Noninvasive Tools to Predict Necrotizing Enterocolitis in Infants with Congenital Heart Diseases: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Vital Parameter Monitoring
3.1.1. Heart Rate (HR), HR Variability, and HR Characteristics (HRCs)
3.1.2. Blood Pressure (BP)
3.1.3. Peripheral Oxygen Saturation (SpO2)
3.1.4. Pulsatility Index (PI)
3.2. Doppler Ultrasonography
3.3. Abdominal Near-Infrared Spectroscopy (aNIRS)
3.4. Abdominal Ultrasound (aUS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of Neonatal Necrotising Enterocolitis in High-Income Countries: A Systematic Review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis—A Systematic Review. J. Pediatr. 2020, 220, 86–92.e3. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Fanaroff, A.; Walsh, M. Fanaroff and Martin’s Neonatal-Perinatal Medicine, 2-Volume Set Diseases of the Fetus and Infant, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- McElhinney, D.B.; Hedrick, H.L.; Bush, D.M.; Pereira, G.R.; Stafford, P.W.; Gaynor, J.W.; Spray, T.L.; Wernovsky, G. Necrotizing Enterocolitis in Neonates with Congenital Heart Disease: Risk Factors and Outcomes. Pediatrics 2000, 106, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.K.; Christensen, R.D.; Henry, E.; Besner, G.E.; Baer, V.L.; Wiedmeier, S.E.; Stoddard, R.A.; Miner, C.A.; Burnett, J. Necrotizing Enterocolitis in Term Neonates: Data from a Multihospital Health-Care System. J. Perinatol. 2007, 27, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Polin, R.A.; Pollack, P.F.; Barlow, B.; Wigger, H.J.; Slovis, T.L.; Santulli, T.V.; Heird, W.C. Necrotizing Enterocolitis in Term Infants. J. Pediatr. 1976, 89, 460–462. [Google Scholar] [CrossRef]
- Behrman, R.E.; Stoll, B.J.; Kanto, W.P.; Glass, R.I.; Nahmias, A.J.; Brann, A.W. Epidemiology of Necrotizing Enterocolitis: A Case Control Study. J. Pediatr. 1980, 96, 447–451. [Google Scholar] [CrossRef]
- Palmer, S.R.; Biffin, A.; Gamsu, H.R. Outcome of Neonatal Necrotising Enterocolitis: Results of the BAPM/CDSC Surveillance Study, 1981–1984. Arch. Dis. Child. 1989, 64, 388–394. [Google Scholar] [CrossRef]
- Velazco, C.S.; Fullerton, B.S.; Hong, C.R.; Morrow, K.A.; Edwards, E.M.; Soll, R.F.; Jaksic, T.; Horbar, J.D.; Modi, B.P. Morbidity and Mortality among “Big” Babies Who Develop Necrotizing Enterocolitis: A Prospective Multicenter Cohort Analysis. J. Pediatr. Surg. 2018, 53, 108–112. [Google Scholar] [CrossRef]
- Burge, K.Y.; Gunasekaran, A.; Makoni, M.M.; Mir, A.M.; Burkhart, H.M.; Chaaban, H. Clinical Characteristics and Potential Pathogenesis of Cardiac Necrotizing Enterocolitis in Neonates with Congenital Heart Disease: A Narrative Review. J. Clin. Med. 2022, 11, 3987. [Google Scholar] [CrossRef]
- Fisher, J.G.; Bairdain, S.; Sparks, E.A.; Khan, F.A.; Archer, J.M.; Kenny, M.; Edwards, E.M.; Soll, R.F.; Modi, B.P.; Yeager, S.; et al. Serious Congenital Heart Disease and Necrotizing Enterocolitis in Very Low Birth Weight Neonates. J. Am. Coll. Surg. 2015, 220, 1018–1026.e14. [Google Scholar] [CrossRef]
- Lau, P.E.; Cruz, S.M.; Ocampo, E.C.; Nuthakki, S.; Style, C.C.; Lee, T.C.; Wesson, D.E.; Olutoye, O.O. Necrotizing Enterocolitis in Patients with Congenital Heart Disease: A Single Center Experience. J. Pediatr. Surg. 2018, 53, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.T.; McMahon, C.J.; James, A. Necrotizing Enterocolitis in Children with Congenital Heart Disease: A Literature Review. Pediatr. Cardiol. 2021, 42, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Carlo, W.F.; Kimball, T.R.; Michelfelder, E.C.; Border, W.L. Persistent Diastolic Flow Reversal in Abdominal Aortic Doppler-Flow Profiles Is Associated With an Increased Risk of Necrotizing Enterocolitis in Term Infants With Congenital Heart Disease. Pediatrics 2007, 119, 330–335. [Google Scholar] [CrossRef]
- Wienecke, L.M.; Cohen, S.; Bauersachs, J.; Mebazaa, A.; Chousterman, B.G. Immunity and Inflammation: The Neglected Key Players in Congenital Heart Disease? Heart Fail. Rev. 2022, 27, 1957–1971. [Google Scholar] [CrossRef]
