Refractory Chylothorax and Ventricular Hypertrophy Treated with Trametinib in a Patient with Noonan Syndrome: 18-Month Follow-Up
Abstract
1. Introduction
2. Materials and Methods
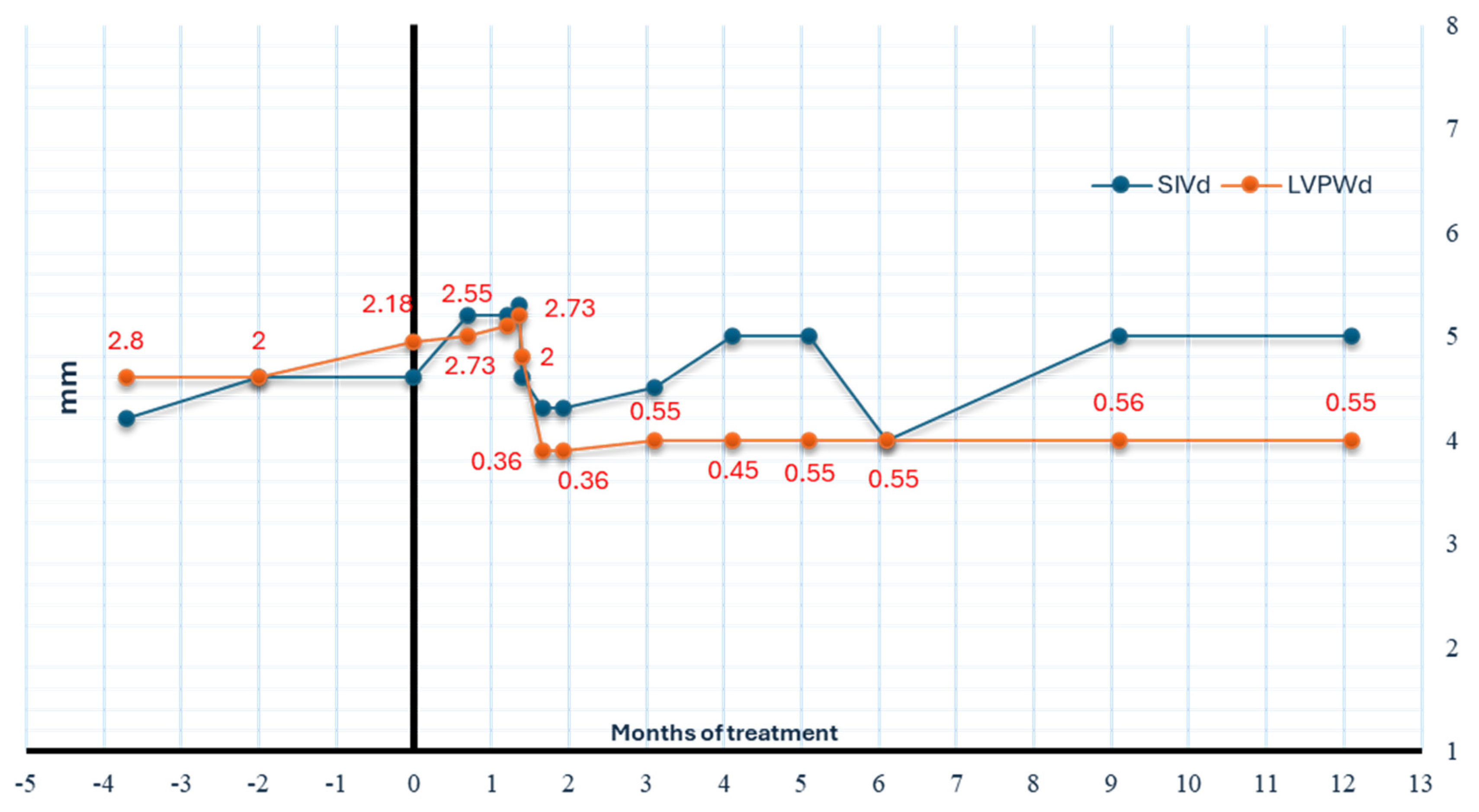
3. Off-Label Trametinib Treatment for Chylothorax
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tartaglia, M.; Mehler, E.L.; Goldberg, R.; Zampino, G.; Brunner, H.G.; Kremer, H.; van der Burgt, I.; Crosby, A.H.; Ion, A.; Jeffery, S.; et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001, 29, 465–468. [Google Scholar] [CrossRef]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef]
- Kouz, K.; Lissewski, C.; Spranger, S.; Mitter, D.; Riess, A.; Lopez-Gonzalez, V.; Luttgen, S.; Aydin, H.; von Deimling, F.; Evers, C.; et al. Genotype and phenotype in patients with Noonan syndrome and a RIT1 mutation. Genet. Med. 2016, 18, 1226–1234. [Google Scholar] [CrossRef]
- Johnston, J.J.; van der Smagt, J.J.; Rosenfeld, J.A.; Pagnamenta, A.T.; Alswaid, A.; Baker, E.H.; Blair, E.; Borck, G.; Brinkmann, J.; Craigen, W.; et al. Autosomal recessive Noonan syndrome associated with biallelic LZTR1 variants. Genet. Med. 2018, 20, 1175–1185. [Google Scholar] [CrossRef]
- van Der Burgt, I.; Brunner, H. Genetic heterogeneity in Noonan syndrome: Evidence for an autosomal recessive form. Am. J. Med. Genet. 2000, 94, 46–51. [Google Scholar] [CrossRef]
- Bhambhani, V.; Muenke, M. Noonan syndrome. Am. Fam. Physician 2014, 89, 37–43. [Google Scholar]
- Tartaglia, M.; Gelb, B.D. Noonan syndrome and related disorders: Genetics and pathogenesis. Annu. Rev. Genom. Hum. Genet. 2005, 6, 45–68. [Google Scholar] [CrossRef]
- Jongmans, M.C.; van der Burgt, I.; Hoogerbrugge, P.M.; Noordam, K.; Yntema, H.G.; Nillesen, W.M.; Kuiper, R.P.; Ligtenberg, M.J.; van Kessel, A.G.; van Krieken, J.H.; et al. Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation. Eur. J. Hum. Genet. 2011, 19, 870–874. [Google Scholar] [CrossRef]
- Shoji, Y.; Ida, S.; Niihori, T.; Aoki, Y.; Okamoto, N.; Etani, Y.; Kawai, M. Genotype-phenotype correlation analysis in Japanese patients with Noonan syndrome. Endocr. J. 2019, 66, 983–994. [Google Scholar] [CrossRef]
- Nakhaei-Rad, S.; Haghighi, F.; Bazgir, F.; Dahlmann, J.; Busley, A.V.; Buchholzer, M.; Kleemann, K.; Schanzer, A.; Borchardt, A.; Hahn, A.; et al. Molecular and cellular evidence for the impact of a hypertrophic cardiomyopathy-associated RAF1 variant on the structure and function of contractile machinery in bioartificial cardiac tissues. Commun. Biol. 2023, 6, 657. [Google Scholar] [CrossRef]
- Gelb, B.D.; Roberts, A.E.; Tartaglia, M. Cardiomyopathies in Noonan syndrome and the other RASopathies. Prog. Pediatr. Cardiol. 2015, 39, 13–19. [Google Scholar] [CrossRef]
- Yaoita, M.; Niihori, T.; Mizuno, S.; Okamoto, N.; Hayashi, S.; Watanabe, A.; Yokozawa, M.; Suzumura, H.; Nakahara, A.; Nakano, Y.; et al. Spectrum of mutations and genotype-phenotype analysis in Noonan syndrome patients with RIT1 mutations. Hum. Genet. 2016, 135, 209–222. [Google Scholar] [CrossRef]
