Cardiopulmonary Exercise Performance of Children Born Non-Extremely Preterm
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Demographics and History
2.3. Physical Activity Status
2.4. Spirometry
2.5. Cardiopulmonary Exercise Testing
2.6. Statistics
3. Results
4. Discussion
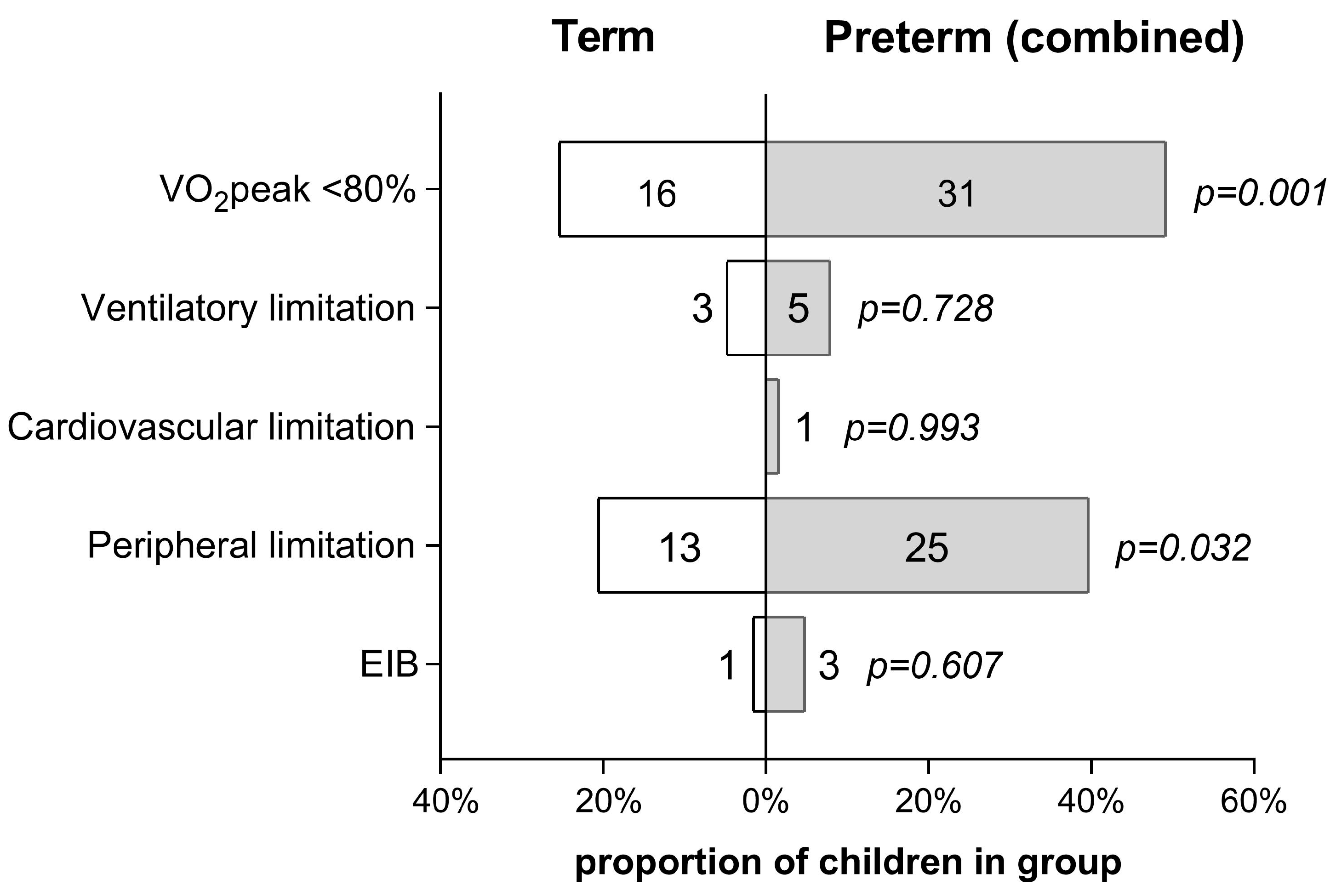
4.1. Effect of Cardiopulmonary Limiting Factors
4.2. Ventilatory Response to Exercise
4.3. Peripheral O2 Utilization
4.4. Mechanisms of Exercise Limitation
4.5. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, G.; Liu, J.; Liu, M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990–2019. JAMA Pediatr. 2022, 176, 787–796. [Google Scholar] [CrossRef]
- Pravia, C.I.; Benny, M. Long-term consequences of prematurity. Clevel. Clin. J. Med. 2020, 87, 759–767. [Google Scholar] [CrossRef]
- Ashorn, P.; Ashorn, U.; Muthiani, Y.; Aboubaker, S.; Askari, S.; Bahl, R.; Black, R.E.; Dalmiya, N.; Duggan, C.P.; Hofmeyr, G.J.; et al. Small vulnerable newborns-big potential for impact. Lancet 2023, 401, 1692–1706. [Google Scholar] [CrossRef]
- Simpson, S.J.; Logie, K.M.; O’Dea, C.A.; Banton, G.L.; Murray, C.; Wilson, A.C.; Pillow, J.J.; Hall, G.L. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 2017, 72, 702–711. [Google Scholar] [CrossRef]
- Welsh, L.; Kirkby, J.; Lum, S.; Odendaal, D.; Marlow, N.; Derrick, G.; Stocks, J.; EPICure Study Group. The EPICure study: Maximal exercise and physical activity in school children born extremely preterm. Thorax 2010, 65, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Bar-Yishay, E.; Prais, D.; Klinger, G.; Mei-Zahav, M.; Mussaffi, H.; Steuer, G.; Hananya, S.; Matyashuk, Y.; Gabarra, N.; et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest 2012, 142, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Rideau Batista Novais, A.; Matecki, S.; Jaussent, A.; Picot, M.C.; Amedro, P.; Guillaumont, S.; Picaud, J.C.; Cambonie, G. Hyperventilation during exercise in very low birth weight school-age children may implicate inspiratory muscle weakness. J. Pediatr. 2012, 160, 415–420.e1. [Google Scholar] [CrossRef] [PubMed]
- Clemm, H.; Røksund, O.; Thorsen, E.; Eide, G.E.; Markestad, T.; Halvorsen, T. Aerobic capacity and exercise performance in young people born extremely preterm. Pediatrics 2012, 129, e97–e105. [Google Scholar] [CrossRef]
