Efficacy and Safety of Orogastric vs. Nasogastric Tube Feeding in Preterm Infants: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- (I)
- Participants: preterm infants (<37 weeks gestation) who can breathe spontaneously and need gastric tube feeding.
- (II)
- Intervention: the intervention group received OG tube feeding.
- (III)
- Control: the control group received NG tube feeding.
- (IV)
- Outcomes:Primary outcomes:
- Establishment of full enteral tube feeds (days): at least 140–160 mL/kg/day;
- Time to regain birth weight (days).
Secondary outcomes:- Apnea: breathing pauses that last for more than 10 s;
- Bradycardia: heart rate < 100/min.
Desaturation: SpO2 < 85%.- Tube displacement: displacement or removal of a feeding tube without intention.
- Aspiration pneumonia: radiological and/or clinical evidence of lower respiratory tract compromise caused by aspiration of stomach contents covertly or evidently [13];
- Gastric residuals: gastric residual volume > 50% of the previous meal [14];
- Necrotizing enterocolitis: any stage (modified Bells staging) [15].
- (V)
- The type of study: randomized controlled trials (RCTs).
2.3. Study Selection and Date Extraction
2.4. Assessment of Risk of Bias
2.5. Statistical Analysis
3. Results
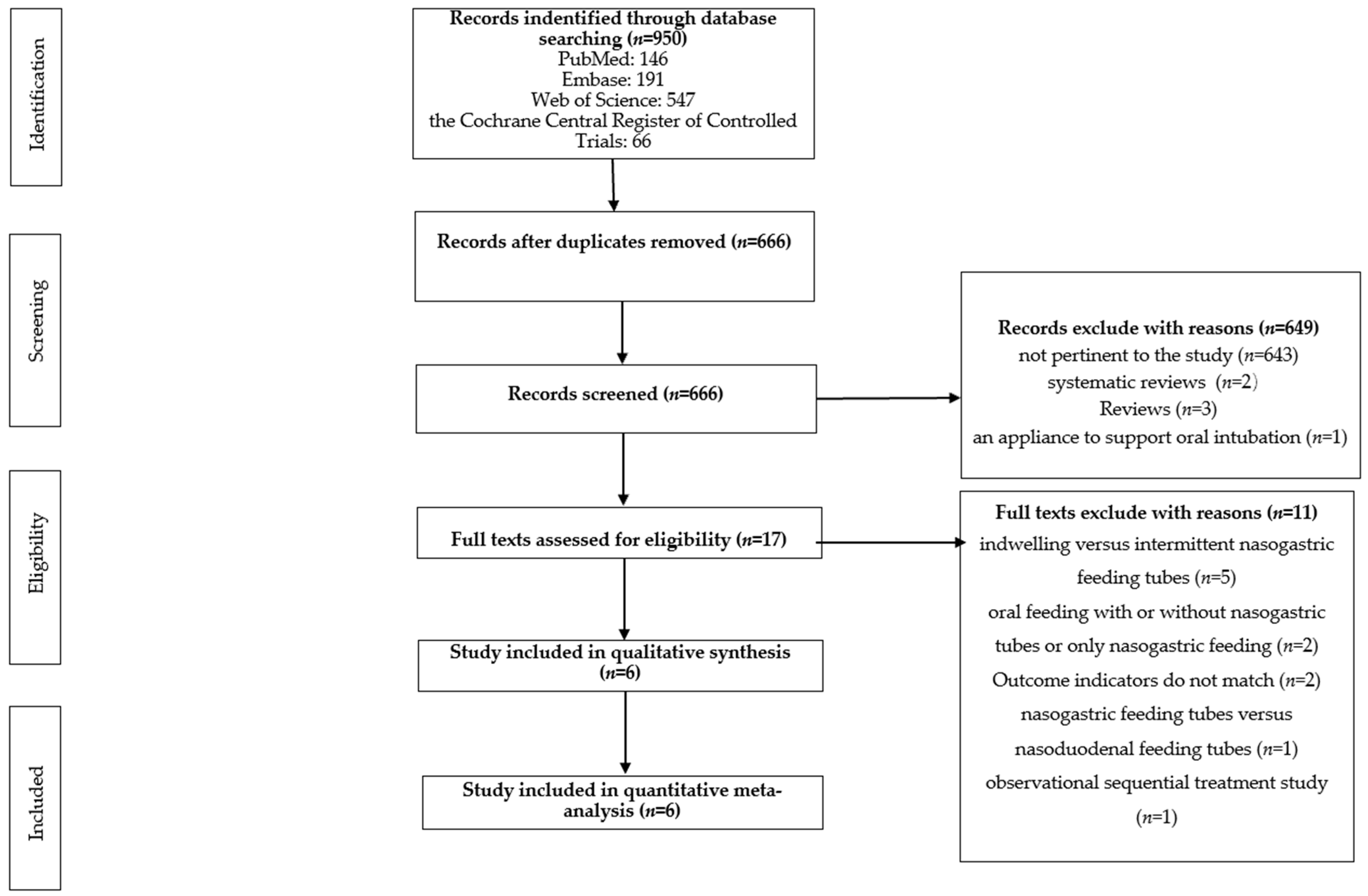
3.1. Literature Search and Screening
3.2. Basic Characteristics of Included Articles
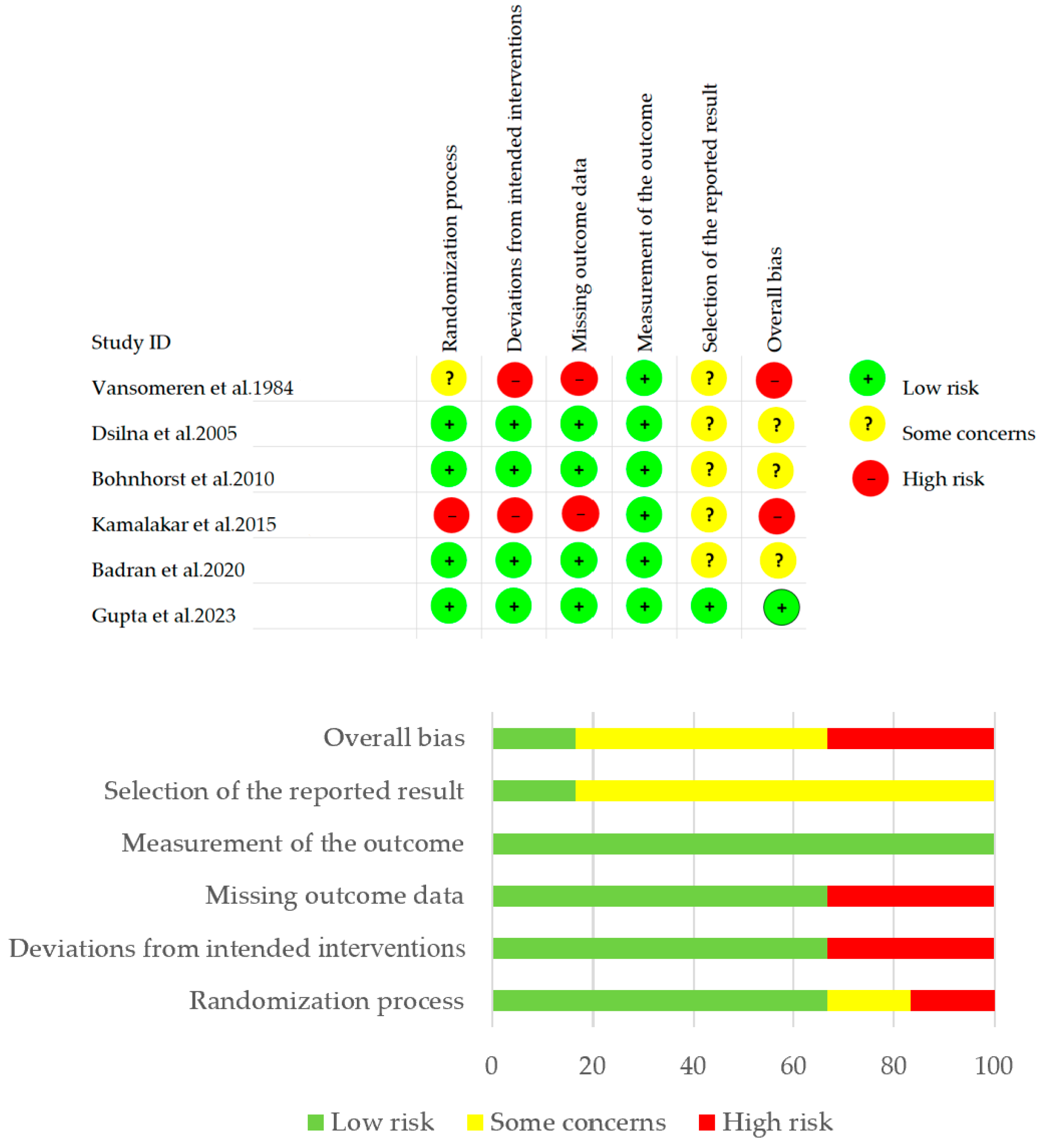
3.3. Risk Assessment of Bias of Included Articles
3.4. Outcomes
3.4.1. Time to Establish Full Enteral Tube Feeding (Days)
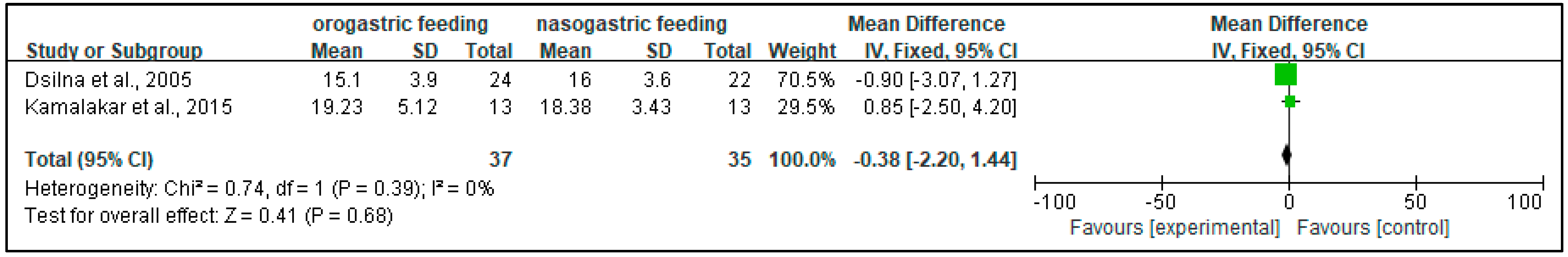
3.4.2. Time to Regain Birth Weight (Days)
3.4.3. Apnea
3.4.4. Bradycardias and Desaturation
3.4.5. Tube Displacement
3.4.6. Aspiration Pneumonia
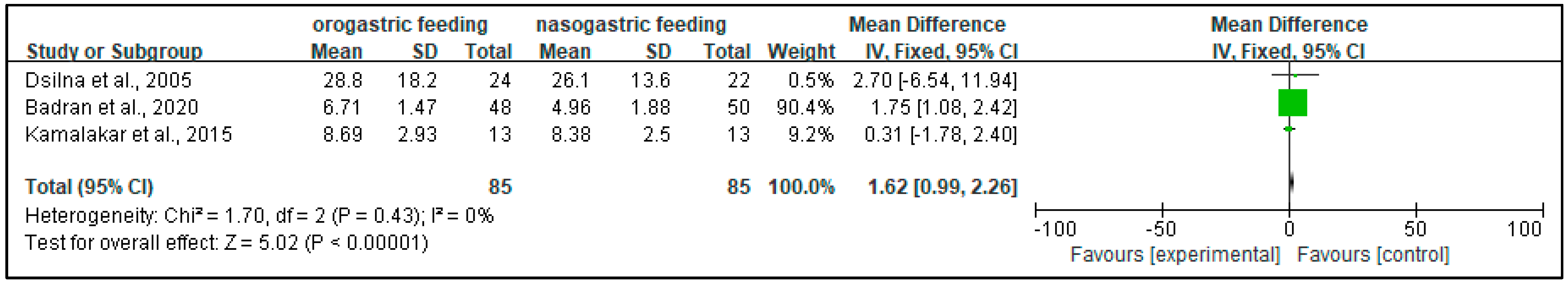
3.4.7. Gastric Residuals
3.4.8. Necrotizing Enterocolitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Watson, J.; McGuire, W. Nasal versus oral route for placing feeding tubes in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2013, 2013, CD003952. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Anderson-Berry, A. Early Enteral Feeding in Preterm Infants: A Narrative Review of the Nutritional, Metabolic, and Developmental Benefits. Nutrients 2021, 13, 2289. [Google Scholar] [CrossRef] [PubMed]