- Stuurman, K.E.; Joosten, M.; van der Burgt, I.; Elting, M.; Yntema, H.G.; Meijers-Heijboer, H.; Rinne, T. Prenatal ultrasound findings of rasopathies in a cohort of 424 fetuses: Update on genetic testing in the NGS era. J. Med. Genet. 2019, 56, 654–661. [Google Scholar] [CrossRef]
- Sleutjes, J.; Kleimeier, L.; Leenders, E.; Klein, W.; Draaisma, J. Lymphatic Abnormalities in Noonan Syndrome Spectrum Disorders: A Systematic Review. Mol. Syndromol. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Witt, D.R.; Hoyme, H.E.; Zonana, J.; Manchester, D.K.; Fryns, J.P.; Stevenson, J.G.; Curry, C.J.; Hall, J.G. Lymphedema in Noonan syndrome: Clues to pathogenesis and prenatal diagnosis and review of the literature. Am. J. Med. Genet. 1987, 27, 841–856. [Google Scholar] [CrossRef]
- Joyce, S.; Gordon, K.; Brice, G.; Ostergaard, P.; Nagaraja, R.; Short, J.; Moore, S.; Mortimer, P.; Mansour, S. The lymphatic phenotype in Noonan and Cardiofaciocutaneous syndrome. Eur. J. Hum. Genet. 2016, 24, 690–696. [Google Scholar] [CrossRef]
- Swarts, J.W.; Kleimeier, L.E.R.; Leenders, E.; Rinne, T.; Klein, W.M.; Draaisma, J.M.T. Lymphatic anomalies during lifetime in patients with Noonan syndrome: Retrospective cohort study. Am. J. Med. Genet. A 2022, 188, 3242–3261. [Google Scholar] [CrossRef]
- Hoffner, B.; Benchich, K. Trametinib: A Targeted Therapy in Metastatic Melanoma. J. Adv. Pract. Oncol. 2018, 9, 741–745. [Google Scholar]
- O’Leary, C.G.; Andelkovic, V.; Ladwa, R.; Pavlakis, N.; Zhou, C.; Hirsch, F.; Richard, D.; O’Byrne, K. Targeting BRAF mutations in non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, 1119–1124. [Google Scholar] [CrossRef]
- Andelfinger, G.; Marquis, C.; Raboisson, M.J.; Theoret, Y.; Waldmuller, S.; Wiegand, G.; Gelb, B.D.; Zenker, M.; Delrue, M.A.; Hofbeck, M. Hypertrophic Cardiomyopathy in Noonan Syndrome Treated by MEK-Inhibition. J. Am. Coll. Cardiol. 2019, 73, 2237–2239. [Google Scholar] [CrossRef]
- Dori, Y.; Smith, C.; Pinto, E.; Snyder, K.; March, M.E.; Hakonarson, H.; Belasco, J. Severe Lymphatic Disorder Resolved with MEK Inhibition in a Patient with Noonan Syndrome and SOS1 Mutation. Pediatrics 2020, 146, e20200167. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Carli, D.; Giorgio, E.; Villar, A.M.; Cardaropoli, S.; Carbonara, C.; Campagnoli, M.F.; Galletto, P.; Palumbo, M.; Olivieri, S.; et al. MEK Inhibition in a Newborn with RAF1-Associated Noonan Syndrome Ameliorates Hypertrophic Cardiomyopathy but Is Insufficient to Revert Pulmonary Vascular Disease. Genes 2021, 13, 6. [Google Scholar] [CrossRef]
