbak, P.; Vijayasekaran, S. Objective assessment of supraglottoplasty outcomes using polysomnography. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1211–1216. [Google Scholar] [CrossRef]
- Powitzky, R.; Stoner, J.; Fisher, T.; Digoy, G.P. Changes in sleep apnea after supraglottoplasty in infants with laryngomalacia. Int. J. Pediatr. Otolaryngol. 2011, 75, 1234–1239. [Google Scholar] [CrossRef]
- Mase, C.A.; Chen, M.L.; Horn, D.L.; Parikh, S.R. Supraglottoplasty for sleep endoscopy diagnosed sleep dependent laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 511–515. [Google Scholar] [CrossRef]
- Ching, H.H.; Spinner, A.G.; Reeve, N.H.; O-Lee, T.J. A novel technique for unilateral supraglottoplasty. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 150–154. [Google Scholar] [CrossRef]
- Cortes, M.C.; Villamor, P.; de la Torre González, C.; Álvarez-Neri, H. Complete polysomnographic parameters in infants with severe laryngomalacia prior to and after supraglottoplasty. Int. J. Pediatr. Otorhinolaryngol. 2019, 119, 131–135. [Google Scholar] [CrossRef]
- Bhushan, B.; Schroeder, J.W., Jr.; Billings, K.R.; Giancola, N.; Thompson, D.M. Polysomnography Outcomes after Supraglottoplasty in Children with Obstructive Sleep Apnea. Otolaryngol. Head. Neck Surg. 2019, 161, 694–698. [Google Scholar] [CrossRef]
- Verkest, V.; Verhulst, S.; Van Hoorenbeeck, K.; Vanderveken, O.; Saldien, V.; Boudewyns, A. Prevalence of obstructive sleep apnoea in children with laryngomalacia and value of polysomnography in treatment decisions. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110255. [Google Scholar] [CrossRef]
- Casellas, N.J.; Shah, S.; Ravikumar, S.; Vandjelovic, N.D.; Faria, J.; Allen, P.D.; McKenna Benoit, M.K. Polysomnogram Outcomes in Patients with Laryngomalacia and Obstructive Sleep Apnoea Treated Surgically vs. Non-Surgically. J. Laryngol. Otol. 2023, 22, 1–21. [Google Scholar] [CrossRef]
- Digoy, G.P.; Shukry, M.; Stoner, J.A. Sleep apnea in children with laryngomalacia: Diagnosis via sedated endoscopy and objective outcomes after supraglottoplasty. Otolaryngol. Head. Neck Surg. 2012, 147, 544–550. [Google Scholar] [CrossRef]
- Kennedy, D.G.; Wilson, N.R.; Mwaura, A.; Carnino, J.M.; Levi, J. An Analysis of Laryngomalacia and Its Interplay with Obesity and Obstructive Sleep Apnea in Pediatric Inpatients. Cureus 2023, 15, e45313. [Google Scholar] [CrossRef]
- Williamson, A.; McArdle, E.H.; Jaffal, H. Findings on drug-induced sleep endoscopy in infants with laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2023, 176, 111775. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of pediatric obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Abel, F.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; et al. ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children. Eur. Respir. J. 2017, 50, 1700985. [Google Scholar] [CrossRef]
- Katz, E.S.; Mitchell, R.B.; D’Ambrosio, C.M. Obstructive sleep apnoea in infants. Am. J. Respir. Crit. Care Med. 2012, 185, 805–816. [Google Scholar] [CrossRef]
- Gislason, T.; Benediktsdottir, B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest 1995, 107, 963–966. [Google Scholar] [CrossRef]
- Farhood, Z.; Ong, A.A.; Nguyen, S.A.; Gillespie, M.B.; Discolo, C.M.; White, D.R. Objective Outcomes of Supraglottoplasty for Children with Laryngomalacia and Obstructive Sleep Apnoea: A Meta- analysis. JAMA Otolaryngol. Head. Neck Surg. 2016, 142, 665–671. [Google Scholar] [CrossRef]