- Lequier, L.L.; Nikaidoh, H.; Leonard, S.R.; Bokovoy, J.L.; White, M.L.; Scannon, P.J.; Giroir, B.P. Preoperative and Postoperative Endotoxemia in Children With Congenital Heart Disease. Chest 2000, 117, 1706–1712. [Google Scholar] [CrossRef]
- Pathan, N.; Burmester, M.; Adamovic, T.; Berk, M.; Ng, K.W.; Betts, H.; Macrae, D.; Waddell, S.; Paul-Clark, M.; Nuamah, R.; et al. Intestinal Injury and Endotoxemia in Children Undergoing Surgery for Congenital Heart Disease. Am. J. Respir. Crit. Care Med. 2011, 184, 1261–1269. [Google Scholar] [CrossRef]
- Granger, D.N.; Seifert, H.; Senchenkova, E. Intestinal Ischemia and Reperfusion: Consequences and Mechanisms. In PanVascular Medicine; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3535–3553. [Google Scholar]
- Miller, T.A.; Minich, L.L.; Lambert, L.M.; Joss-Moore, L.; Puchalski, M.D. Abnormal Abdominal Aorta Hemodynamics Are Associated with Necrotizing Enterocolitis in Infants With Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2014, 35, 616–621. [Google Scholar] [CrossRef]
- van der Heide, M.; Mebius, M.J.; Bos, A.F.; Roofthooft, M.T.R.; Berger, R.M.F.; Hulscher, J.B.F.; Kooi, E.M.W. Hypoxic/Ischemic Hits Predispose to Necrotizing Enterocolitis in (near) Term Infants with Congenital Heart Disease: A Case Control Study. BMC Pediatr. 2020, 20, 553. [Google Scholar] [CrossRef]
- Bubberman, J.M.; van Zoonen, A.; Bruggink, J.L.M.; van der Heide, M.; Berger, R.M.F.; Bos, A.F.; Kooi, E.M.W.; Hulscher, J.B.F. Necrotizing Enterocolitis Associated with Congenital Heart Disease: A Different Entity? J. Pediatr. Surg. 2019, 54, 1755–1760. [Google Scholar] [CrossRef]
- Becker, K.; Hornik, C.; Cotten, C.; Clark, R.; Hill, K.; Smith, P.; Lenfestey, R. Necrotizing Enterocolitis in Infants with Ductal-Dependent Congenital Heart Disease. Am. J. Perinatol. 2014, 32, 633–638. [Google Scholar] [CrossRef]
- Iannucci, G.J.; Oster, M.E.; Mahle, W.T. Necrotising Enterocolitis in Infants with Congenital Heart Disease: The Role of Enteral Feeds. Cardiol. Young 2013, 23, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Chen, X.; Wang, L.; Zhang, M.; Nappi, F.; Zampi, J.D.; Zheng, J.; Xu, Z.; Bao, N. Analysis of Clinical Features of Neonates with Congenital Heart Disease Who Develop Necrotizing Enterocolitis: A Retrospective Case-Control Study. Ann. Transl. Med. 2022, 10, 879. [Google Scholar] [CrossRef]
- Natarajan, G.; Anne, S.R.; Aggarwal, S. Outcomes of Congenital Heart Disease in Late Preterm Infants: Double Jeopardy? Acta Paediatr. 2011, 100, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Frid, G.; Reppucci, M.; Lum, T.; Paul, M.; Seiden, H.; Coakley, B.A. Comparison of Necrotizing Enterocolitis in Pre-Mature Infants vs. Term-Born Infants With Congenital Heart Disease. Front. Pediatr. 2021, 9, 802607. [Google Scholar] [CrossRef]
- Cozzi, C.; Aldrink, J.; Nicol, K.; Nicholson, L.; Cua, C. Intestinal Location of Necrotizing Enterocolitis among Infants with Congenital Heart Disease. J. Perinatol. 2013, 33, 783–785. [Google Scholar] [CrossRef]
- Siano, E.; Lauriti, G.; Ceccanti, S.; Zani, A. Cardiogenic Necrotizing Enterocolitis: A Clinically Distinct Entity from Classical Necrotizing Enterocolitis. Eur. J. Pediatr. Surg. 2019, 29, 014–022. [Google Scholar] [CrossRef]
- Motta, C.; Scott, W.; Mahony, L.; Koch, J.; Wyckoff, M.; Reisch, J.; Burchfield, P.J.; Brion, L.P. The Association of Congenital Heart Disease with Necrotizing Enterocolitis in Preterm Infants: A Birth Cohort Study. J. Perinatol. 2015, 35, 949–953. [Google Scholar] [CrossRef]
- Singh, G.K.; Fong, L.V.; Salmon, A.P.; Keeton, B.R. Study of Low Dosage Prostaglandin—Usages and Complications. Eur. Heart J. 1994, 15, 377–381. [Google Scholar] [CrossRef]
- Johnson, J.N.; Ansong, A.K.; Li, J.S.; Xu, M.; Gorentz, J.; Hehir, D.A.; del Castillo, S.L.; Lai, W.W.; Uzark, K.; Pasquali, S.K. Celiac Artery Flow Pattern in Infants with Single Right Ventricle Following the Norwood Procedure with a Modified Blalock-Taussig or Right Ventricle to Pulmonary Artery Shunt. Pediatr. Cardiol. 2011, 32, 479–486. [Google Scholar] [CrossRef]
- Jeffries, H.E.; Wells, W.J.; Starnes, V.A.; Wetzel, R.C.; Moromisato, D.Y. Gastrointestinal Morbidity After Norwood Palliation for Hypoplastic Left Heart Syndrome. Ann. Thorac. Surg. 2006, 81, 982–987. [Google Scholar] [CrossRef]