- MacLean, J.E.; DeHaan, K.; Fuhr, D.; Hariharan, S.; Kamstra, B.; Hendson, L.; Adatia, I.; Majaesic, C.; Lovering, A.T.; Thompson, R.B.; et al. Altered breathing mechanics and ventilatory response during exercise in children born extremely preterm. Thorax 2016, 71, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Kriemler, S.; Keller, H.; Saigal, S.; Bar-Or, O. Aerobic and lung performance in premature children with and without chronic lung disease of prematurity. Clin. J. Sport Med. 2005, 15, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Pianosi, P.T.; Fisk, M. Cardiopulmonary exercise performance in prematurely born children. Pediatr. Res. 2000, 47, 653–658. [Google Scholar] [CrossRef]
- Joshi, S.; Powell, T.; Watkins, W.J.; Drayton, M.; Williams, E.M.; Kotecha, S. Exercise-induced bronchoconstriction in school-aged children who had chronic lung disease in infancy. J. Pediatr. 2013, 162, 813–818.e1. [Google Scholar] [CrossRef]
- O’Dea, C.A.; Logie, K.; Maiorana, A.; Wilson, A.C.; Pillow, J.J.; Banton, G.L.; Simpson, S.J.; Hall, G.L. Increased prevalence of expiratory flow limitation during exercise in children with bronchopulmonary dysplasia. ERJ Open Res. 2018, 4, 00048-2018. [Google Scholar] [CrossRef]
- Ruf, K.; Thomas, W.; Brunner, M.; Speer, C.P.; Hebestreit, H. Diverging effects of premature birth and bronchopulmonary dysplasia on exercise capacity and physical activity—A case control study. Respir. Res. 2019, 20, 260. [Google Scholar] [CrossRef]
- Edwards, M.O.; Kotecha, S.J.; Lowe, J.; Watkins, W.J.; Henderson, A.J.; Kotecha, S. Effect of preterm birth on exercise capacity: A systematic review and meta-analysis. Pediatr. Pulmonol. 2015, 50, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hochwald, O.; Bentur, L.; Haddad, Y.; Hanna, M.; Zucker-Toledano, M.; Mainzer, G.; Haddad, J.; Gur, M.; Borenstein-Levin, L.; Kugelman, A.; et al. Cardiopulmonary exercise testing in childhood in late preterms: Comparison to early preterms and term-born controls. J. Pers. Med. 2022, 12, 1547. [Google Scholar] [CrossRef]
- Weigelt, A.; Bleck, S.; Huebner, M.J.; Rottermann, K.; Waellisch, W.; Morhart, P.; Abu-Tair, T.; Dittrich, S.; Schoeffl, I. Impact of premature birth on cardiopulmonary function in later life. Eur. J. Pediatr. 2023, 182, 3265–3274. [Google Scholar] [CrossRef] [PubMed]
- Vrijlandt, E.J.; Gerritsen, J.; Boezen, H.M.; Grevink, R.G.; Duiverman, E.J. Lung function and exercise capacity in young adults born prematurely. Am. J. Respir. Crit. Care Med. 2006, 173, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modeling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2021; National Vital Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2023; Volume 72, Number 1. [CrossRef]
- NHS. Physical Activity Guidelines for Children and Young People. 2019. Available online: https://www.nhs.uk/live-well/exercise/physical-activity-guidelines-children-and-young-people/ (accessed on 22 December 2023).