- Muzumdar, H.; Arens, R. Diagnostic issues in pediatric obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 263–273. [Google Scholar] [CrossRef]
- Nosetti, L.; Zaffanello, M.; De Bernardi, F.; Piacentini, G.; Roberto, G.; Salvatore, S.; Simoncini, D.; Pietrobelli, A.; Agosti, M. Age and Upper Airway Obstruction: A Challenge to the Clinical Approach in Pediatric Patients. Int. J. Environ. Res. Public. Health. 2020, 17, 3531. [Google Scholar]
- Fard, D.; Rohlfing, M.L.; Razak, A.; Cohen, M.B.; Levi, J.R. Prevalence and natural history of obstructive sleep apnea in pediatric patients with laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2020, 133, 109967. [Google Scholar] [CrossRef] [PubMed]
- Belmont, J.R.; Grundfast, K. Congenital laryngeal stridor (laryngomalacia): Etiologic factors and associated disorders. Ann. Otol. Rhinol. Laryngol. 1984, 93, 430–437. [Google Scholar] [CrossRef]
- Amin, R.S. Gastroesophageal reflux and infant apnea. J. Pediatr. 2000, 137, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M. Abnormal sensorimotor integrative function of the larynx in con- genital laryngomalacia: A new theory of etiology. Laryngoscope 2007, 117, 1–33. [Google Scholar] [CrossRef]
- Golz, A.; Goldenberg, D.; Westerman, S.T.; Catalfumo, F.J.; Netzer, A.; Westerman, L.M.; Joachims, H.Z. Laser partial epiglottidectomy as a treatment for obstructive sleep apnea and laryngomalacia. Ann. Otol. Rhinol. Laryngol. 2000, 109, 1140–1145. [Google Scholar] [PubMed]
- Hui, Y.; Gaffney, R.; Crysdale, W.S. Laser aryepiglottoplasty for treatment of neur- asthenic laryngomalacia in cerebral palsy. Ann. Otol. Rhinol. Laryngol. 1995, 104, 432–436. [Google Scholar] [CrossRef]
- Gan, R.W.C.; Moustafa, A.; Turner, K.; Knight, L. Histopathology of laryngomalacia. Acta Otolaryngol. 2021, 141, 85–88. [Google Scholar] [CrossRef]
- Matthews, B.L.; Little, J.P.; Mcguirt, W.F., Jr.; Koufman, J.A. Reflux in infants with laryngomalacia: Results of 24-hour double-probe pH monitoring. Otolaryngol. Head. Neck Surg. 1999, 120, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Garritano, F.G.; Carr, M.M. Characteristics of patients undergoing supraglottoplasty for laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Arad-Cohen, N.; Cohen, A.; Tirosh, E. The relationship between gastroesophageal reflux and apnea in infants. J. Pediatr. 2000, 137, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.P.; Schefft, G.L.; Thach, B.T. Apnea associated with regurgitation in infants. J. Pediatr. 1985, 106, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Tamana, S.K.; Smithson, L.; Ding, L.; Lau, A.; Chikuma, J.; Mariasine, J.; Lefebvre, D.L.; Subbarao, P.; Becker, A.B.; et al. Phenotypes of sleep-disordered breathing symptoms to two years of age based on age of onset and duration of symptoms. Sleep. Med. 2018, 48, 93–100. [Google Scholar] [CrossRef]
- Simons, J.P.; Greenberg, L.L.; Mehta, D.K.; Fabio, A.; Maguire, R.C.; Mandell, D.L. Laryngomalacia and swallowing function in children. Laryngoscope 2016, 126, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; McCoy, J.L.; Shaffer, A.D.; Tobey, A.B.; Dohar, J.E.; Simons, J.P.; Maguire, R.C.; Padia, R. Initiation of acid suppression therapy for laryngomalacia. Am. J. Otolaryngol. 2022, 43, 103434. [Google Scholar] [CrossRef]
