Assessment of Sleep Quality in Adolescents with Overweight and Obesity Using the Adolescent Sleep Hygiene Scale (ASHS)
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Anthropometric Data and Laboratory Investigation
2.3. The Adolescent Sleep Hygiene Scale (ASHS) Questionnaire
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 August 2024).
- Presentation Maps|World Obesity Federation Global Obesity Observatory. Available online: https://data.worldobesity.org/maps/?area=trends&group=G&year=2020 (accessed on 3 April 2023).
- Patton, G.C.; Sawyer, S.M.; Santelli, J.S.; Ross, D.A.; Afifi, R.; Allen, N.B.; Arora, M.; Azzopardi, P.; Baldwin, W.; Bonell, C.; et al. Our Future: A Lancet Commission on Adolescent Health and Wellbeing. Lancet 2016, 387, 2423–2478. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2017; Volume 92, pp. 251–265. [Google Scholar] [CrossRef]
- Rogers, E.M.; Banks, N.F.; Jenkins, N.D.M. The Effects of Sleep Disruption on Metabolism, Hunger, and Satiety, and the Influence of Psychosocial Stress and Exercise: A Narrative Review. Diabetes Metab. Res. Rev. 2024, 40, e3667. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Valsamakis, G.; Chrousos, G.; Mastorakos, G. Stress, Female Reproduction and Pregnancy. Psychoneuroendocrinology 2019, 100, 48–57. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Rodriguez, D.; Schmitz, K.H.; Audrain-McGovern, J. Sleep Duration and Adolescent Obesity. Pediatrics 2013, 131, e1428. [Google Scholar] [CrossRef]
- Fatima, Y.; Doi, S.A.R.; Mamun, A.A. Sleep Quality and Obesity in Young Subjects: A Meta-Analysis. Obes. Rev. 2016, 17, 1154–1166. [Google Scholar] [CrossRef]