- Gordon, K.; Moore, M.; Van Zanten, M.; Pearce, J.; Itkin, M.; Madden, B.; Ratnam, L.; Mortimer, P.S.; Nagaraja, R.; Mansour, S. Case Report: Progressive central conducting lymphatic abnormalities in the RASopathies. Two case reports, including successful treatment by MEK inhibition. Front. Genet. 2022, 13, 1001105. [Google Scholar] [CrossRef]
- Nakano, T.A.; Rankin, A.W.; Annam, A.; Kulungowski, A.M.; McCallen, L.M.; Hill, L.R.; Chatfield, K.C. Trametinib for Refractory Chylous Effusions and Systemic Complications in Children with Noonan Syndrome. J. Pediatr. 2022, 248, 81–88.e1. [Google Scholar] [CrossRef]
- Leegaard, A.; Gregersen, P.A.; Nielsen, T.O.; Bjerre, J.V.; Handrup, M.M. Succesful MEK-inhibition of severe hypertrophic cardiomyopathy in RIT1-related Noonan Syndrome. Eur. J. Med. Genet. 2022, 65, 104630. [Google Scholar] [CrossRef] [PubMed]
- Hribernik, I.; Brooks, T.; Dunlop-Jones, A.; Bentham, J.R. Successful treatment of refractory chylothorax with MEK inhibitor trametinib in a child with Noonan syndrome: Case report. Eur. Heart J. Case Rep. 2023, 7, ytad190. [Google Scholar] [CrossRef]
- Leenders, E.; Kleimeier, L.E.R.; Weeke, L.C.; Coppens, C.H.; Klein, W.M.; Draaisma, J.M.T. Trametinib restores the central conducting lymphatic flow in a premature infant with Noonan syndrome. Clin. Case Rep. 2024, 12, e9164. [Google Scholar] [CrossRef]
- Kiamanesh, O.; Greenway, S.C.; Dicke, F.; Ballantyne, B.; Mitrovic, S.; McGrath, K.; White, J.A.; Kent, W.D.T.; Andelfinger, G. Treatment of RAF1-Related Obstructive Hypertrophic Cardiomyopathy by MEK Inhibition Using Trametinib. JACC Case Rep. 2024, 29, 102379. [Google Scholar] [CrossRef]
- Meisner, J.K.; Bradley, D.J.; Russell, M.W. Molecular Management of Multifocal Atrial Tachycardia in Noonan’s Syndrome with MEK1/2 Inhibitor Trametinib. Circ. Genom. Precis. Med. 2021, 14, e003327. [Google Scholar] [CrossRef]
- Lioncino, M.; Fusco, A.; Monda, E.; Colonna, D.; Sibilio, M.; Caiazza, M.; Magri, D.; Borrelli, A.C.; D’Onofrio, B.; Mazzella, M.L.; et al. Severe Lymphatic Disorder and Multifocal Atrial Tachycardia Treated with Trametinib in a Patient with Noonan Syndrome and SOS1 Mutation. Genes 2022, 13, 1503. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Gelb, B.D.; Yohe, M.E.; Wolf, C.; Andelfinger, G. New prospectives on treatment opportunities in RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 541–560. [Google Scholar] [CrossRef] [PubMed]
- Soto-Martinez, M.; Massie, J. Chylothorax: Diagnosis and management in children. Paediatr. Respir. Rev. 2009, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Anderson, B.K.; Mahajan, P.; Fernandes, C.J.; Margolin, J.F.; Iacobas, I. Sirolimus efficacy in the treatment of critically ill infants with congenital primary chylous effusions. Pediatr. Blood Cancer 2022, 69, e29510. [Google Scholar] [CrossRef]
- Tamaoka, S.; Osada, A.; Kin, T.; Arimitsu, T.; Hida, M. Midodrine, an Oral Alpha-1 Adrenoreceptor Agonist, Successfully Treated Refractory Congenital Chylous Pleural Effusion and Ascites in a Neonate. Chest 2021, 159, e189–e191. [Google Scholar] [CrossRef]