- del Castillo, S.L.; McCulley, M.E.; Khemani, R.G.; Jeffries, H.E.; Thomas, D.W.; Peregrine, J.; Wells, W.J.; Starnes, V.A.; Moromisato, D.Y. Reducing the Incidence of Necrotizing Enterocolitis in Neonates with Hypoplastic Left Heart Syndrome with the Introduction of an Enteral Feed Protocol. Pediatr. Crit. Care Med. 2009, 11, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Luce, W.A.; Schwartz, R.M.; Beauseau, W.; Giannone, P.J.; Boettner, B.L.; Cheatham, J.P.; Galantowicz, M.E.; Cua, C.L. Necrotizing Enterocolitis in Neonates Undergoing the Hybrid Approach to Complex Congenital Heart Disease. Pediatr. Crit. Care Med. 2011, 12, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Torres, A. Classic Norwood versus Sano Modification versus Hybrid Approach: Necrotizing Enterocolitis or No Necrotizing Enterocolitis? Pediatr. Crit. Care Med. 2011, 12, 109–110. [Google Scholar] [CrossRef]
- Thiene, G.; Frescura, C. Anatomical and Pathophysiological Classification of Congenital Heart Disease. Cardiovasc. Pathol. 2010, 19, 259–274. [Google Scholar] [CrossRef]
- Kumar, N.; Akangire, G.; Sullivan, B.; Fairchild, K.; Sampath, V. Continuous Vital Sign Analysis for Predicting and Preventing Neonatal Diseases in the Twenty-First Century: Big Data to the Forefront. Pediatr. Res. 2020, 87, 210–220. [Google Scholar] [CrossRef]
- Stone, M.L.; Tatum, P.M.; Weitkamp, J.-H.; Mukherjee, A.B.; Attridge, J.; McGahren, E.D.; Rodgers, B.M.; Lake, D.E.; Moorman, J.R.; Fairchild, K.D. Abnormal Heart Rate Characteristics before Clinical Diagnosis of Necrotizing Enterocolitis. J. Perinatol. 2013, 33, 847–850. [Google Scholar] [CrossRef]
- Doheny, K.K.; Palmer, C.; Browning, K.N.; Jairath, P.; Liao, D.; He, F.; Travagli, R.A. Diminished Vagal Tone Is a Predictive Biomarker of Necrotizing Enterocolitis-Risk in Preterm Infants. Neurogastroenterol. Motil. 2014, 26, 832–840. [Google Scholar] [CrossRef]
- Fairchild, K.; Lake, D. Cross-Correlation of Heart Rate and Oxygen Saturation in Very Low Birthweight Infants: Association with Apnea and Adverse Events. Am. J. Perinatol. 2018, 35, 463–469. [Google Scholar] [CrossRef]
- Leung, M.P.; Chau, K.; Hui, P.; Tam, A.Y.C.; Chan, F.L.; Lai, C.; Yeung, C. Necrotizing Enterocolitis in Neonates with Symptomatic Congenital Heart Disease. J. Pediatr. 1988, 113, 1044–1049. [Google Scholar] [CrossRef]
- Hebra, A.; Brown, M.F.; Hirschl, R.B.; McGeehin, K.; O’Neill, J.A.; Norwood, W.I.; Ross, A.J. Mesenteric Ischemia in Hypoplastic Left Heart Syndrome. J. Pediatr. Surg. 1993, 28, 606–611. [Google Scholar] [CrossRef]
- Choi, G.-J.; Song, J.; Kim, H.; Huh, J.; Kang, I.-S.; Chang, Y.S.; Sung, S.I.; Hyun, M.C. Development of Necrotizing Enterocolitis in Full-Term Infants with Duct Dependent Congenital Heart Disease. BMC Pediatr. 2022, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Niño, J.A. Perfusion Index Trajectory Analysis: Predictive Ability for Short-Term Complications in Preterm Newborns. Neonatol. Clin. Pediatr. 2021, 8, 067. [Google Scholar] [CrossRef] [PubMed]
- Hashem, R.H.; Mansi, Y.A.; Almasah, N.S.; Abdelghaffar, S. Doppler Ultrasound Assessment of the Splanchnic Circulation in Preterms with Neonatal Sepsis at Risk for Necrotizing Enterocolitis. J. Ultrasound 2017, 20, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Guang, Y.; Ying, D.; Sheng, Y.; Yiyong, F.; Jun, W.; Shuqiang, G.; Rong, J. Early Doppler Ultrasound in the Superior Mesenteric Artery and the Prediction of Necrotizing Enterocolitis in Preterm Neonates. J. Ultrasound Med. 2019, 38, 3283–3289. [Google Scholar] [CrossRef]
- Kempley, S.T.; Gamsu, H.R. Superior Mesenteric Artery Blood Flow Velocity in Necrotising Enterocolitis. Arch. Dis. Child. 1992, 67, 793–796. [Google Scholar] [CrossRef]
- Coombs, R.C.; Morgan, M.E.I.; Durbin, G.M.; Booth, I.W.; McNeish, A.S. Abnormal Gut Blood Flow Velocities in Neonates at Risk of Necrotising Enterocolitis. J. Pediatr. Gastroenterol. Nutr. 1992, 15, 13–19. [Google Scholar] [CrossRef]