- Anvekar, A.; Athikarisamy, S.; Rao, S.; Gill, A.; Nathan, E.; Doherty, D.; Lam, G. Time to regain birth weight—A marker to predict the severity of retinopathy of prematurity? BMC Pediatr. 2021, 21, 540. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Ehsan, L.; Jones, M.; Khan, M.; Middleton, J.; Vergales, B.; Perks, P.; Syed, S. Time to regain birth weight predicts neonatal growth velocity: A single-center experience. Clin. Nutr. ESPEN 2020, 38, 165–171. [Google Scholar] [CrossRef]
- El Rafei, R.; Jarreau, P.H.; Norman, M.; Maier, R.F.; Barros, H.; Van Reempts, P.; Pedersen, P.; Cuttini, M.; Costa, R.; Zemlin, M.; et al. Association between postnatal growth and neurodevelopmental impairment by sex at 2 years of corrected age in a multi-national cohort of very preterm children. Clin. Nutr. 2021, 40, 4948–4955. [Google Scholar] [CrossRef]
- De Rose, D.U.; Cota, F.; Gallini, F.; Bottoni, A.; Fabrizio, G.C.; Ricci, D.; Romeo, D.M.; Mercuri, E.; Vento, G.; Maggio, L. Extra-uterine growth restriction in preterm infants: Neurodevelopmental outcomes according to different definitions. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2021, 33, 135–145. [Google Scholar] [CrossRef]
- Stocks, J. Effect of nasogastric tubes on nasal resistance during infancy. Arch. Dis. Child. 1980, 55, 17–21. [Google Scholar] [CrossRef]
- Greenspan, J.S.; Wolfson, M.R.; Holt, W.J.; Shaffer, T.H. Neonatal gastric intubation: Differential respiratory effects between nasogastric and orogastric tubes. Pediatr. Pulmonol. 1990, 8, 254–258. [Google Scholar] [CrossRef]
- Tonkin, S.L.; Partridge, J.; Beach, D.; Whiteney, S. The pharyngeal effect of partial nasal obstruction. Pediatrics 1979, 63, 261–271. [Google Scholar] [CrossRef]
- Cortines, A.A.O.; Costa, L.R. Associated factors and persistence of palatal groove in preterm infants: A cohort study. BMC Pediatr. 2016, 16, 143. [Google Scholar] [CrossRef]