- Irwin, M.R. Why Sleep Is Important for Health: A Psychoneuroimmunology Perspective. Annu. Rev. Psychol. 2015, 66, 143. [Google Scholar] [CrossRef]
- Yang, C.; Yan, P.; Wu, X.; Zhang, W.; Cui, H.; Zhang, L.; Xu, Z.; Peng, S.; Tang, M.; Wang, Y.; et al. Associations of Sleep with Cardiometabolic Risk Factors and Cardiovascular Diseases: An Umbrella Review of Observational and Mendelian Randomization Studies. Sleep Med. Rev. 2024, 77, 101965. [Google Scholar] [CrossRef]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W.; Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016, 3, 67–104. [Google Scholar] [CrossRef]
- Oster, H.; Chaves, I. Effects of Healthy Lifestyles on Chronic Diseases: Diet, Sleep and Exercise. Nutrients 2023, 15, 4627. [Google Scholar] [CrossRef] [PubMed]
- Dutil, C.; Chaput, J.-P. Inadequate Sleep as a Contributor to Type 2 Diabetes in Children and Adolescents. Nutr. Diabetes 2017, 7, e266. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Patel, P.; Babu, J.R.; Wang, X.; Geetha, T. The Importance of Sleep in Overcoming Childhood Obesity and Reshaping Epigenetics. Biomedicines 2024, 12, 1334. [Google Scholar] [CrossRef] [PubMed]
- Lewien, C.; Genuneit, J.; Meigen, C.; Kiess, W.; Poulain, T. Sleep-Related Difficulties in Healthy Children and Adolescents. BMC Pediatr. 2021, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Trosman, I.; Ivanenko, A. Classification and Epidemiology of Sleep Disorders in Children and Adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2021, 30, 47–64. [Google Scholar] [CrossRef]