- Deeg, K.-H.; Rupprecht, T.; Schmid, E. Doppler Sonographic Detection of Increased Flow Velocities in the Celiac Trunk and Superior Mesenteric Artery in Infants with Necrotizing Enterocolitis. Pediatr. Radiol. 1993, 23, 578–582. [Google Scholar] [CrossRef]
- Coombs, R.C.; Morgan, M.E.; Durbin, G.M.; Booth, I.W.; McNeish, A.S. Gut Blood Flow Velocities in the Newborn: Effects of Patent Ductus Arteriosus and Parenteral Indomethacin. Arch. Dis. Child. 1990, 65, 1067–1071. [Google Scholar] [CrossRef]
- Havranek, T.; Rahimi, M.; Hall, H.; Armbrecht, E. Feeding Preterm Neonates with Patent Ductus Arteriosus (PDA): Intestinal Blood Flow Characteristics and Clinical Outcomes. J. Matern.-Fetal Neonatal Med. 2015, 28, 526–530. [Google Scholar] [CrossRef]
- Harrison, A.M.; Davis, S.; Reid, J.R.; Morrison, S.C.; Arrigain, S.; Connor, J.T.; Temple, M.E. Neonates with Hypoplastic Left Heart Syndrome Have Ultrasound Evidence of Abnormal Superior Mesenteric Artery Perfusion before and after Modified Norwood Procedure. Pediatr. Crit. Care Med. 2005, 6, 445–447. [Google Scholar] [CrossRef]
- Cozzi, C.T.; Galantowicz, M.; Cheatham, J.P.; Nicholson, L.; Fernandez, R.; Backes, C.H.; McCaw, C.; Cua, C.L. Ultrasound Assessment of Mesenteric Blood Flow in Neonates with Hypoplastic Left Heart before and after Hybrid Palliation. Cardiol. Young 2015, 25, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.; Ho, M.H.K.; Cheng, V.Y.W. Mesenteric Blood Flow Response to Feeding after Systemic-to-Pulmonary Arterial Shunt Palliation. Ann. Thorac. Surg. 2003, 75, 947–951. [Google Scholar] [CrossRef] [PubMed]
- del Castillo, S.L.; Moromisato, D.Y.; Dorey, F.; Ludwick, J.; Starnes, V.A.; Wells, W.J.; Jeffries, H.E.; Wong, P.C. Mesenteric Blood Flow Velocities in the Newborn with Single-Ventricle Physiology: Modified Blalock-Taussig Shunt versus Right Ventricle-Pulmonary Artery Conduit. Pediatr. Crit. Care Med. 2006, 7, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, B.; Berger, G.; Fenstermaker, B.; Rowland, D.G.; Boettner, B.; Olshove, V.; Galantowicz, M.; Cheatham, J.P.; Cua, C.L. Echocardiographic Parameters That Predict Outcome in Aortic Atresia Patients Undergoing Comprehensive Stage II Procedure. Congenit. Heart Dis. 2010, 5, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, C.; Stines, J.; Luce, W.A.; Hayes, J.; Cheatham, J.P.; Galantowicz, M.; Cua, C.L. Diastolic Flow Parameters Are Not Sensitive in Predicting Necrotizing Enterocolitis in Patients Undergoing Hybrid Procedure. Congenit. Heart Dis. 2013, 8, 234–239. [Google Scholar] [CrossRef]
- Papneja, K.; Laks, J.; Szabo, A.B.; Grosse-Wortmann, L. Low Descending Aorta Flow Is Associated with Adverse Feeding Outcomes in Neonates with Small Left-Sided Structures. Int. J. Cardiovasc. Imaging 2021, 37, 269–273. [Google Scholar] [CrossRef]
- Pham, M.; Dean, P.; McCulloch, M.; Vergales, J. Association Between Pulsatility Index and the Development of Necrotizing Enterocolitis in Infants with Congenital Heart Disease. Pediatr. Cardiol. 2022, 43, 1156–1162. [Google Scholar] [CrossRef]
- Moore, J.E. Newer Monitoring Techniques to Determine the Risk of Necrotizing Enterocolitis. Clin. Perinatol. 2013, 40, 125–134. [Google Scholar] [CrossRef]
- Seager, E.; Longley, C.; Aladangady, N.; Banerjee, J. Measurement of Gut Oxygenation in the Neonatal Population Using Near-Infrared Spectroscopy: A Clinical Tool? Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 76–86. [Google Scholar] [CrossRef]
- Cerbo, R.M.; Maragliano, R.; Pozzi, M.; Strocchio, L.; Mostert, M.; Manzoni, P.; Stronati, M. Global Perfusion Assessment and Tissue Oxygen Saturation in Preterm Infants: Where Are We? Early Hum. Dev. 2013, 89, S44–S46. [Google Scholar] [CrossRef]