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hell. J. Cardiol. 2009, 50, 283–294. [Google Scholar]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, S.; Davies, C.T.; Wozniak, E.; Barnes, C.A. Cardio-respiratory response to exercise in normal children. Clin. Sci. 1971, 40, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Paridon, S.M.; Alpert, B.S.; Boas, S.R.; Cabrera, M.E.; Caldarera, L.L.; Daniels, S.R.; Kimball, T.R.; Knilans, T.K.; Nixon, P.A.; Rhodes, J.; et al. Clinical stress testing in the pediatric age group: A statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Circulation 2006, 113, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Pianosi, P.T.; Smith, J.R. Ventilatory limitation of exercise in pediatric subjects evaluated for exertional dyspnea. Front. Physiol. 2019, 10, 20. [Google Scholar] [CrossRef]
- Burstein, D.S.; McBride, M.G.; Min, J.; Paridon, A.A.; Perelman, S.; Huffman, E.M.; O’Malley, S.; Del Grosso, J.; Groepenhoff, H.; Paridon, S.M.; et al. Normative values for cardiopulmonary exercise stress testing using ramp cycle ergometry in children and adolescents. J. Pediatr. 2021, 229, 61–69.e5. [Google Scholar] [CrossRef]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Lagiou, O.; Fouzas, S.; Lykouras, D.; Sinopidis, X.; Karatza, A.; Karkoulias, K.; Dimitriou, G.; Anthracopoulos, M.B. Exercise limitation in children and adolescents with mild-to-moderate asthma. J. Asthma Allergy 2022, 15, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Romer, L.; Rodman, J.; Miller, J.; Smith, C. Consequences of exercise-induced respiratory muscle work. Respir. Physiol. Neurobiol. 2006, 151, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Melamed, K.H.; Santos, M.; Oliveira, R.K.F.; Urbina, M.F.; Felsenstein, D.; Opotowsky, A.R.; Waxman, A.B.; Systrom, D.M. Unexplained exertional intolerance associated with impaired systemic oxygen extraction. Eur. J. Appl. Physiol. 2019, 119, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Nagashima, M.; Goto, M.; Nagano, Y.; Yokota, M.; Tauchi, N.; Nishibata, K. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996, 28, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K. The anaerobic threshold: Definition, physiological significance and identification. Adv. Cardiol. 1986, 35, 1–23. [Google Scholar] [PubMed]
| Term | Preterm (Combined) | Late Preterm (GA 340/7–366/7 Weeks) | Preterm (GA 290/7–336/7 Weeks) | |
|---|---|---|---|---|
| n | 63 | 63 | 34 | 29 |
| Male sex, n (%) | 40 (63.5) | 40 (63.5) | 21 (61.8) | 19 (65.5) |
| Age, years | 9.7 ± 2.1 (7–12) | 9.7 ± 2.1 (7–12) | 9.9 ± 1.9 (7–12) | 9.3 ± 2.5 (7–11) |
| Height, cm | 144.1 ± 8.0 (130–165) | 141.9 ± 7.8 (129–163) | 143 ± 7.3 (127–162) | 140.6 ± 8 (123–155) |
| Weight, kg | 48.5 ± 11.9 (32.5–79) | 44.0 ± 10.1 (31–75) | 47.6 ± 10 (33–75) | 39.8 ± 12 (31–65.4) |
| BMI, kg/m2 | 24.7 ± 4.4 (16.8–34) | 22.1 ± 3.5 (15.6–29.5) | 23.6 ± 3.8 (16.3–29.5) | 20.3 ± 3.3 (15.6–28) |
| Overweight, n (%) | 18 (28.6) | 11 (17.4) | 6 (17.6) | 5 (17.2) |
| Obese, n (%) | 7 (11.1) | 6 (9.5) | 4 (11.8) | 2 (6.9) |
| GA, weeks | 38.4 ± 1.1 (37–41) | 35.1 ± 2.2 (29–36,9) | 35.9 ± 1 (34–36.9) | 33 ± 1.2 (29–33.9) |
| NICU admission, n (%) | 2 (3.2) | 40 (63.5) | 11 (32.4) | 29 (100) |
| Need of MV, n (%) | 0 (0) | 14 (22.2) | 5 (14.7) | 9 (31) |
| Need of O2 support, n (%) a | 0 (0) | 30 (47.6) | 10 (29.4) | 20 (69) |
| Days of O2 support, n (%) | 0 (0) | 4 ± 5.8 (0.5–18) | 3.2 ± 4 (0.5–11) | 4.7 ± 6.2 (0.5–18) |
| History of asthma, n (%) b | 10 (15.9) | 15 (23.8) | 7 (20.6) | 8 (27.6) |
| Sports activities, n (%) c | 39 (61.9) | 43 (68.3) | 20 (58.8) | 23 (79.3) |
| IPAQ “active”, n (%) | 50 (79.4) | 44 (69.8) | 24 (70.5) | 20 (68.9) |
| Term | Preterm (Combined) | Late Preterm (GA 340/7–366/7 Weeks) | Preterm (GA 290/7–336/7 Weeks) | |
|---|---|---|---|---|
| FEV1, % pred. | 97.8 ± 8.2 (82–114) | 95.9 ± 9.3 (80–115) | 96.6 ± 9.6 (80–115) | 95 ± 9.2 (80–109) |
| FVC, % pred. | 94.4 ± 11.1 (80–108) | 93.2 ± 10.8 (73–108) | 94 ± 10.5 (73–108) | 92.3 ± 11.1 (73–101) |