- Kansagra, S. Sleep Disorders in Adolescents. Pediatrics 2020, 145 (Suppl. S2), S204–S209. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s Updated Sleep Duration Recommendations: Final Report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Fadzil, A. Factors Affecting the Quality of Sleep in Children. Children 2021, 8, 122. [Google Scholar] [CrossRef]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Edwards, K.C.A.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- LeBourgeois, M.K.; Giannotti, F.; Cortesi, F.; Wolfson, A.R.; Harsh, J. The Relationship between Reported Sleep Quality and Sleep Hygiene in Italian and American Adolescents. Pediatrics 2005, 115, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Harsh, J.; Easley, A.; LeBourgeois, M.K. A Measure of Children’s Sleep Hygiene. Sleep 2002, 25, A316. [Google Scholar]
- Storfer-Isser, A.; Lebourgeois, M.K.; Harsh, J.; Tompsett, C.J.; Redline, S.; Redline, S. Psychometric Properties of the Adolescent Sleep Hygiene Scale (ASHS). J. Sleep Res. 2013, 22, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, V.; Kotsikogianni, D.; Tsitsika, A.K.; Vlachakis, D.; Chrousos, G.P.; Charmandari, E.; Bacopoulou, F. Validation of the Greek Version of the Adolescent Sleep Hygiene Scale (ASHS). EMBnet. J. 2021, 26, e979. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Frías, C.; Figueroa-Vega, N.; Malacara, J.M. Sleep Extension Increases the Effect of Caloric Restriction Over Body Weight and Improves the Chronic Low-Grade Inflammation in Adolescents With Obesity. J. Adolesc. Health 2020, 66, 575–581. [Google Scholar] [CrossRef]