- van der Heide, M.; Dotinga, B.M.; Stewart, R.E.; Kalteren, W.S.; Hulscher, J.B.F.; Reijneveld, S.A.; Bos, A.F.; Kooi, E.M.W. Regional Splanchnic Oxygen Saturation for Preterm Infants in the First Week after Birth: Reference Values. Pediatr. Res. 2021, 90, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Cortez, J.; Gupta, M.; Amaram, A.; Pizzino, J.; Sawhney, M.; Sood, B.G. Noninvasive Evaluation of Splanchnic Tissue Oxygenation Using Near-Infrared Spectroscopy in Preterm Neonates. J. Matern.-Fetal Neonatal Med. 2011, 24, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Lazar, D.A.; Burrin, D.G.; Smith, E.O.; Magliaro, T.J.; Stark, A.R.; Brandt, M.L.; Zamora, I.J.; Sheikh, F.; Akinkuotu, A.C.; et al. Abdominal Near-Infrared Spectroscopy Measurements Are Lower in Preterm Infants at Risk for Necrotizing Enterocolitis. Pediatr. Crit. Care Med. 2014, 15, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Schat, T.E.; Schurink, M.; van der Laan, M.E.; Hulscher, J.B.F.; Hulzebos, C.V.; Bos, A.F.; Kooi, E.M.W. Near-Infrared Spectroscopy to Predict the Course of Necrotizing Enterocolitis. PLoS ONE 2016, 11, e0154710. [Google Scholar] [CrossRef]
- Schat, T.E.; van der Laan, M.E.; Schurink, M.; Hulscher, J.B.F.; Hulzebos, C.V.; Bos, A.F.; Kooi, E.M.W. Abdominal Near-Infrared Spectroscopy in Preterm Infants: A Comparison of Splanchnic Oxygen Saturation Measurements at Two Abdominal Locations. Early Hum. Dev. 2014, 90, 371–375. [Google Scholar] [CrossRef]
- Howarth, C.; Banerjee, J.; Leung, T.; Aladangady, N. Could Near Infrared Spectroscopy (NIRS) Be the New Weapon in Our Fight against Necrotising Enterocolitis? Front. Pediatr. 2022, 10, 1024566. [Google Scholar] [CrossRef]
- Bailey, S.; Hendricks-Munoz, K.; Mally, P. Cerebral, Renal, and Splanchnic Tissue Oxygen Saturation Values in Healthy Term Newborns. Am. J. Perinatol. 2013, 31, 339–344. [Google Scholar] [CrossRef]
- Bailey, S.M.; Hendricks-Muñoz, K.D.; Mally, P. Splanchnic-Cerebral Oxygenation Ratio (SCOR) Values in Healthy Term Infants as Measured by near-Infrared Spectroscopy (NIRS). Pediatr. Surg. Int. 2013, 29, 591–595. [Google Scholar] [CrossRef]
- Le Bouhellec, J.; Prodhomme, O.; Mura, T.; Jacquot, A.; Combes, C.; Gamon, L.; Durand, S.; Filleron, A.; Cambonie, G. Near-Infrared Spectroscopy: A Tool for Diagnosing Necrotizing Enterocolitis at Onset of Symptoms in Preterm Neonates with Acute Gastrointestinal Symptoms? Am. J. Perinatol. 2021, 38, e299–e308. [Google Scholar] [CrossRef]
- Adams, P.S.; Vargas, D.; Baust, T.; Saenz, L.; Koh, W.; Blasiole, B.; Callahan, P.M.; Phadke, A.S.; Nguyen, K.N.; Domnina, Y.; et al. Associations of Perioperative Renal Oximetry Via Near-Infrared Spectroscopy, Urinary Biomarkers, and Postoperative Acute Kidney Injury in Infants After Congenital Heart Surgery. Pediatr. Crit. Care Med. 2019, 20, 27–37. [Google Scholar] [CrossRef]
- Hickok, R.L.; Spaeder, M.C.; Berger, J.T.; Schuette, J.J.; Klugman, D. Postoperative Abdominal NIRS Values Predict Low Cardiac Output Syndrome in Neonates. World J. Pediatr. Congenit. Heart Surg. 2016, 7, 180–184. [Google Scholar] [CrossRef]
- Stapleton, G.E.; Eble, B.K.; Dickerson, H.A.; Andropoulos, D.B.; Chang, A.C. Mesenteric Oxygen Desaturation in an Infant with Congenital Heart Disease and Necrotizing Enterocolitis. Tex. Heart Inst. J. 2007, 34, 442–444. [Google Scholar]
- Kim, J.-W.; Shin, W.-J.; Park, I.; Chung, I.-S.; Gwak, M.; Hwang, G.-S. Splanchnic Oxygen Saturation Immediately After Weaning From Cardiopulmonary Bypass Can Predict Early Postoperative Outcomes in Children Undergoing Congenital Heart Surgery. Pediatr. Cardiol. 2014, 35, 587–595. [Google Scholar] [CrossRef]
- Kaufman, J.; Almodovar, M.C.; Zuk, J.; Friesen, R.H. Correlation of Abdominal Site Near-Infrared Spectroscopy with Gastric Tonometry in Infants Following Surgery for Congenital Heart Disease*. Pediatr. Crit. Care Med. 2008, 9, 62–68. [Google Scholar] [CrossRef]
- Bhalala, U.S.; Nishisaki, A.; McQueen, D.; Bird, G.L.; Morrison, W.E.; Nadkarni, V.M.; Nathan, M.; Starr, J.P. Change in Regional (Somatic) near-Infrared Spectroscopy Is Not a Useful Indictor of Clinically Detectable Low Cardiac Output in Children after Surgery for Congenital Heart Defects. Pediatr. Crit. Care Med. 2012, 13, 529–534. [Google Scholar] [CrossRef]
- DeWitt, A.G.; Charpie, J.R.; Donohue, J.E.; Yu, S.; Owens, G.E. Splanchnic Near-Infrared Spectroscopy and Risk of Necrotizing Enterocolitis After Neonatal Heart Surgery. Pediatr. Cardiol. 2014, 35, 1286–1294. [Google Scholar] [CrossRef]