| FEV1/FVC, % | 0.92 ± 0.04 (0.83–0.99) | 0.92 ± 0.04 (0.82–0.99) | 0.92 ± 0.04 (0.82–0.99) | 0.91 ± 0.03 (0.82–0.99) |
| FEF25–75, % pred. | 96.6 ± 12.5 (77–132) | 92.8 ± 11.4 (69–123) | 94 ± 12.1 (73–123) | 91.4 ± 10.2 (69–110) |
| Term | Preterm (Combined) | Late Preterm (GA 340/7–366/7 Weeks) | Preterm (GA 290/7–336/7 Weeks) | |
|---|---|---|---|---|
| RR (breaths per min) | 20.5 ± 3.7 (14–29) | 21.4 ± 4.1 (14–31) | 21.4 ± 3.9 (15–31) | 21.3 ± 4.5 (14–30) |
| VT (L) | 0.53 ± 0.05 (0.44–0.61) | 0.52 ± 0.06 (0.39–0.62) | 0.53 ± 0.05 (0.43–0.61) | 0.51 ± 0.06 (0.39–0.62) |
| VE (L/min) | 11.4 ± 2.3 (7.5–14.8) | 11.2 ± 2.2 (7.8–15.4) | 11.5 ± 2.3 (7.8–15.4) | 10.9 ± 2.1 (7.9–14.7) |
| HR (beats per min) | 84.9 ± 9.3 (71–102) | 84.9 ± 9.3 (71–102) | 85.2 ± 9.2 (74–103) | 87.5 ± 8.9 (75–105) |
| SpO2 (%) | 99.3 ± 0.7 (98–100) | 99.1 ± 0.8 (97–100) | 99.3 ± 0.8 (97–100) | 98.9 ± 0.8 (98–100) |
| RER | 0.81 ± 0.05 (0.73–0.9) | 0.82 ± 0.04 (0.75–0.91) | 0.82 ± 0.05 (0.75–0.9) | 0.83 ± 0.04 (0.77–0.91) |
| Term | Preterm (Combined) | Late Preterm (GA 340/7–366/7 Weeks) | Preterm (GA 290/7–336/7 Weeks) | |
|---|---|---|---|---|
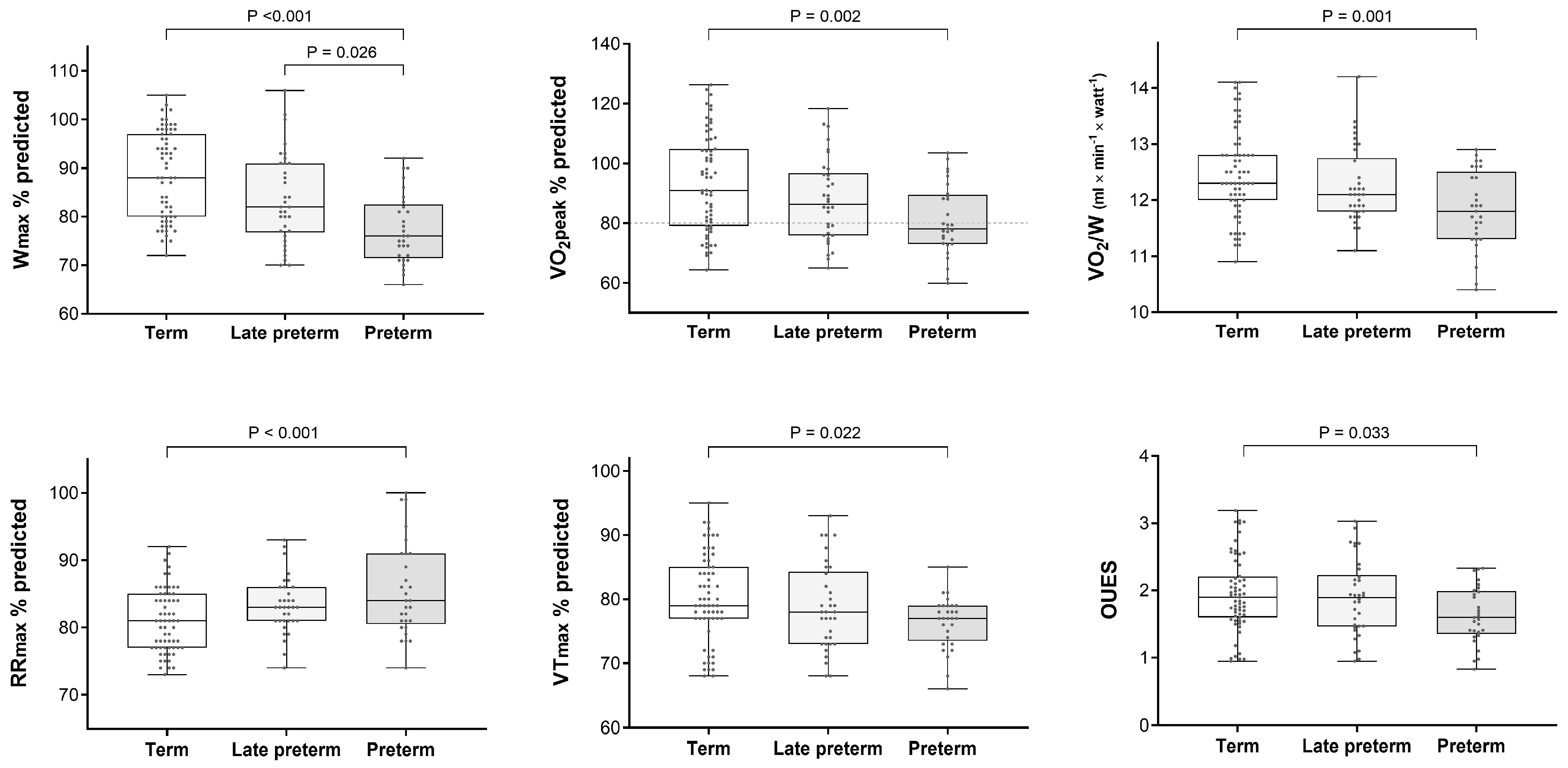
| Wmax, % pred. | 88.6 ± 9 (72–105) a,b | 80.8 ± 8.9 (66–106) a | 83.7 ± 9.3 (70–106) b | 77.4 ± 7.1 (66–92) b |
| HRmax, % pred. | 92.1 ± 2.8 (87–103) | 92.2 ± 3.3 (86–100) | 92.1 ± 3.3 (86–100) | 92.4 ± 3.4 (87–99) |
| VO2peak, % pred. | 93.5 ± 16.3 (64–126) c,d | 84.6 ± 13.3 (60–118) c | 87.8 ± 13.9 (65–118) d | 80.9 ± 11.7 (60–104) d |
| VO2 AT, % pred. | 92 ± 13.7 (58–123) | 89 ± 13 (59–112) | 89.7 ± 13.6 (59–112) | 88.2 ± 12.4 (60–109) |
| VO2/W, mL/min/Watt | 12.5 ± 0.8 (10.9–14.1) e,d | 12.1 ± 0.8 (10.4–14.2) e | 12.3 ± 0.7 (11.1–14.2) d | 11.8 ± 0.7 (10.4–12.9) d |
| O2 pulse, % pred. | 100 ± 6.6 (86–114) | 99.1 ± 7.1 (79–113) | 99 ± 5.8 (88–111) | 99.2 ± 8.5 (79–113) |
| RRmax, % pred. | 81 ± 4.7 (73–92) f,g | 84.3 ± 5.6 (74–100) f | 83.3 ± 4.1 (74–93) g | 85.5 ± 6.9 (74–100) g |