- Tanphaichitr, A.; Tanphaichitr, P.; Apiwattanasawee, P.; Brockbank, J.; Rutter, M.J.; Simakajornboon, N. Prevalence and risk factors for central sleep apnea in infants with laryngomalacia. Otolaryngol. Head. Neck Surg. 2014, 150, 677–683. [Google Scholar] [CrossRef]
- Bonsignore, M.R. Obesity and obstructive sleep apnea. Handb. Exp. Pharmacol. 2022, 274, 181–201. [Google Scholar]
- Rifai, H.A.; Benoit, M.; El-Hakim, H. Secondary Airway Lesions in Laryngomalacia. Otolaryngol. Head. Neck Surg. 2010, 144, 268–273. [Google Scholar] [CrossRef]
- Dickson, J.M.; Richter, G.T.; Meinzen-Derr, J.; Rutter, M.J.; Thompson, D.M. Secondary Airway Lesions in Infants with Laryngomalacia. Ann. Otol. Rhinol. Laryngol. 2009, 118, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bredun, S.; Kotowski, M.; Mezydlo, J.; Szydlowski, J. Characteristics of Patients with Laryngomalacia: A Tertiary Referral Center Experience of 106 Cases. Diagnostics 2023, 13, 3180. [Google Scholar] [CrossRef] [PubMed]
- Krashin, E.; Ben-Ari, J.; Springer, C.; Derowe, A.; Avital, A.; Sivan, Y. Synchronous airway lesions in laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 501–507. [Google Scholar] [CrossRef] [PubMed]
- McSwiney, P.F.; Cavanagh, N.P.; Languth, P. Outcome in congenital stridor (laryngomalacia). Arch. Dis. Child. 1977, 52, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.; Dikkers, F.G.; Halmos, G.B. The groningen laryngomalacia classification system--based on systematic review and dynamic airway changes. Pediatr. Pulmonol. 2015, 50, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Rahbar, R.; Brigger, M.; Chan, K.; Cheng, A.; Daniel, S.J.; De Alarcon, A.; Garabedian, N.; Hart, C.; Hartnick, C.; et al. International Pediatric ORL Group (IPOG) laryngomalacia consensus recommendations. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Dunn, B.; Torre, C.; Sasaki, J.; Gonzales, R.; Liu, S.Y.; Chan, D.K.; Certal, V.; Cable, B.B. Supraglottoplasty for laryngomalacia with obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2016, 126, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Boudewyns, A.; Saldien, V.; Van de Heyning, P.; Verhulst, S. Drug-induced sedation endoscopy in surgically naïve infants and children with obstructive sleep apnea: Impact on treatment decision and outcome. Sleep. Breath. 2018, 22, 503–510. [Google Scholar] [CrossRef]
- Litman, R.S.; Weissend, E.E.; Shrier, D.A.; Ward, D.S. Morphologic changes in the upper airway of children during awakening from propofol administration. Anesthesiology 2002, 96, 607–611. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gunter, J.; Donnelly, L.F.; Wang, Y.; Nick, T.G.; Sadhasivam, S. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth. Analg. 2009, 109, 745–753. [Google Scholar] [CrossRef]
- Goldberg, S.; Shatz, A.; Picard, E.; Wexler, I.; Schwartz, S.; Swed, E.; Zilber, L.; Kerem, E. Endoscopic findings in children with obstructive sleep apnea: Effects of age and hypotonia. Pediatr. Pulmonol. 2005, 40, 205–210. [Google Scholar] [CrossRef]