- Miller, M.A.; Kruisbrink, M.; Wallace, J.; Ji, C.; Cappuccio, F.P. Sleep Duration and Incidence of Obesity in Infants, Children, and Adolescents: A Systematic Review and Meta-Analysis of Prospective Studies. Sleep 2018, 41, zsy018. [Google Scholar] [CrossRef]
- Overberg, J.; Kalveram, L.; Keller, T.; Krude, H.; Kühnen, P.; Wiegand, S. ARTICLE Interactions between Nocturnal Melatonin Secretion, Metabolism, and Sleeping Behavior in Adolescents with Obesity. Int. J. Obes. 2022, 46, 1051–1058. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Rifas-Shiman, S.L.; Quante, M.; Redline, S.; Oken, E.; Taveras, E.M. Chronotype, Social Jet Lag, and Cardiometabolic Risk Factors in Early Adolescence. JAMA Pediatr. 2019, 173, 1049–1057. [Google Scholar] [CrossRef]
- Chen, P.; Baylin, A.; Lee, J.; Dunietz, G.L.; Cantoral, A.; Tellez Rojo, M.M.; Peterson, K.E.; Jansen, E.C. The Association Between Sleep Duration and Sleep Timing and Insulin Resistance Among Adolescents in Mexico City. J. Adolesc. Health 2021, 69, 57–63. [Google Scholar] [CrossRef]
- Thumann Id, B.F.; Michels, N.; Felső, R.; Hunsberger, M.; Kaprio Id, J.; Moreno, L.A.; Siani, A.; Tornaritis, M.; Veidebaum, T.; De Henauw, S.; et al. Associations between Sleep Duration and Insulin Resistance in European Children and Adolescents Considering the Mediating Role of Abdominal Obesity. PLoS ONE 2020, 15, e0235049. [Google Scholar] [CrossRef]
- Javaheri, S.; Storfer-Isser, A.; Rosen, C.L.; Redline, S. The Association of Short and Long Sleep Durations with Insulin Sensitivity In Adolescents. J. Pediatr. 