| VTmax, % pred. | 80.2 ± 6.8 (68–95) h,i | 77.7 ± 5.7 (66–93) h | 78.7 ± 6.7 (68–93) i | 76.4 ± 4 (66–85) i |
| VEmax, % pred. | 80.5 ± 5.8 (68–93) | 80.7 ± 5.2 (68–94) | 80.2 ± 4.9 (71–94) | 81.1 ± 5.7 (68–93) |
| Breathing reserve, % | 29.9 ± 10.7 (9–53) | 28.8 ± 9.7 (5–50) | 28.9 ± 10.3 (5–50) | 28.7 ± 9.1 (10–45) |
| VE/VCO2 slope, % pred. | 99.4 ± 10.4 (67.4–126.2) | 101.3 ± 11.9 (77–126.8) | 100.4 ± 12.3 (77–126.8) | 102.3 ± 11.6 (80–124.3) |
| OUES | 1.95 ± 0.56 (0.95–3.2) j | 1.82 ± 0.54 (0.83–3.03) | 1.89 ± 0.56 (0.95–3.03) j | 1.63 ± 0.42 (0.83–2.33) j |
| SpO2max | 98.4 ± 0.9 (97–100) | 98 ± 1 (96–100) | 98 ± 0.9 (97–100) | 97.9 ± 1.2 (96–100) |
| PETCO2max (mmHg) | 36.1 ± 2.7 (31–41) | 35.9 ± 3.1 (30–42) | 35.9 ± 3 (30–42) | 35.8 ± 2.6 (30–41) |
| RER | 1.16 ± 0.06 (1.06–1.26) | 1.15 ± 0.05 (1.06–1.22) | 1.15 ± 0.05 (1.06–1.23) | 1.15 ± 0.06 (1.05–1.22) |
| Borg scale | 7.3 ± 0.5 (6–9) | 7.2 ± 0.5 (6–9) | 7.2 ± 0.5 (6–9) | 7 ± 0.4 (6–8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouzas, S.; Nourloglou, A.; Vervenioti, A.; Karatza, A.; Anthracopoulos, M.B.; Dimitriou, G. Cardiopulmonary Exercise Performance of Children Born Non-Extremely Preterm. Children 2024, 11, 198. https://doi.org/10.3390/children11020198
Fouzas S, Nourloglou A, Vervenioti A, Karatza A, Anthracopoulos MB, Dimitriou G. Cardiopulmonary Exercise Performance of Children Born Non-Extremely Preterm. Children. 2024; 11(2):198. https://doi.org/10.3390/children11020198
Chicago/Turabian StyleFouzas, Sotirios, Aikaterini Nourloglou, Aggeliki Vervenioti, Ageliki Karatza, Michael B. Anthracopoulos, and Gabriel Dimitriou. 2024. "Cardiopulmonary Exercise Performance of Children Born Non-Extremely Preterm" Children 11, no. 2: 198. https://doi.org/10.3390/children11020198
APA StyleFouzas, S., Nourloglou, A., Vervenioti, A., Karatza, A., Anthracopoulos, M. B., & Dimitriou, G. (2024). Cardiopulmonary Exercise Performance of Children Born Non-Extremely Preterm. Children, 11(2), 198. https://doi.org/10.3390/children11020198