- Boudewyns, A.; Abel, F.; Alexopoulos, E.; Evangelisti, M.; Kaditis, A.; Miano, S.; Villa, M.P.; Verhulst, S.L. Adenotonsillectomy to treat obstructive sleep apnea: Is it enough? Pediatr. Pulmonol. 2017, 52, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Olney, D.R.; Greinwald, J.H., Jr.; Smith, R.J.H.; Bauman, N.M. Laryngomalacia and its treatment. Laryngoscope 1999, 109, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Love, H.; Slaven, J.E.; Mitchell, R.M.; Bandyopadhyay, A. Outcomes of OSA in surgically naïve young children with and without DISE identified laryngomalacia. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110351. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.E.; Lawlor, C.M.; Wu, E.L.; Rodriguez, K.H. Utility of polysomnography in determination of laryngomalacia severity. Int. J. Pediatr. Otorhinolaryngol. 2017, 93, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ratanakorn, W.; Brockbank, J.; Ishman, S.; Tadesse, D.G.; Hossain, M.M.; Simakajornboon, N. The maturation changes of sleep-related respiratory abnormalities in infants with laryngomalacia. J. Clin. Sleep. Med. 2021, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Cotton, R.T.; Richardson, M.A. Congenital laryngeal anomalies. Otolaryngol. Clin. N. Am. 1981, 14, 203–218. [Google Scholar] [CrossRef]
- Sivan, Y.; Ben-Ari, J.; Schonfeld, T.M. Laryngomalacia: A cause for early near miss for SIDS. Int. J. Pediatr. Otorhinolaryngol. 1991, 21, 59–64. [Google Scholar] [CrossRef]
- Thorne, M.C.; Garetz, S.L. Laryngomalacia: Review and summary of current clinical practice in 2015. Paediatr. Respir. Rev. 2016, 17, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Holinger, L.D.; Konior, R.J. Surgical management of severe laryngomalacia. Laryngoscope 1989, 99, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Iglauer, S. Epiglottidectomy for the relief of congenital laryngeal stridor, with report of a case. Laryngoscope 1922, 32, 56–59. [Google Scholar] [CrossRef]
- Marcus, C.L.; Crockett, D.M.; Ward, S.L. Evaluation of epiglottoplasty as treatment for severe laryngomalacia. J. Pediatr. 1990, 117, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.C.; Inglis, A.F.; Mouzakes, J.; Carron, J.; Perkins, J.A. Laryngeal anatomic differences in pediatric patients with severe laryngomalacia. Arch. Otolaryngol. Head. Neck Surg. 2005, 131, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, F.; Pignataro, L.; Gaini, R.M.; Garavello, W. Drug induced sleep endoscopy in the decision-making process of children with obstructive sleep apnea. Sleep. Med. 2015, 16, 331–335. [Google Scholar] [CrossRef]
- Manickam, P.V.; Shott, S.R.; Boss, E.F.; Cohen, A.P.; Meinzen-Derr, J.K.; Amin, R.S.; Ishman, S.L. Systematic review of site of obstruction identification and non-CPAP treatment options for children with persistent pediatric obstructive sleep apnea. Laryngoscope 2016, 126, 491–500. [Google Scholar] [CrossRef]
- Preciado, D.; Zalzal, G. A systematic review of supraglottoplasty outcomes. Arch. Otolaryngol. Head. Neck Surg. 2012, 138, 718–721. [Google Scholar] [CrossRef]
- Friedman, M.; Wilson, M.; Lin, H.C.; Chang, H.W. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol. Head. Neck Surg. 2009, 140, 800–808. [Google Scholar] [CrossRef]
| Theory | Hypothesis | Evidence |
|---|---|---|
| Anatomic theory | Presence of supraglottic anatomic anomalies | Flaccid epiglottis, omega-shaped, posterior displacement of epiglottis short aryepiglottic fold, redundant arytenoideal mucosa |
| Cartilagineous theory | Alteration of the cartilaginous framework of larynx | Histological immaturity is seen in specimens of children who underwent supraglottoplasty |