- van Druten, J.; Khashu, M.; Chan, S.S.; Sharif, S.; Abdalla, H. Abdominal Ultrasound Should Become Part of Standard Care for Early Diagnosis and Management of Necrotising Enterocolitis: A Narrative Review. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F551–F559. [Google Scholar] [CrossRef]
- Singh, Y.; Tissot, C.; Fraga, M.V.; Yousef, N.; Cortes, R.G.; Lopez, J.; Sanchez-de-Toledo, J.; Brierley, J.; Colunga, J.M.; Raffaj, D.; et al. International Evidence-Based Guidelines on Point of Care Ultrasound (POCUS) for Critically Ill Neonates and Children Issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit. Care 2020, 24, 65. [Google Scholar] [CrossRef]
- Lazow, S.P.; Tracy, S.A.; Estroff, J.A.; Parad, R.B.; Castro-Aragon, I.M.; Fujii, A.M.; Staffa, S.J.; Zurakowski, D.; Chen, C. A Role for Abdominal Ultrasound in Discriminating Suspected Necrotizing Enterocolitis in Congenital Heart Disease Patients. Pediatr. Surg. Int. 2022, 38, 225–233. [Google Scholar] [CrossRef]
- Abraham, B.P.; Sachdeva, R.; Vyas, P.G.; Thomas Collins, R. An Unusual Presentation of Necrotizing Enterocolitis on an Echocardiogram. Pediatr. Cardiol. 2012, 33, 1427–1429. [Google Scholar] [CrossRef]
- Müller, B.; Stahr, N.; Knirsch, W.; Hoigné, I.; Frey, B. Bubbles in the Heart as First Sign of Gastric Pneumatosis. Eur. J. Pediatr. 2014, 173, 1587–1589. [Google Scholar] [CrossRef]
- Cheong, C.Y.; Ong, G.Y.; Chor, Y.K. Bedside Ultrasound Detection of Systemic Air Embolism Secondary to Fulminant Necrotizing Enterocolitis in a Neonate With Congenital Heart Disease: A Case Report. Cureus 2022, 14, e22970. [Google Scholar] [CrossRef]
- Diez, S.; Tielesch, L.; Weiss, C.; Halbfass, J.; Müller, H.; Besendörfer, M. Clinical Characteristics of Necrotizing Enterocolitis in Preterm Patients With and Without Persistent Ductus Arteriosus and in Patients With Congenital Heart Disease. Front. Pediatr. 2020, 8, 257. [Google Scholar] [CrossRef]

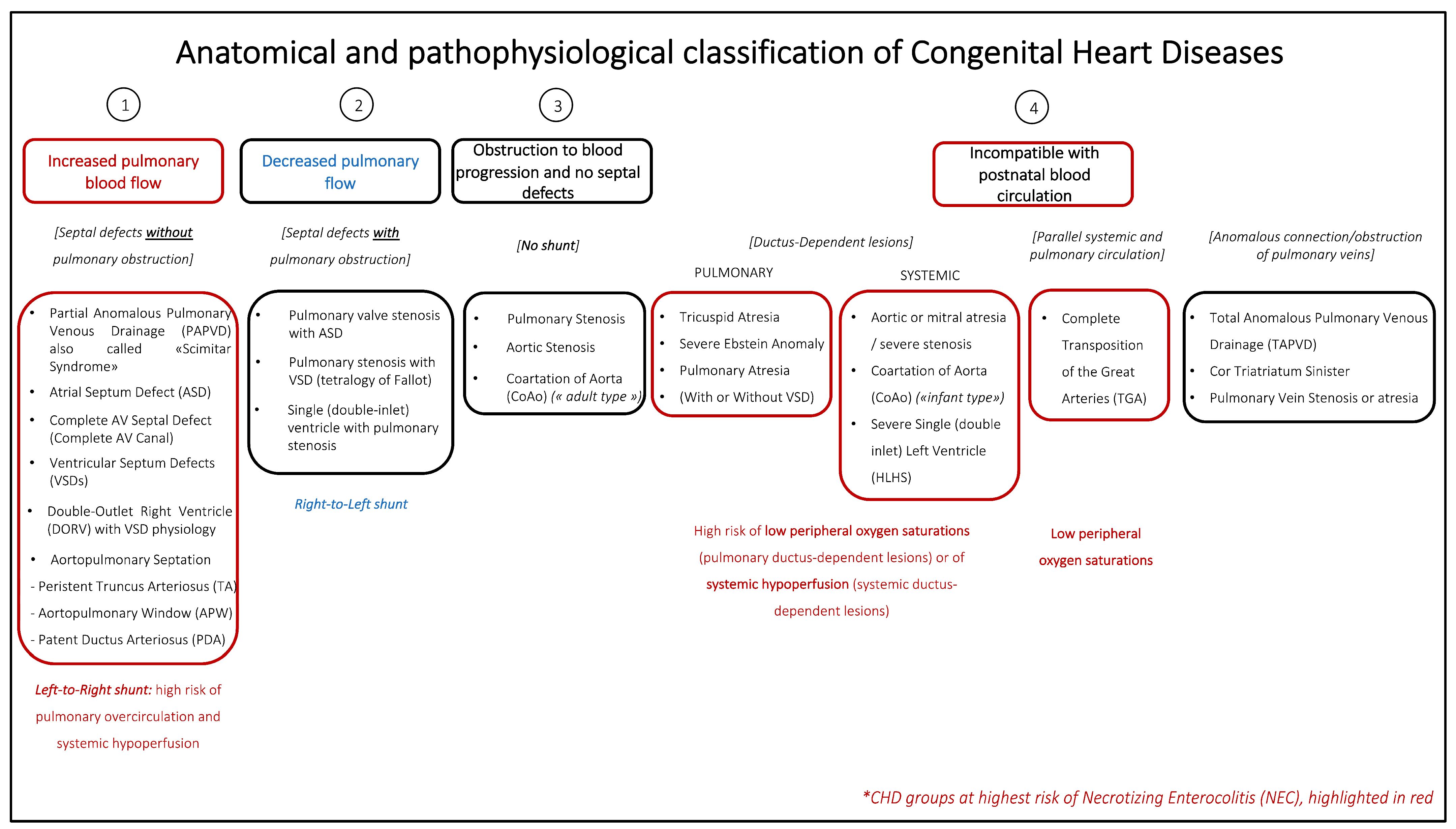
| Classical NEC | Cardiac NEC | |
|---|---|---|
| Population | Preterm | Preterm, term |
| Onset | Later presentation (4–5 weeks of life) | Earlier presentation (1–3 weeks of life) |
| Risk factors | -Low BW and GA -Feeding -Dysbiosis -Asphyxia | -Low BW and GA -Systemic ductal-dependent lesions (CoAo, AAI) -Low cardiac output state/shock -High dose (>0.05 μg/kg/minute) or long duration of PGE1 -Specific CHD pathologies (HLHS, TA, APW, AVSD) -Trisomy 21 |
| Localization | Ileus, colon, pan-NEC | Colon, distal ileus, pan-NEC |
| Potential Predictive Tool | Article Included Population | Study Type and Population | Main Findings |
|---|---|---|---|
| SpO2 | Van der Heide et al. BMC Pediatr 2020 [20] Neonates | Retrospective case-control study on neonates > 35 GW with CHD; 16 CHD patients with NEC (GA 38.6, BW 2937 g) vs. 16 matched CHD controls without NEC (GA 39.1, BW 3323 g) (TGA, ToF, CoAo, IAA, PVS) | No difference in median diastolic and systolic blood pressure and post-ductal SpO2 during the first 48 h of hospitalization and before the onset of NEC between cases and controls |
| Choi et al. BMC Pediatr 2022 [43] Neonates | Retrospective case-control study; 355 full-term infants (≥37 GW) with DD-CHD receiving PGE1 within 7 days from birth (10 CHD-NEC, GA 37.9, BW 2783.5 g; 325 no-NEC, GA 39.1, BW 3170.9) | Clinical risk factors: GA < 38 weeks, BW < 2500 g, need for MV, parenteral nutrition, functional single ventricle; no difference in SpO2 in the 12 h before the onset of NEC and in the 12 h before the end of PGE1 infusion between infants with or without NEC | |
| Blood pressure or episodes of hypotension | Leung et al. J Pediatr 1988 [41] Infants | Prospective observational study; 133 infants with symptomatic CHD (7 with NEC) (GA, BW, time of evaluation not found) | PGE1 infusion (p = 0.02), and apnea (p < 0.01) and hypotensive episodes (p < 0.001) induced by PGE1 infusion as risk factors for NEC |
| Hebra et al. J Pediatr Surg 1993 [42] Infants | Retrospective case-control study; 387 patients undergoing Norwood stage I for HLHS (31 with mesenteric ischemia, mean age at evaluation 17.5 weeks, 87% full-term, mean BW 3 kg) | Significant hypotension or low perfusion state documented within 48 h prior to the onset of mesenteric ischemia in 80% of patients (mortality of 97%) | |