- Hawes, J.; McEwan, P.; McGuire, W. Nasal versus oral route for placing feeding tubes in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2004, CD003952. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.G.; Shin, J.; Ryu, H.G. Pneumonitis and pneumonia after aspiration. J. Dent. Anesth. Pain. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Lin, H.C.; Torrazza, R.M.; Parker, L.; Talaga, E.; Neu, J. Gastric residual evaluation in preterm neonates: A useful monitoring technique or a hindrance? Pediatr. Neonatol. 2014, 55, 335–340. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kliegman, R.M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. N. Am. 1986, 33, 179–201. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Vansomeren, V.; Linnett, S.J.; Stothers, J.K.; Sullivan, P.G. An investigation into the benefits of resiting nasoenteric feeding tubes. Pediatrics 1984, 74, 379–383. [Google Scholar] [CrossRef]
- Dsilna, A.; Christensson, K.; Alfredsson, L.; Lagercrantz, H.; Blennow, M. Continuous feeding promotes gastrointestinal tolerance and growth in very low birth weight infants. J. Pediatr. 2005, 147, 43–49. [Google Scholar] [CrossRef]
- Bohnhorst, B.; Cech, K.; Peter, C.; Doerdelmann, M. Oral versus nasal route for placing feeding tubes: No effect on hypoxemia and bradycardia in infants with apnea of prematurity. Neonatology 2010, 98, 143–149. [Google Scholar] [CrossRef]
- Kamalakar, S.N.; Udaykanth, S. Nasal versus oral route for placing feeding tubes in preterm or low birth weight infants. MRIMS J. Health Sci. 2015, 3, 190–195. [Google Scholar]
- Badran, A.T.; Hashish, M.; Ali, A.; Shokeir, M.; Shabaan, A. Nasogastric versus Orogastric Bolus Tube Feeding in Preterm Infants: Pilot Randomized Clinical Trial. Am. J. Perinatol. 2021, 38, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.P.; Ahmad, Z.S.; Mittal, R.; Kukreja, S.; Jha, C.; Raheja, K. Nasogastric vs Orogastric Feeding in Stable Preterm (≤32 Weeks) Neonates: A Randomized Open-Label Controlled Trial. Indian Pediatr. 