| Neurologic theory | Possible lack of neuromotor coordination during breathing and swallowing. | Neurological comorbidities or prematurity are often associated. Weakness of laryngeal tone, presence of central sleep apneas. Possible spontaneous improvement during growth is reported. |
| Combination | Inflammation, airway pressure modification due to motor dysfunction could induce anatomical and cartilaginous modification of supraglottic anatomy. | GER seems to trigger all these factors (anatomic, cartilagineous, and neurological). Obesity. |
| Autor/Year | N | Mean Age | Type of Instrument | Follow-Up Period | Post-Op Complication | Revision Surgery | Mean AHI Pre-Op | Mean AHI Post-Op |
|---|---|---|---|---|---|---|---|---|
| Valera 2006 [16] | 7 | 6.8 mo. | Cold Knife | 3 mo. | 2 cases fail to extubation | 2 cases tracheostomy | 11.7 | 2.2 |
| Zafereo 2008 [17] | 10 | 4 mo. | Cold Knife | 11 weeks | No | NR | 12.2 | 4.2 |
| O’Connor 2009 [18] | 10 | 2.6 mo. | Cold Knife | 3 mo. | 1 case lung collapse | NR | 42.7 | 4.5 |
| Powitzky 2011 [19] | 20 | 3.9 mo. | CO2 laser | 9.5 mo. | NR | 1 case supraglottoplasty 6 cases adenotonsillectomy | 11.2 * | 4.7 * |
| Digoy 2012 [26] | 36 | 56 mo. | CO2 laser | 3 mo. | NR | NR | 13.3 | 4.1 |
| Chan 2012 [3] | 22 | 7.4 yrs. | CO2 laser | NR | No | NR | 10.4 | 2.9 |
| Mase 2015 [20] | 9 | 17 mo. | CO2 laser Cold knife Microdebrider | 155 days | NR | NR | 23.5 | 4.8 |
| Ching 2017 [21] | 12 | 13.1 mo. | CO2 laser, Cold knife BRA | 6 mo. | No | 1 case tracheostomy | 19.3 | 4 |
| Cortes 2019 [22] | 9 | 5.5 mo. | Cold knife | 1 mo. | 1 case foreign body reaction to epiglottopexy suture | No | 34.87 | 9.44 |
| Bhushan 2019 [23] | 41 | 1.3 yrs. | CO2 laser | 12.1 mo. | No | No | 26.62 | 7.27 |
| Verkest 2020 [24] | 44 | NR | Cold knife | 3 mo. | 4 cases, temporary feeding problems 2 cases fever/infection | NR | 8.9 * | 2.4 * |
| Casellas 2022 [25] | 30 | 13.28 mo. | NR | NR | NR | NR | MiO 3.98 MoO: 6.8 SO: 29.6 | MiO: 2.4 MoO: 2.2 SO: 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerritelli, L.; Migliorelli, A.; Larini, A.; Catalano, A.; Caranti, A.; Bianchini, C.; Ciorba, A.; Stomeo, F.; Vicini, C.; Pelucchi, S. Laryngomalacia and Obstructive Sleep Apnea in Children: From Diagnosis to Treatment. Children 2024, 11, 284. https://doi.org/10.3390/children11030284
Cerritelli L, Migliorelli A, Larini A, Catalano A, Caranti A, Bianchini C, Ciorba A, Stomeo F, Vicini C, Pelucchi S. Laryngomalacia and Obstructive Sleep Apnea in Children: From Diagnosis to Treatment. Children. 2024; 11(3):284. https://doi.org/10.3390/children11030284
Chicago/Turabian StyleCerritelli, Luca, Andrea Migliorelli, Alessio Larini, Andrea Catalano, Alberto Caranti, Chiara Bianchini, Andrea Ciorba, Francesco Stomeo, Claudio Vicini, and Stefano Pelucchi. 2024. "Laryngomalacia and Obstructive Sleep Apnea in Children: From Diagnosis to Treatment" Children 11, no. 3: 284. https://doi.org/10.3390/children11030284
APA StyleCerritelli, L., Migliorelli, A., Larini, A., Catalano, A., Caranti, A., Bianchini, C., Ciorba, A., Stomeo, F., Vicini, C., & Pelucchi, S. (2024). Laryngomalacia and Obstructive Sleep Apnea in Children: From Diagnosis to Treatment. Children, 11(3), 284. https://doi.org/10.3390/children11030284