- Hebron, K.E.; Hernandez, E.R.; Yohe, M.E. The RASopathies: From pathogenetics to therapeutics. Dis. Models Mech. 2022, 15, dmm049107. [Google Scholar] [CrossRef]
- Casey, D.; Demko, S.; Sinha, A.; Mishra-Kalyani, P.S.; Shen, Y.L.; Khasar, S.; Goheer, M.A.; Helms, W.S.; Pan, L.; Xu, Y.; et al. FDA Approval Summary: Selumetinib for Plexiform Neurofibroma. Clin. Cancer Res. 2021, 27, 4142–4146. [Google Scholar] [CrossRef]
- Perreault, S.; Larouche, V.; Tabori, U.; Hawkin, C.; Lippe, S.; Ellezam, B.; Decarie, J.C.; Theoret, Y.; Metras, M.E.; Sultan, S.; et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer 2019, 19, 1250. [Google Scholar] [CrossRef]
- Infante, J.R.; Fecher, L.A.; Falchook, G.S.; Nallapareddy, S.; Gordon, M.S.; Becerra, C.; DeMarini, D.J.; Cox, D.S.; Xu, Y.; Morris, S.R.; et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 773–781. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, Y.; Ma, Y.C.; Zhang, Z.; Wu, J.W.; Du, S.; Li, W.Y.; Lu, X.H.; Ma, Y.; Wang, R.L. Exploring the mechanism of the potent allosteric inhibitor compound2 on SHP2 (WT) and SHP2(F285S) by molecular dynamics study. J. Mol. Graph. Model. 2021, 103, 107807. [Google Scholar] [CrossRef]
- Watanabe, D.; Hasebe, Y.; Kasai, S.; Shinohara, T.; Maebayashi, Y.; Katsumata, N.; Nemoto, A.; Naitoh, A. PTPN11 c.853T>C (p.Phe285Leu) mutation in Noonan syndrome with chylothorax. Nagoya J. Med. Sci. 2022, 84, 871–876. [Google Scholar] [PubMed]
- Kondyli, M.; Larouche, V.; Saint-Martin, C.; Ellezam, B.; Pouliot, L.; Sinnett, D.; Legault, G.; Crevier, L.; Weil, A.; Farmer, J.P.; et al. Trametinib for progressive pediatric low-grade gliomas. J. Neurooncol. 2018, 140, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Geoerger, B.; Moertel, C.; Whitlock, J.A.; Aerts, I.; Hargrave, D.; Osterloh, L.; Tan, E.; Choi, J.; Russo, M.; et al. Efficacy and Safety of Trametinib Monotherapy or in Combination with Dabrafenib in Pediatric BRAF V600-Mutant Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.R.; Pehlivan, K.C.; Milburn, M.; Yeh-Nayre, L.; Elster, J.; Crawford, J.R. Trametinib-based Treatment of Pediatric CNS Tumors: A Single Institutional Experience. J. Pediatr. Hematol. Oncol. 2020, 42, e730–e737. [Google Scholar] [CrossRef]



| Mutations | Heart Disease/ Arrhythmia | Lymphatic Abnormalities/ DCMRL | Other Clinical Features | ABT | Dose | Duration of Treatment | Results | Adverse Events | References |
|---|---|---|---|---|---|---|---|---|---|
| RIT1 (NM_006912.5): c.104G>C, p.Ser35Thr (het, d.n.) | HCM, SBS | Not reported | Prenatal dysplasia of all four valves, polyhydramnios, postnatal macrosomia, hypertelorism, and low-set ears. | 14 wks | 0.02 mg/kg/day | 17 mo | Regression of HCM, improvement SBS, bettering of growth. | None | Andelfinger et al., 2019 [20] |