2023, 60, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Blau, J.; Sridhar, S.; Fau-Mathieson, S.; Mathieson, S.; Fau-Chawla, A.; Chawla, A. Effects of protein/nonprotein caloric intake on parenteral nutrition associated cholestasis in premature infants weighing 600–1000 grams. J. Parenter. Enter. Nutr. 2007, 31, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Pivodic, A.; Holmström, G.; Smith, L.E.H.; Hård, A.L.; Löfqvist, C.; Al-Hawasi, A.; Larsson, E.; Lundgren, P.; Gränse, L.; Tornqvist, K.; et al. Prognostic Value of Parenteral Nutrition Duration on Risk of Retinopathy of Prematurity: Development and Validation of the Revised DIGIROP Clinical Decision Support Tool. JAMA Ophthalmol. 2023, 141, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Zingg, W.; Tomaske, M.; Fau-Martin, M.; Martin, M. Risk of parenteral nutrition in neonates—An overview. Nutrients 2012, 4, 1490–1503. [Google Scholar] [CrossRef]
- Buchman, A.L.; Moukarzel Aa Fau-Bhuta, S.; Bhuta, S.; Fau-Belle, M.; Belle, M.; Fau-Ament, M.E.; Ament Me Fau-Eckhert, C.D.; Eckhert Cd Fau-Hollander, D.; Hollander, D.; Fau-Gornbein, J.; et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J. Parenter. Enter. Nutr. 1995, 19, 453–460. [Google Scholar] [CrossRef]
- Hansen, C.F.; Thymann, T.; Andersen, A.D.; Holst, J.J.; Hartmann, B.; Hilsted, L.; Langhorn, L.; Jelsing, J.; Sangild, P.T. Rapid gut growth but persistent delay in digestive function in the postnatal period of preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G550–G560. [Google Scholar] [CrossRef]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; de Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018, 83, 102–110. [Google Scholar] [CrossRef]
- Esubalew, H.; Messelu, M.A.; Tarekegn, B.T.; Admasu, A.T.; Abrha, N.N.; Terefe, B. Time to full enteral feeding and its predictors among very low birth weight (VLBW) neonates admitted to the neonatal intensive care units (NICU) in comprehensive specialized hospitals in Northwest Ethiopia. BMC Pediatr. 2024, 24, 366. [Google Scholar] [CrossRef]
- Imam, Z.O.; Nabwera, H.M.; Tongo, O.O.; Andang’o, P.E.A.; Abdulkadir, I.; Ezeaka, C.V.; Ezenwa, B.N.; Fajolu, I.B.; Mwangome, M.K.; Umoru, D.D.; et al. Time to full enteral feeds in hospitalised preterm and very low birth weight infants in Nigeria and Kenya. PLoS ONE 2024, 19, e0277847. [Google Scholar] [CrossRef]