2011, 158, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Levitt Katz, L.E.; Brar, P.C.; Gallagher, P.R.; Berkowitz, R.I.; Brooks, L.J. Sleep Architecture and Glucose and Insulin Homeostasis in Obese Adolescents. Diabetes Care 2011, 34, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Y.; Li, G.; Han, L.; Li, Y.; Li, L.; Feng, D.; Wu, Y.; Xiao, X.; Li, M.; et al. Childhood Sleep Duration Modifies the Polygenic Risk for Obesity in Youth through Leptin Pathway: The Beijing Child and Adolescent Metabolic Syndrome Cohort Study. Int. J. Obes. 2019, 43, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Sun, J.; Wang, M.; Zhao, M.; Magnussen, C.G.; Xi, B. Association between Short Sleep Duration and Metabolic Syndrome in Chinese Children and Adolescents. Sleep Med. 2020, 74, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Matziridis, A.; Tsiptsios, D.; Manolis, A.; Ouranidis, A.; Triantafyllis, A.S.; Tsamakis, K.; Serdari, A.; Leontidou, E.; Terzoudi, A.; Dragioti, E.; et al. Sleep Insufficiency and Incident Diabetes Mellitus among Indigenous and Minority Populations in Greece. Sleep Sci. 2021, 14, 101. [Google Scholar] [CrossRef]
- Tsiptsios, D.; Leontidou, E.; Fountoulakis, P.N.; Ouranidis, A.; Matziridis, A.; Manolis, A.; Triantafyllis, A.S.; Tsamakis, K.; Serdari, A.; Terzoudi, A.; et al. Association between Sleep Insufficiency and Dyslipidemia: A Cross-Sectional Study among Greek Adults in the Primary Care Setting. Sleep Sci. 2022, 15, 49. [Google Scholar] [CrossRef]
- De Azevedo Abreu, G.; Barufaldi, L.A.; Bloch, K.V.; Szklo, M. A Systematic Review on Sleep Duration and Dyslipidemia in Adolescents:Understanding Inconsistencies. Arq. Bras. Cardiol. 2015, 105, 418. [Google Scholar] [CrossRef]