| RIT1 (NM_006912.5): c.246T>G, p.Phe82Leu (het, d.n.) | Progressive biventricular HCM, SBS, pulmonary congestion with postcapillary pulmonary hypertension. | Bilateral chylothorax/ Not performed | Polyhydramnios | 13 wks | 0.02 mg/kg/day | 17 mo | Regression of HCM, improvement of SBS, regression of pulmonary edema, resolution of chylous effusions, bettering of growth. | None | Andelfinger et al., 2019 [20] |
| SOS1 (NM_005633): c.2536G>A, p.Glu846Lys (het) | Not reported | Persistent left chylothorax, protein-losing enteropathy with hypoalbuminemia, anemia, abnormal electrolytes levels/Diffusely abnormal central lymphatic system with retrograde mesenteric flow. Extensive perfusion of the left chest and lung. | Difficulty in gaining weight, chronic fatigue, Hashimoto thyroiditis, delayed puberty, growth hormone deficiency, Attention-deficit/ hyperactivity disorder. | 15 y | 0.01 mg/kg per dose (0.5 mg daily) for tolerability for 1 week; then 1 mg/day | 6 mo | Disappearance of the left sided pulmonary interstitial and intercostal lymphatic networks, reduction in retrograde mesenteric flow and resolution of the lymphatic leaks, bettering of growth | None | Dori et al., 2020 [21] |
| RAF1 (NM_002880.4): c.770C>T, p.Ser257Leu (het, d.n.) | Biventricular HCM, pulmonary valve stenosis atrial septal defect/ MAT, fibrillation and polymorphic ventricular tachycardia | Chylothorax/not performed | Not reported | 20 wks | 0.025 mg/kg/day | 6 mo | Resolution of MAT, Improvement of biventricular HCM | None | Meisner et al., 2021 [29] |
| RAF1 (NM_002880.4): c.770C>T, p.Ser257Leu (het, d.n.) | Severe biventricular obstructive HCM, dysplastic pulmonary valve, pulmonary hypertension | Not reported | Cerebral ventricular hemorrhage (Grade II) with post-hemorrhagic hydrocephalus | 47th day of life | 0.022 mg/kg/day | Death on day + 57. | Initially improvement in the overall clinical conditions, reduction in the septal thickness and nt-proBNP. Finally developed pulmonary hypertension and severe congestive heart failure | None | Mussa et al., 2021 [22] |
| BRAF (NM_004333.6): c.770A>G, p.Gln257Arg (het) | HCM | Genital and bilateral lower limb lymphoedema, bilateral refractory chylothorax, intestinal lymphangiectasia/ Moderate left to side pleural effusion | Feeding difficulties, mild to moderate learning difficulties, short stature, epilepsy | Never started | - | - | Death due to prolonged seizure resulting in cardiac arrest | None | Gordon et al., 2022 [23] |
| RIT1 (NM_006912.5): c.246T>G, p.Phe82Leu (het, d.n.) | Mild SBS | Progressive lower limb and genital lymphoedema, Chylothorax, pericardial effusion with cardiac tamponade, protein-losing enteropathy, ascites/ Reflux of lymphatic fluid into the penoscrotal mass, leakage of contrast bilaterally into the pleural effusions and into the small bowel mesentery | Not reported | 22 y | 1 mg/day for a month then 2 mg/day | 22 mo | Total resolution of ascites in 3 months and of pericardial effusion in 18 months, bettering of the nutritional state | Nausea, gastritis, constipation, eczema of the lower legs, iron deficiency anemia | Gordon et al., 2022 [23] |