- Al Tawil, K.; Sumaily, H.; Ahmed, I.A.; Sallam, A.; Al Zaben, A.; Al Namshan, M.; Crankson, S. Risk factors, characteristics and outcomes of necrotizing enterocolitis in late preterm and term infants. J. Neonatal Perinat. Med. 2013, 6, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Weaver, M.; Murgas Torrazza, R.J.; Shuster, J.; Li, N.; Krueger, C.; Neu, J. Effect of Gastric Residual Evaluation on Enteral Intake in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Rochow, N.; Chessell, L.; Wilson, J.; Cunningham, K.; Fusch, C.; Dutta, S.; Thomas, S. Gastric Residual Volume in Feeding Advancement in Preterm Infants (GRIP Study): A Randomized Trial. J. Pediatr. 2018, 200, 79–83.e1. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Thanigainathan, S.; Ramaswamy, V.V.; Rajaiah, B.; Ramakrishnan, S. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2023, 6, Cd012937. [Google Scholar] [CrossRef]
| Study | Group | Patients (n) | Gestational Age (Week) | Postnatal Age (d) | Birth Weight (g) | Intervention | Follow-Up | Outcome Indicators |
|---|---|---|---|---|---|---|---|---|
| Vansomeren et al., 1984 [17] | experiment group | 20 | 31 (30–34) * | 6 (2.5–12.5) * | 1420 (1280–1740) * | OG feeding | 14 days | (1) apneas (the number of episodes per hour on 3 days, 7 days) |
| control group | 22 | 32 (31–34) * | 2 (1–6) * | 1370 (1320–1760) * | NG feeding | |||
| Dsilna et al., 2005 [18] | experiment group | 24 | 26.8 (1.4) ‡ | within 30 h of birth | 899 (179) ‡ | OG feeding | birth to postmenstrual age (32 weeks) | (1) time to achieve full enteral feeding (140 to 160 mL/kg/day) (2) time to regain birth weight (3) gastric residuals (the total number of the occasions) |
| control group | 22 | 26.6 (1.2) ‡ | 833 (177) ‡ | NG feeding | ||||
| Bohnhorst et al., 2010 [19] | experiment group | 32 | 29 (24–31) * | postmenstrual age < 36 weeks | 1195 (465–1885) * | OG feeding | 12 h | (1) apneas (the frequency of episodes per hour) (2) bradycardia and desaturation (the frequency of episodes per hour) |
| control group | NG feeding | |||||||