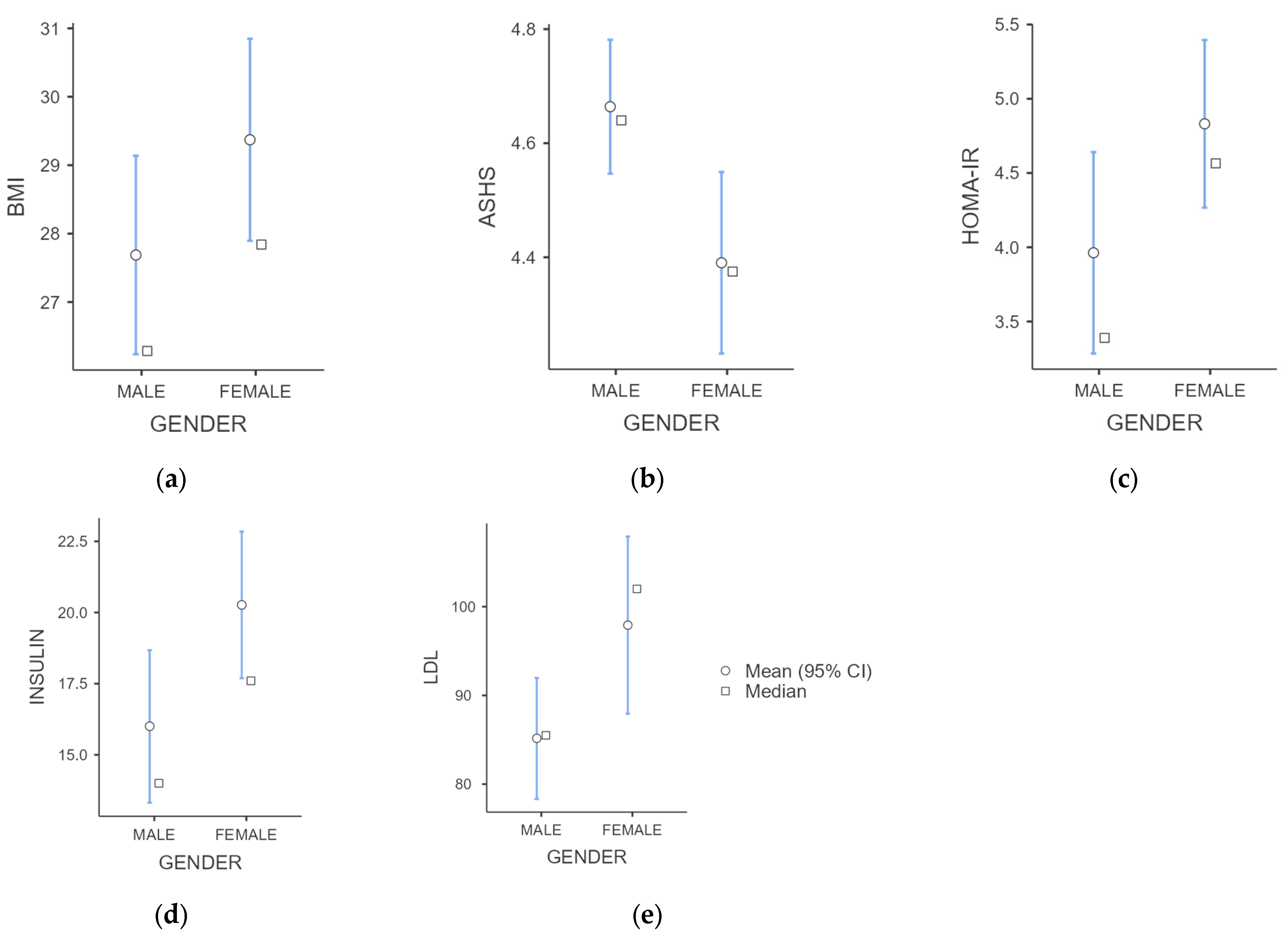


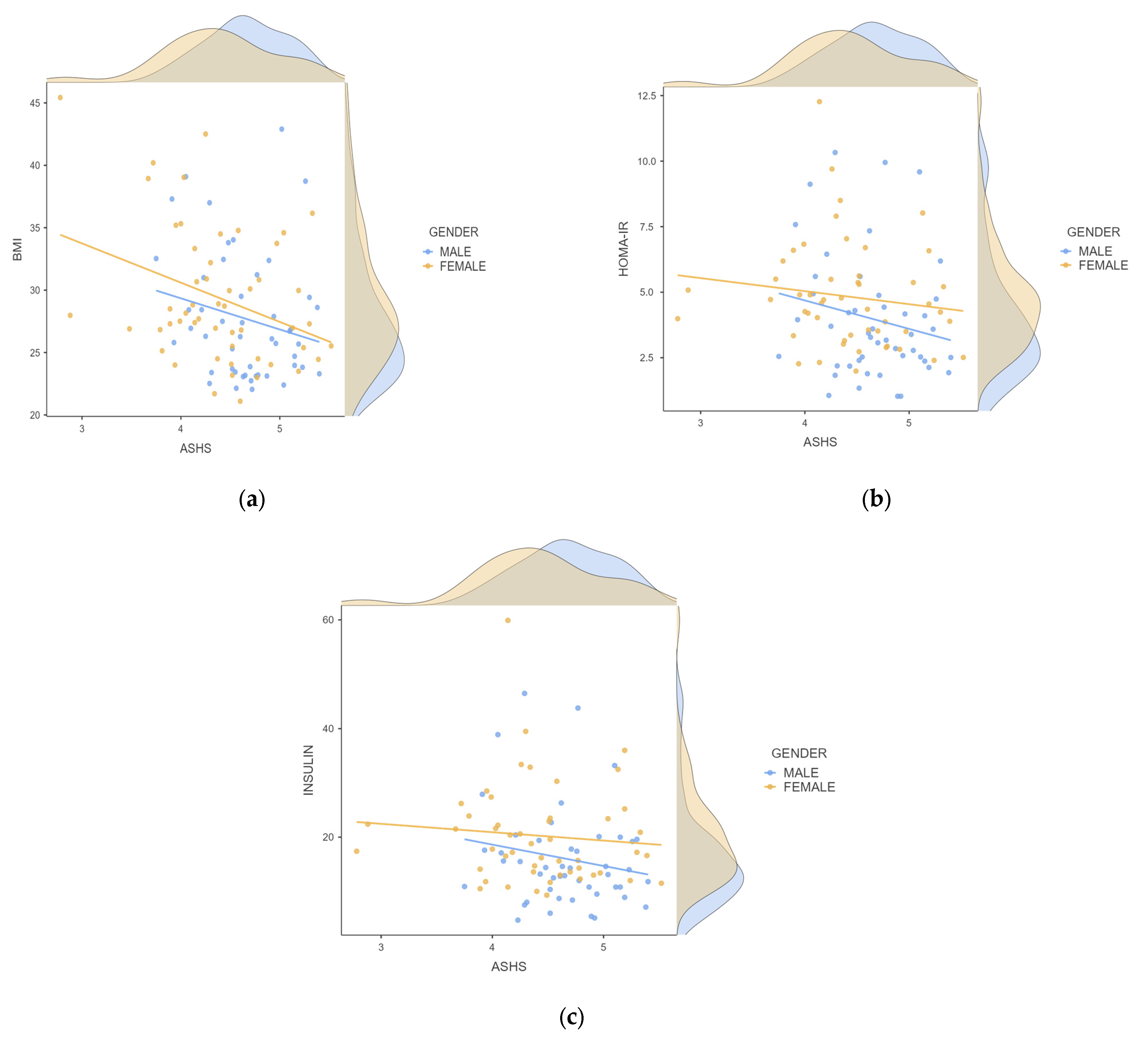
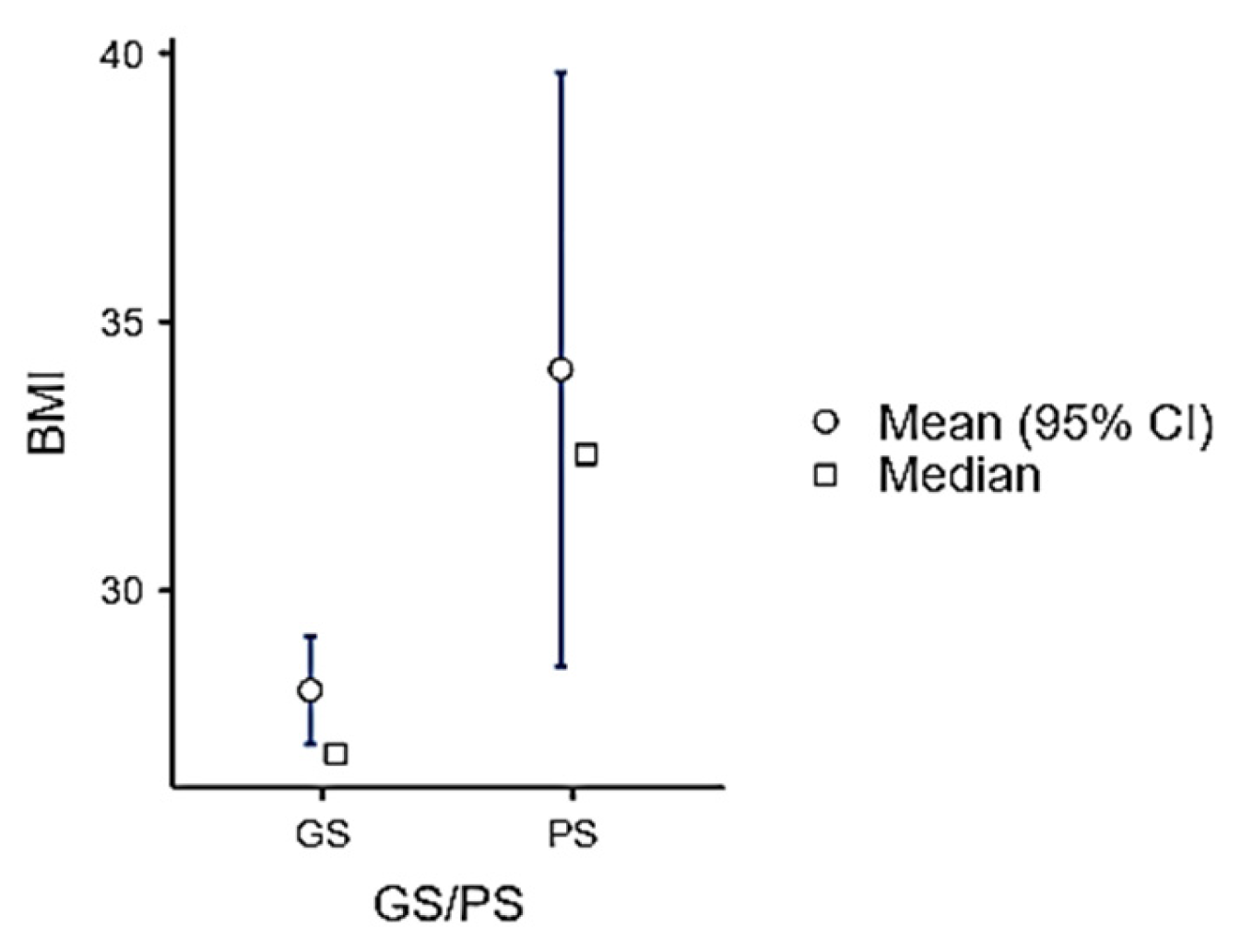
| Male (n = 48) | Female (n = 52) | p-Value | |
|---|---|---|---|
| Age (years) | 13.60 ± 1.88 | 14.00 ± 2.15 | 0.306 |
| BMI (kg/m2) | 27.69 ± 5.13 | 29.37 ± 5.43 | 0.049 |
| WC (cm) | 94.90 ± 12.26 | 92.86 ± 11.96 | 0.414 |
| Glucose (mg/dL) | 97.81 ± 13.33 | 94.60 ± 11.96 | 0.310 |
| Insulin (μIU/mL) | 16.00 ± 9.35 | 20.27 ± 9.30 | 0.003 |
| HbA1c% | 5.28 ± 0.27 | 5.25 ± 0.33 | 0.616 |
| HOMA-IR | 3.96 ± 2.37 | 4.83 ± 2.04 | 0.008 |
| TC (mg/dL) | 151.69 ± 29.90 | 148.76 ± 31.31 | 0.517 |
| HDL (mg/dL) | 50.68 ± 12.04 | 46.98 ± 10.95 | 0.095 |
| LDL (mg/dL) | 85.16 ± 24.04 | 97.92 ± 36.78 | 0.009 |
| TG (mg/dL) | 79.10 ± 38.11 | 83.29 ± 25.39 | 0.237 |
| non-HDL (mg/dL) | 101.00 ± 28.35 | 101.76 ± 28.97 | 0.785 |
| Uric acid (mg/dL) | 5.18 ± 1.19 | 5.08 ± 1.25 | 0.612 |
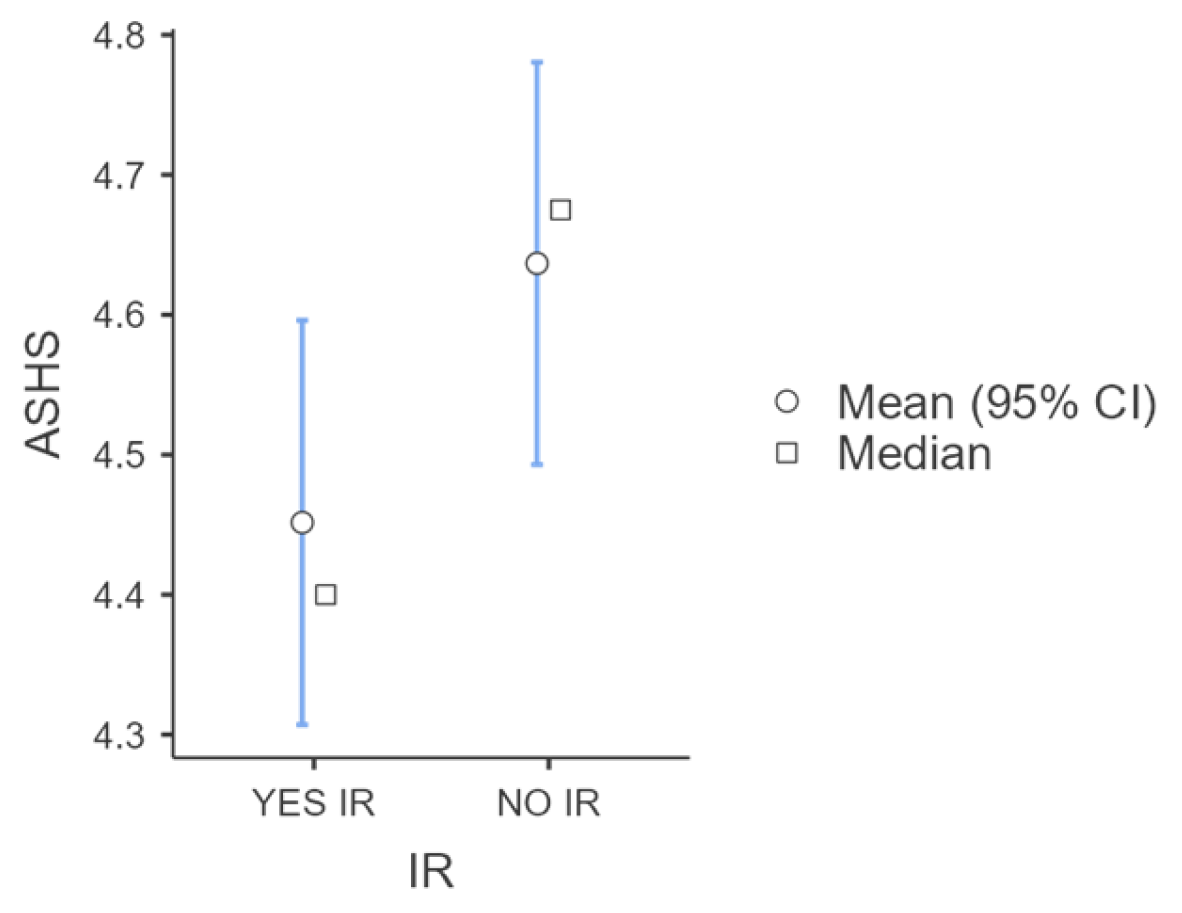
| ASHS Total Score | 4.66 ± 0.42 | 4.39 ± 0.59 | 0.010 |
| ASHS Score Total | Domain 1 Bed/Bedroom Sharing | Domain 2 Phycological | Domain 3 Cognitive | Domain 4 Emotional | Domain 5 Sleep Environment | Domain 6 Substances | Domain 7 Sleep Routine | Domain 8 Daytime Sleep | Domain 9 Sleep Stability | |
|---|---|---|---|---|---|---|---|---|---|---|
| Min | 2.78 | 1.00 | 3.00 | 1.00 | 1.00 | 2.75 | 5.50 | 4.00 | 1.00 | 1.00 |
| Max | 5.52 | 6.00 | 5.80 | 5.17 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Median | 4.52 | 6.00 | 4.80 | 3.67 | 4.33 | 5.25 | 6.00 | 5.00 | 5.00 | 3.50 |
| Mean | 4.52 | 5.05 | 4.84 | 3.57 | 4.41 | 4.95 | 5.52 | 4.20 | 4.66 | 3.49 |