| RIT1 (NM_006912.5): c.246T>G p.Phe82Leu (het) | Severe HCM with severe left ventricular outflow tract obstruction, SBS, mitral valve dysplasia with moderate stenosis and mild to moderate regurgitation. | Refractory bilateral chylous effusion/ dilated lymphatics along the bilateral iliac vessels. | Not reported | 4 y | 0.018 mg/kg/day initially every other day for 3 days, then every 36 h for 3 days, then daily | 1 y | No progression of HCM, improvement of respiratory function, lymphatic dysplasia and growth. | None | Nakano et al., 2022 [24] |
| SOS1 (NM_001382395.1): c.1322G>A, p.Cys441Tyr (het) | Moderate SBS | Ascites, bilateral chylous pleural effusion/ Markedly dilated and malformed lymphatics in the bilateral hila, intercostal spaces, and lungs extending to the left side of neck, to retroperitoneum and pelvis | Esophageal atresia with tracheoesophageal fistula | 3 mo | 0.026 mg/kg/day for 3 days, then every 36 h for 3 days, then daily | 1 y | Resolution of pleural effusion (no recurrence of chylous effusion), improvement of respiratory function, lymphatic dysplasia, SBS and growth | Grade 2 skin irritation | Nakano et al., 2022 [24] |
| PTPN11 (NM_001330437): c.854T>C, p.Phe285Ser (het) | Moderate to severe HCM, moderate SBS. | Persistent chylous effusion/ Dilated retroperitoneal and intrathoracic lymphatics with chylolymphatic reflux into the intercostal lymphatics, hila, and pulmonary parenchyma | Myeloproliferative disorder | 4 mo | 0.023 mg/kg/day for 3 days, then every 36 h for 3 days, and then daily | 1 y | Reducing of left ventricular mass, decreased NT-proBNP, slight improvement in the pulmonary valve gradient, bettering of respiratory function, chylous effusion and ascites Death several weeks after discharge for a sudden cardiac event. | None | Nakano et al., 2022 [24] |
| SOS1 (NM_005633.4): c.1655G>C, p.Arg552Thr (het) | Small muscular interventricular septal defects, mild SPS/ MAT | Bilateral pleural effusion | Not reported | 9 wks | 0.02 mg/kg/day | 4 mo | Resolution of pleural effusion and MAT (in 72 h), improvement in respiratory function | None | Lioncino et al., 2022 [30] |
| RIT1 (NM_006912.5): c.170C >G, p.Ala57Gly (het, d.n.) | HCM, Increased right ventricular outflow tract obstruction, SBS, dysplastic parachute mitral valve | Not reported | Not reported | 6 mo | 0.025 mg/kg/day, (maximal dosage of 0.04 mg/kg/day) | 30 mo | Normalizing of left ventricular wall thickness, decreasing of right ventricular outflow tract obstruction, bettering of growth | Dry and skin rash | Leegaard et al., 2022 [25] |