| McElhinney et al. Pediatrics 2000 [4] Neonates | Retrospective case-control study; original cohort: 643 patients < 1 month of age with structural, electrical, and/or myocardial/metabolic heart disease; of these, 21 patients with NEC (GA 36.7, BW 3 kg, age at admission 5.1 d) and 70 matched controls (GA 38.1, BW 2.8 kg) | Clinical risk factors: prematurity (<36 weeks), and GA; episodes of low cardiac output (based on laboratory criteria: metabolic acidosis with arterial pH < 7.2, serum creatinine > 1.2 mg/dL; and serum aspartate aminotransferase and serum alanine aminotransferase > 300 U/L) or clinical shock (not meeting these criteria) (p = 0.005, OR 6.5), or highest PGE dose > 0.05 mcg/kg/min (p = 0.04, OR 3.9) correlated with the development of NEC; | |
| Heart rate and heart rate variability | No studies including neonates/infants with CHD | ||
| Perfusion index | No studies including neonates/infants with CHD | ||
| Doppler indices of abdominal and mesenteric vessels | Johnson et al. Pediatr Cardiol 2011 [31] Neonates | Observational study of 44 patients from 5 centers belonging to the Pediatric Heart Network Single Ventricle Reconstruction Trial (MBTS: n = 19, GA 38, BW 3.15 kg, age at surgery 4 d; RVPAS n = 25, GA 38, BW 3.1, age at surgery 6) | Median RI of the CA higher in the MBTS group than in the RV-to-PAS group (1 vs. 0.82, p = 0.02); no difference in NEC or feeding intolerance episodes |
| Cozzi et al. Congenit Heart Dis. 2013 [57] Neonates | Retrospective chart review of 69 patients undergoing the hybrid procedure for single-ventricle palliation (8 with NEC Bell’s stage > or =2, GA 35.5, BW 2.6 kg vs. GA 38, BW 3.1 kg) | After the hybrid procedure, no differences between the NEC and No NEC groups for the echocardiographic indices (antegrade VTI, retrograde VTI, effective VTI, VTI regurgitant fraction, VTI retrograde/VTI antegrade ratio, CO, peak antegrade velocity through PDA stent, retrograde/antegrade time ratio, % regurgitant time) | |
| Carlo et al. Pediatrics 2007 [14] Neonates | Retrospective case-control study of CHD patients (variable diagnoses), 18 with NEC Bell’s stage > or =2 (GA 37.4, BW 2952 g, age at onset 5 d (1–24)) and 20 controls (GA 37.8, BW 3103 g) | Persistent diastolic flow reversal in the descending aorta associated with increased likelihood of NEC in term neonates with CHD (47% vs. 15%, OR 5.04) | |
| Papneja et al. In J Cardiovasc Imaging 2021 [58] Neonates | Retrospective study of 51 neonates with small left-sided structures (including borderline left ventricle and HLHS), with preoperative cardiac MRI and abdominal Doppler US, of which 13 with feeding intolerance and 2 with NEC (composite outcome) (45 term neonates, 5 born 35–37 weeks, 1 born 33 + 5 weeks; mean age at evaluation 4.6 d) | Significant lower mean DAo flow by MRI in patients with the composite outcome vs. controls (0.89 L/min/m2 vs. 1.23 L/min/m2, p = 0.007); a DAo mean flow of 0.91 L/min/m2 identified patients with the composite outcome with Se 61% and Sp 76%; no correlation between DAo flow by MRI and AUS; no association between DAo flow by AUS (PI, PSV or MV) and the composite outcome | |
| Van der Heide et al. BMC Pediatr 2020 [20] Neonates | Retrospective case-control study of 32 neonates with CHD; 16 CHD patients with NEC (GA 38.6, BW 2937 g) vs. 16 matched CHD controls without NEC (GA 39.1, BW 3323 g) (TGA, ToF, CoAo, IAA, PVS) | No difference in diastolic backflow in the DAo between the two groups | |
| Miller et al. Pediatr Cardiol 2014 [19] Neonates/Infants | Retrospective cohort study of 61 neonates/infants undergoing stage I palliation for HLHS or other right single-ventricle anomaly at any time frame until Fontan correction; 11 with NEC (BW 3126 g) and 50 controls (BW 3130 g) (mean follow-up 3.8 y); NEC occurred pre-operative (n = 1), prior to stage I discharge (n = 3), between stage I and stage II (n = 5), post-stage II (n = 1), and post-Fontan (n = 1) | No difference in gender, BW, baseline anatomy, surgical procedure, bypass time or cross-clamp time; at both pre-operative and routine post-operative stage I echo, lower abdominal aorta PI in the NEC group (any time of onset) compared to the no NEC group (pre-operative: 3.38 vs. 3.89 with p < 0.05; post-operative: 2.21 vs. 3.05 with p = 0.01); no difference in RV function nor GLS | |