| SD | 0.53 | 1.45 | 0.59 | 0.95 | 1.13 | 0.82 | 0.78 | 1.74 | 1.31 | 1.03 |
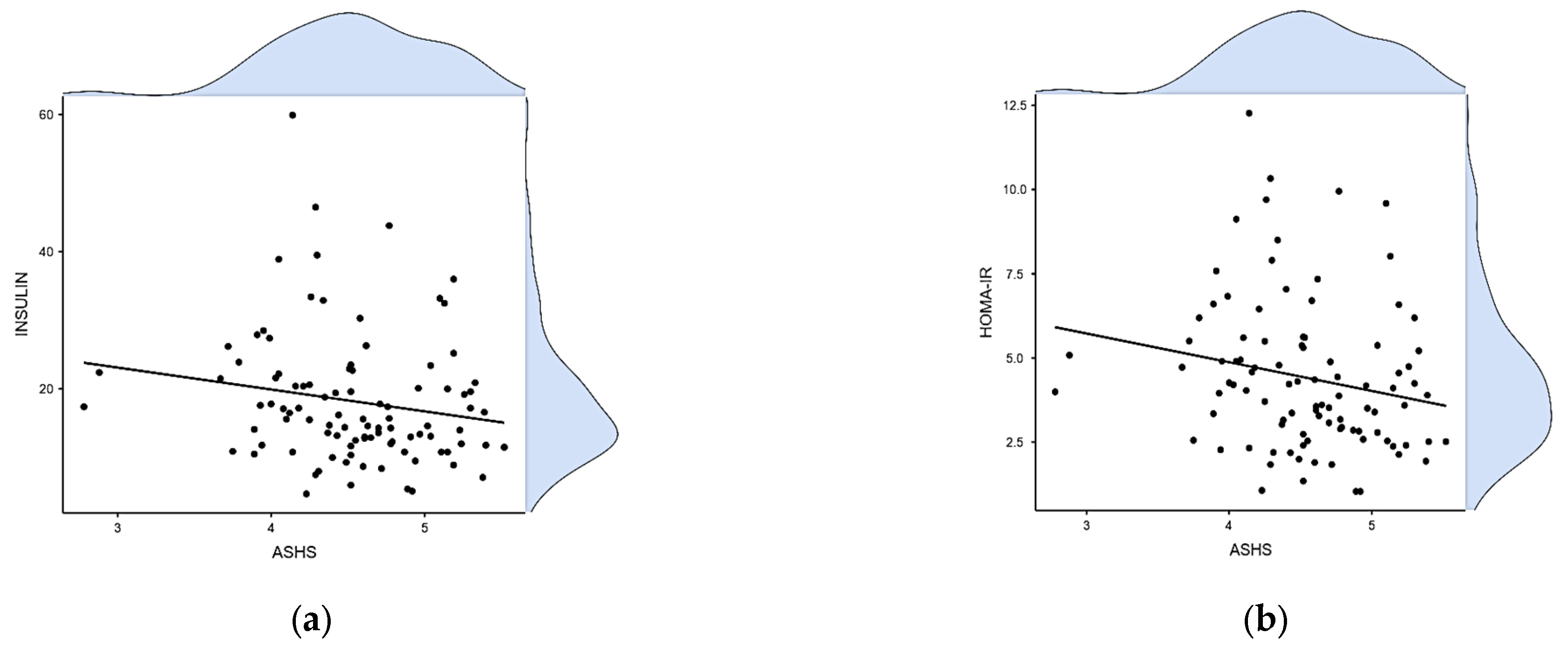
| ASHS | BMI | WC | Glucose | Insulin | HbA1c% | HOMA-IR | TC | HDL | LDL | TG | Non-HDL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASHS | - | |||||||||||
| - | ||||||||||||
| BMI | −0.306 | - | ||||||||||
| 0.002 | - | |||||||||||
| WC | −0.101 | 0.834 | - | |||||||||
| 0.330 | <0.001 | - | ||||||||||
| Glucose | 0.003 | 0.006 | 0.031 | - | ||||||||
| 0.977 | 0.950 | 0.765 | - | |||||||||
| Insulin | −0.224 | 0.355 | 0.224 | 0.055 | - | |||||||
| 0.027 | <0.001 | 0.032 | 0.589 | - | ||||||||
| HbA1c% | −0.095 | 0.016 | 0.116 | 0.137 | 0.092 | - | ||||||
| 0.359 | 0.879 | 0.272 | 0.182 | 0.379 | - | |||||||
| HOMA-IR | −0.260 | 0.369 | 0.274 | 0.324 | 0.888 | 0.087 | - | |||||
| 0.010 | <0.001 | 0.008 | 0.001 | <0.001 | 0.405 | - | ||||||
| TC | 0.202 | −0.089 | 0.021 | −0.234 | 0.083 | 0.031 | −0.003 | - | ||||
| 0.045 | 0.379 | 0.837 | 0.020 | 0.422 | 0.768 | 0.975 | - | |||||
| HDL | 0.061 | −0.347 | −0.272 | −0257 | −0.261 | −0.094 | −0.338 | 0.322 | - | |||
| 0.547 | <0.001 | 0.008 | 0.010 | 0.010 | 0.367 | <0.001 | 0.001 | - | ||||
| LDL | 0.087 | 0.069 | 0.096 | −0.147 | 0.209 | 0.062 | 0.160 | 0.865 | −0.033 | - | ||
| 0.392 | 0.498 | 0.355 | 0.144 | 0.040 | 0.547 | 0.117 | <0.001 | 0.745 | - | |||
| TG | 0.042 | 0.261 | 0.292 | −0.088 | 0.335 | −0.009 | 0.279 | 0.593 | −0.286 | 0.663 | - | |
| 0.682 | 0.009 | 0.004 | 0.388 | <0.001 | 0.932 | 0.006 | <0.001 | 0.004 | <0.001 | - | ||
| non-HDL | 0.154 | 0.057 | 0.144 | −0.138 | 0.192 | 0.070 | 0.136 | 0.919 | −0.040 | 0.944 | 0.747 | - |