| RIT1 (NM_006912.5): not reported (het) | HCM, left ventricular outflow tract obstruction, severe right ventricular outflow tract obstruction, SBS | Bilateral chylothorax | Bowel perforation | 3 y and 4 mo | 0.032 mg/kg/day for 3 mo. | 11 mo | Reduction in left ventricular mass, no chylothorax recurrence | Eczema of the arms, legs, and trunk Increasing of stoma output | Hribernik et al., 2023 [26] |
| RAF1 (NM_002880.4): c.770C>T, p.Ser257Leu (het, d.n.) | HCM, increased LVOT gradient, apical aneurysm, ectatic coronary arteries | Not reported | Obstructive sleep apnea | 18 y | 0.01 mg/kg | 18 mo | Reduction LVOT gradient and N-terminal pro–B-type natriuretic peptide | Not reported | Kiamanesh, et al., 2024 [28] |
| PTPN11 (NM_001330437): c.922A>G, p.Asn308Asp (het) | None | Bilateral chylothorax/antegrade retroperitoneal lymph flow and dermal backflow, Hydrops | Not reported | 2 wks | 0.0125 mg/Kg/day for 2 wks, after 0.0125 mg/kg/day (max dose 0.018 mg/kg/day) | 18 mo | Resolution of chylothorax and hydrops | Eczema | Leenders et al., 2024 [27] |
| PTPN11 (NM_001330437): c.854T>C, p.Phe285Ser (het) | biventricular HCM, SBS | Right pleural chylous effusion | Ptosis, down-slanted palpebral fissures, marked webbed neck, epicanthic folds, low-set posteriorly rotated ears and prominent frontal bossing | 118th day of life | 0.025 mg/kg daily | 18 mo | Resolution of chylothorax, reduction in left ventricular wall thickness | Transient eczema | Our case report |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascarella, A.; Limongelli, G.; De Falco, A.; Minale, E.M.P.; Di Nardo, G.; Di Marco, G.M.; Zito Marinosci, G.; Olimpico, G.; Siani, P.; De Brasi, D. Refractory Chylothorax and Ventricular Hypertrophy Treated with Trametinib in a Patient with Noonan Syndrome: 18-Month Follow-Up. Children 2024, 11, 1342. https://doi.org/10.3390/children11111342
Pascarella A, Limongelli G, De Falco A, Minale EMP, Di Nardo G, Di Marco GM, Zito Marinosci G, Olimpico G, Siani P, De Brasi D. Refractory Chylothorax and Ventricular Hypertrophy Treated with Trametinib in a Patient with Noonan Syndrome: 18-Month Follow-Up. Children. 2024; 11(11):1342. https://doi.org/10.3390/children11111342
Chicago/Turabian StylePascarella, Antonia, Giuseppe Limongelli, Alessandro De Falco, Elia Marco Paolo Minale, Giangiacomo Di Nardo, Giovanni Maria Di Marco, Geremia Zito Marinosci, Giorgia Olimpico, Paolo Siani, and Daniele De Brasi. 2024. "Refractory Chylothorax and Ventricular Hypertrophy Treated with Trametinib in a Patient with Noonan Syndrome: 18-Month Follow-Up" Children 11, no. 11: 1342. https://doi.org/10.3390/children11111342
APA StylePascarella, A., Limongelli, G., De Falco, A., Minale, E. M. P., Di Nardo, G., Di Marco, G. M., Zito Marinosci, G., Olimpico, G., Siani, P., & De Brasi, D. (2024). Refractory Chylothorax and Ventricular Hypertrophy Treated with Trametinib in a Patient with Noonan Syndrome: 18-Month Follow-Up. Children, 11(11), 1342. https://doi.org/10.3390/children11111342