| Pham et al. Pediatr Cardiol 2022 [59] Neonates | Retrospective single-institution case-control study of patients with CHD (<30 days of age, variable diagnoses) undergoing surgical repair within the first month of life, 30 developing NEC (GA 38.4, BW 3.3 kg) and 50 controls (GA 38.8, BW 3.2 kg) | From Doppler of the DAo, median log PI of the NEC group higher than the lowest control PI (0.68 vs. 0.48, p = 0.03), and lower than the highest control PI (0.61 vs. 0.98, p = 0.05); higher median log modified PI (MODPI) of the NEC group vs. that of the control group (3.9 vs. 3.1, p = 0.01, where negative values due to retrograde flow are substituted with zero) | |
| Abdominal (splanchnic) NIRS | Stapleton et al. Tex Heart Inst J 2007 [74] Neonate | Single case report of infant with PA and IVS and MAPCAs developing NEC at 4 weeks of life (term neonate, 2.4 kg; 120 cc/kg/day oral feeds at NEC diagnosis) | Low mesenteric SO2 compared to cerebral SO2 measured by splanchnic NIRS 48 h after the diagnosis of NEC (24.5% vs. 53.4%) |
| DeWitt et al. Pediatr Cardiol 2014 [78] | Prospective study on 64 neonates undergoing BV repair or SV palliation for CHD, monitored postoperatively by rsSO2 before and during initiation of enteral feeds; 11 developing NEC, 23 not developing NEC (Weight at surgery 3.3 vs. 3.3 kg) |
| |
| Abdominal US | Lazow et al. Pediatr Surg Int 2022 [81] Neonates | Multicenter retrospective review of 86 patients with NEC with CHD (n = 18, GA 37, BW 2.4 kg) vs. without CHD (n = 68, GA 27.6, BW 1 kg) |
|
| Abraham et al. Pediatr Cardiol 2012 [82] Neonate | Single case report of a 5-day-old term neonate with CAVC with Down syndrome (term neonate, BW 3 kg) | Stream of spontaneous air contrast tracked from the hepatic veins/portal veins and entering the heart via the inferior vena cava, proven to be present because of pneumatosis at diagnosis of NEC | |
| Müller et al. Eur J Pediatr 2014 [83] Neonate/Infant | Single case report of a 5-week-old boy with PA and IVS (GA 39, BW 3.5 kg) | Free air in right atrium as suspicious sign of NEC at the time of diagnosis | |
| Cheong et al. Cureus 14(3):e22970 [84] Neonate | Single case report of a 6-day-old neonate with postnatal diagnosis of complex CHD (IAA type B, VSD) developing fulminant NEC after obstructive shock with end-organ damage (term neonate, BW 2.7 kg) | Systemic air embolism (widespread intra-vascular microbubbles entering from the portal veins, liver parenchyma, hepatic veins, right atrium, across the ventricular septal defect, and eventually into the systemic circulation) in the presence of NEC (day 20 of life) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschino, L.; Guiducci, S.; Duci, M.; Meggiolaro, L.; Nardo, D.; Bonadies, L.; Salvadori, S.; Verlato, G.; Baraldi, E. Noninvasive Tools to Predict Necrotizing Enterocolitis in Infants with Congenital Heart Diseases: A Narrative Review. Children 2024, 11, 1343. https://doi.org/10.3390/children11111343
Moschino L, Guiducci S, Duci M, Meggiolaro L, Nardo D, Bonadies L, Salvadori S, Verlato G, Baraldi E. Noninvasive Tools to Predict Necrotizing Enterocolitis in Infants with Congenital Heart Diseases: A Narrative Review. Children. 2024; 11(11):1343. https://doi.org/10.3390/children11111343
Chicago/Turabian StyleMoschino, Laura, Silvia Guiducci, Miriam Duci, Leonardo Meggiolaro, Daniel Nardo, Luca Bonadies, Sabrina Salvadori, Giovanna Verlato, and Eugenio Baraldi. 2024. "Noninvasive Tools to Predict Necrotizing Enterocolitis in Infants with Congenital Heart Diseases: A Narrative Review" Children 11, no. 11: 1343. https://doi.org/10.3390/children11111343
APA StyleMoschino, L., Guiducci, S., Duci, M., Meggiolaro, L., Nardo, D., Bonadies, L., Salvadori, S., Verlato, G., & Baraldi, E. (2024). Noninvasive Tools to Predict Necrotizing Enterocolitis in Infants with Congenital Heart Diseases: A Narrative Review. Children, 11(11), 1343. https://doi.org/10.3390/children11111343






