Reference Range of Quantitative MRI Metrics Corrected T1 and Liver Fat Content in Children and Young Adults: Pooled Participant Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Imaging Protocol and Post-Processing
2.3. Statistical Analysis
3. Results
3.1. Demographics
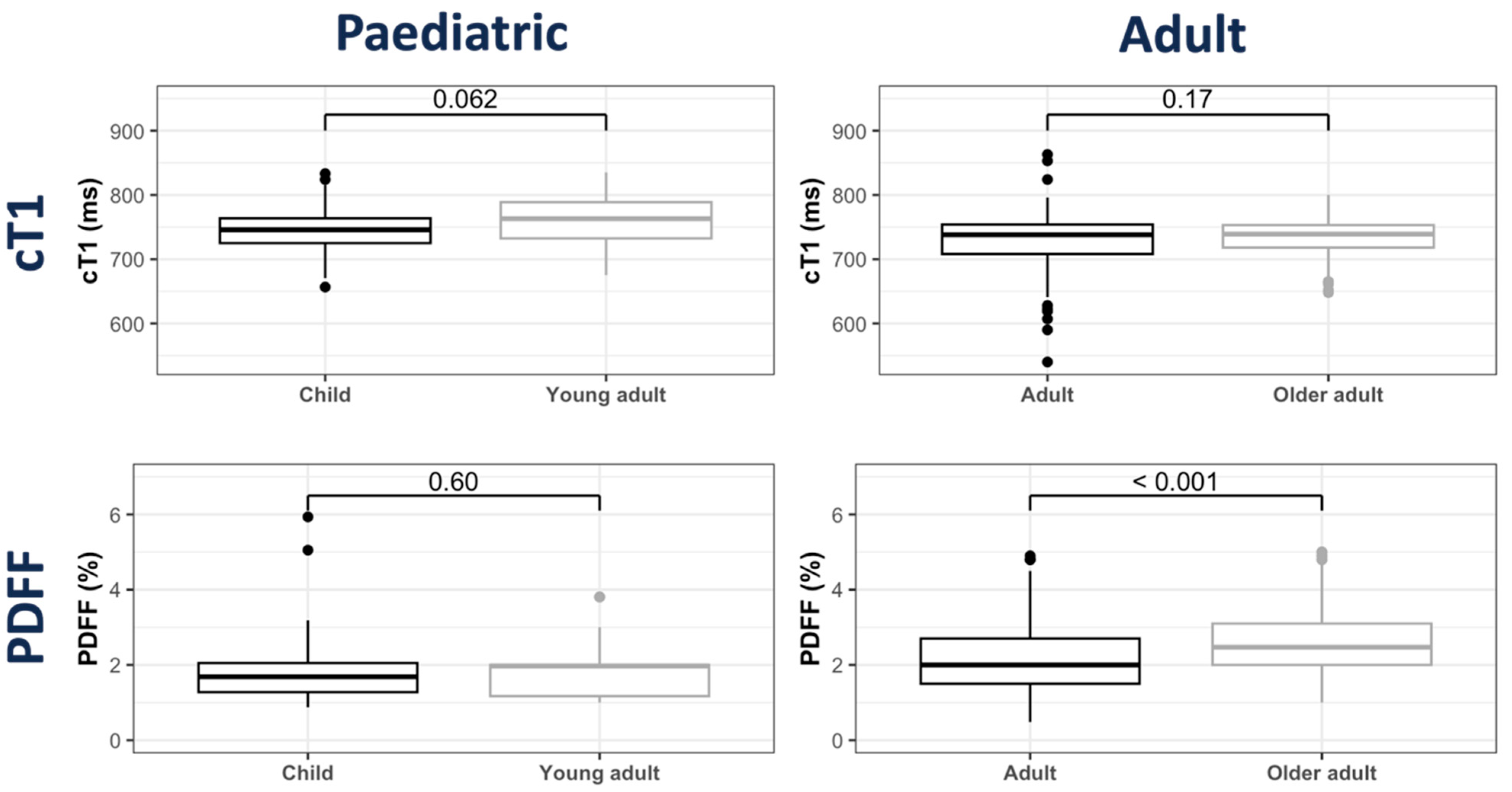
3.2. cT1 Distribution
3.3. PDFF Distribution
3.4. Sex and Ethnicity Characteristics
3.5. Technical Performance: Inter- and Intra-Reader Variation in cT1 and PDFF Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cioffi, C.E.; Welsh, J.A.; Cleeton, R.L.; Caltharp, S.A.; Romero, R.; Wulkan, M.L.; Konomi, J.V.; Frediani, J.K.; Vos, M.B. Natural History of NAFLD Diagnosed in Childhood: A Single-Center Study. Children 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Hartjes, K.; Shah, U.; Khalili, H.; Arnell, H.; Ludvigsson, J.F. Non-aflcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J. Hepatol. 2021, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; Lavine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016, 170, e161971. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Shapiro, W.L.; Noon, S.L.; Schwimmer, J.B. Recent advances in the epidemiology of nonalcoholic fatty liver disease in children. Pediatr. Obes. 2021, 16, e12849. [Google Scholar] [CrossRef]
- Pan, J.J.; Fallon, M.B. Gender and racial differences in nonalcoholic fatty liver disease. World J. Hepatol. 2014, 6, 274–283. [Google Scholar] [CrossRef]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Berentzen, T.L.; Gamborg, M.; Holst, C.; Sørensen, T.I.; Baker, J.L. Body mass index in childhood and adult risk of primary liver cancer. J. Hepatol. 2014, 60, 325–330. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef]
- BSPGHAN. UK Fatty Liver Guidelines. 2020. Available online: https://bspghan.org.uk/abstract-booklet.pdf (accessed on 15 May 2024).
- Gomes, N.; Torres, U.S.; Ferraz, M.L.C.G.; D’Ippolito, G. Advanced MR imaging for detection of liver fibrosis and inflammation in autoimmune hepatitis: A state-of-the-art review. In Seminars in Ultrasound, CT and MRI; WB Saunders: Edinburgh, UK, 2024. [Google Scholar] [CrossRef]
- Thomaides-Brears, H.; Barragán, E.; de Celis Alonso, B.; Dies-Suarez, P.; Pickles, E.; Hidalgo-Tobon, S.; Klunder, M.K.; McKay, A.; de Celis Alonso, B.; Miranda Lora, L.; et al. Prevalence of non-alcoholic fatty liver disease in a Mexican paediatric population diagnosed non-invasively by multiparametric magnetic resonance imaging. In Proceedings of the ISMRM Workshop on MRI of Obesity & Metabolic Disorders, Singapore, 21–24 July 2019. [Google Scholar]