| 0.128 | 0.578 | 0.167 | 0.172 | 0.061 | 0.498 | 0.187 | <0.001 | 0.696 | <0.001 | <0.001 | - | |
| Uric acid | 0.061 | 0.335 | 0.366 | 0.004 | 0.147 | −0.077 | 0.024 | −0.080 | −0.106 | −0.108 | 0.111 | −0.081 |
| 0.613 | 0.004 | 0.002 | 0.971 | 0.220 | 0.526 | 0.843 | 0.507 | 0.378 | 0.370 | 0.358 | 0.503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampani, E.; Kotanidou, E.P.; Tsinopoulou, V.R.; Sapountzi, E.; Ntouma, S.; Pavlou, E.; Galli-Tsinopoulou, A. Assessment of Sleep Quality in Adolescents with Overweight and Obesity Using the Adolescent Sleep Hygiene Scale (ASHS). Children 2024, 11, 1372. https://doi.org/10.3390/children11111372
Kampani E, Kotanidou EP, Tsinopoulou VR, Sapountzi E, Ntouma S, Pavlou E, Galli-Tsinopoulou A. Assessment of Sleep Quality in Adolescents with Overweight and Obesity Using the Adolescent Sleep Hygiene Scale (ASHS). Children. 2024; 11(11):1372. https://doi.org/10.3390/children11111372
Chicago/Turabian StyleKampani, Eleftheria, Eleni P. Kotanidou, Vasiliki Rengina Tsinopoulou, Evdoxia Sapountzi, Stergianna Ntouma, Evangelos Pavlou, and Assimina Galli-Tsinopoulou. 2024. "Assessment of Sleep Quality in Adolescents with Overweight and Obesity Using the Adolescent Sleep Hygiene Scale (ASHS)" Children 11, no. 11: 1372. https://doi.org/10.3390/children11111372
APA StyleKampani, E., Kotanidou, E. P., Tsinopoulou, V. R., Sapountzi, E., Ntouma, S., Pavlou, E., & Galli-Tsinopoulou, A. (2024). Assessment of Sleep Quality in Adolescents with Overweight and Obesity Using the Adolescent Sleep Hygiene Scale (ASHS). Children, 11(11), 1372. https://doi.org/10.3390/children11111372