| Kamalakar et al., 2015 [20] | experiment group | 13 | <32 | between day 1 and day 7 of birth | <1500 g | OG feeding | until infants achieved full enteral feeds | (1) time to achieve full enteral feeding (at least 150 mL/kg/day) (2) time to regain birth weight (3) incidence of apnea, tube displacement, aspiration, necrotizing enterocolitis |
| control group | 13 | NG feeding | ||||||
| Badran et al., 2020 [21] | experiment group | 48 | 33.27 (1.08) ‡ | postmenstrual age range 30–35 weeks | 1753.3 (414.51) ‡ | OG feeding | until infants achieved full enteral feeds | (1) time to achieve full enteral feeding (140 to 160 mL/kg/day) (2) time to regain birth weight (3) incidence of apnea, tube displacement, aspiration, necrotizing enterocolitis, bradycardia, desaturation, gastric residuals |
| control group | 50 | 33.32 (1.57) ‡ | 1859.6 (307.05) ‡ | NG feeding | ||||
| Gupta et al., 2023 [22] | experiment group | 21 | ≤32 | postmenstrual age was 31.3 (1.8) ‡ | 1112.5 (450) ‡ | orogastric feeding | from the time of insertion till the time needed to be changed | (1) bradycardia and desaturations (episodes per hour) |
| control group | nasogastric feeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Fei, Q.; Yuan, T. Efficacy and Safety of Orogastric vs. Nasogastric Tube Feeding in Preterm Infants: A Systematic Review and Meta-Analysis. Children 2024, 11, 1289. https://doi.org/10.3390/children11111289
Liu H, Fei Q, Yuan T. Efficacy and Safety of Orogastric vs. Nasogastric Tube Feeding in Preterm Infants: A Systematic Review and Meta-Analysis. Children. 2024; 11(11):1289. https://doi.org/10.3390/children11111289
Chicago/Turabian StyleLiu, Huazi, Qiang Fei, and Tianming Yuan. 2024. "Efficacy and Safety of Orogastric vs. Nasogastric Tube Feeding in Preterm Infants: A Systematic Review and Meta-Analysis" Children 11, no. 11: 1289. https://doi.org/10.3390/children11111289
APA StyleLiu, H., Fei, Q., & Yuan, T. (2024). Efficacy and Safety of Orogastric vs. Nasogastric Tube Feeding in Preterm Infants: A Systematic Review and Meta-Analysis. Children, 11(11), 1289. https://doi.org/10.3390/children11111289