- Trout, A.T.; Taylor, A.E.; Kasten, J.L.; Castro-Rojas, C.; Mahalingam, N.; Miethke, A.G.; Dillman, J.R. Corrected T1 Mapping in Children and Young Adults With Autoimmune Liver Disease: Correlation With Histology. AJR Am. J. Roentgenol. 2023, 220, 901–902. [Google Scholar] [CrossRef]
- Janowski, K.; Shumbayawonda, E.; Dennis, A.; Kelly, M.; Bachtiar, V.; DeBrota, D.; Langford, C.; Thomaides-Brears, H.; Pronicki, M.; Grajkowska, W.; et al. Multiparametric MRI as a Noninvasive Monitoring Tool for Children With Autoimmune Hepatitis. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 108–114. [Google Scholar] [CrossRef]
- Rijnberg, F.M.; Westenberg, J.J.M.; van Assen, H.C.; Juffermans, J.F.; Kroft, L.J.M.; Boogaard, P.J.v.D.; Monteros, C.T.E.d.L.; Warmerdam, E.G.; Leiner, T.; Grotenhuis, H.B.; et al. 4D flow cardiovascular magnetic resonance derived energetics in the Fontan circulation correlate with exercise capacity and CMR-derived liver fibrosis/congestion. J. Cardiovasc. Magn. Reson. 2022, 24, 21. [Google Scholar] [CrossRef]
- Levick, C.; Phillips-Hughes, J.; Collier, J.; Banerjee, R.; Cobbold, J.F.; Wang, L.M.; Piechnik, S.K.; Robson, M.D.; Neubauer, S.; Barnes, E.; et al. Non-invasive assessment of portal hypertension by multi-parametric magnetic resonance imaging of the spleen: A proof of concept study. PLoS ONE 2019, 14, e0221066. [Google Scholar] [CrossRef]
- Dillman, J.R.; Serai, S.D.; Trout, A.T.; Singh, R.; Tkach, J.A.; Taylor, A.E.; Blaxall, B.C.; Fei, L.; Miethke, A.G. Diagnostic performance of quantitative magnetic resonance imaging biomarkers for predicting portal hypertension in children and young adults with autoimmune liver disease. Pediatr. Radiol. 2019, 49, 332–341. [Google Scholar] [CrossRef]
- Dillman, J.R.; Tkach, J.A.; Gandhi, D.; Singh, R.; Miethke, A.G.; Jayaswal, A.; Trout, A.T. Relationship between magnetic resonance imaging spleen T1 relaxation and other radiologic and clinical biomarkers of liver fibrosis in children and young adults with autoimmune liver disease. Abdom. Radiol. 2020, 45, 3709–3715. [Google Scholar] [CrossRef]
- Mojtahed, A.; Kelly, C.J.; Herlihy, A.H.; Kin, S.; Wilman, H.R.; McKay, A.; Kelly, M.; Milanesi, M.; Neubauer, S.; Thomas, E.L.; et al. Reference range of liver corrected T1 values in a population at low risk for fatty liver disease-a UK Biobank sub-study, with an appendix of interesting cases. Abdom. Radiol. 2019, 44, 72–84. [Google Scholar] [CrossRef]
- Bachtiar, V.; Kelly, M.D.; Wilman, H.R.; Jacobs, J.; Newbould, R.; Kelly, C.J.; Gyngell, M.L.; Groves, K.E.; McKay, A.; Herlihy, A.H.; et al. Repeatability and reproducibility of multiparametric magnetic resonance imaging of the liver. PLoS ONE 2019, 14, e0214921. [Google Scholar] [CrossRef]
- Hutton, C.; Gyngell, M.L.; Milanesi, M.; Bagur, A.; Brady, M. Validation of a standardized MRI method for liver fat and T2* quantification. PLoS ONE 2018, 13, e0204175. [Google Scholar] [CrossRef]
- Arndtz, K.; Shumbayawonda, E.; Hodson, J.; Eddowes, P.J.; Dennis, A.; Thomaides-Brears, H.; Mouchti, S.; Kelly, M.D.; Banerjee, R.; Neubauer, S.; et al. Multiparametric Magnetic Resonance Imaging, Autoimmune Hepatitis, and Prediction of Disease Activity. Hepatol. Commun. 2021, 5, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Roca-Fernandez, A.; Banerjee, R.; Thomaides-Brears, H.; Telford, A.; Sanyal, A.; Neubauer, S.; Nichols, T.E.; Raman, B.; McCracken, C.; Petersen, S.E.; et al. Liver disease is a significant risk factor for cardiovascular outcomes—A UK Biobank study. J. Hepatol. 2023, 79, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Sammer, M.B.; Akbari, Y.S.; Barth, R.A.; Blumer, S.L.; Dillman, J.R.; Farmakis, S.G.; Frush, D.P.; Gokli, A.; Halabi, S.S.; Iyer, R.; et al. Use of Artificial Intelligence in Radiology: Impact on Pediatric Patients, a White Paper from the ACR Pediatric AI Workgroup. J. Am. Coll. Radiol. 2023, 20, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kalveram, L.; Baumann, U.; De Bruyne, R.; Draijer, L.; Janczyk, W.; Kelly, D.; Koot, B.G.; Lacaille, F.; Lefere, S.; Lev, H.M.; et al. Noninvasive scores are poorly predictive of histological fibrosis in paediatric fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
- Archer, A.J.; Belfield, K.J.; Orr, J.G.; Gordon, F.H.; Abeysekera, K.W. EASL clinical practice guidelines: Non-invasive liver tests for evaluation of liver disease severity and prognosis. Front. Gastroenterol. 2022, 13, 436–439. [Google Scholar] [CrossRef]
- Mjelle, A.B.; Mulabecirovic, A.; Havre, R.F.; Rosendahl, K.; Juliusson, P.B.; Olafsdottir, E.; Gilja, O.H.; Vesterhus, M. Normal Liver Stiffness Values in Children: A Comparison of Three Different Elastography Methods. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 706–712. [Google Scholar] [CrossRef]
- Torres, U.S.; D’Ippolito, G. Multiparametric magnetic resonance imaging of the liver: Bridging the gap between theory and practice—A bridge too far? Radiol. Bras. 2021, 54, V–VI. [Google Scholar] [CrossRef] [PubMed]
- Muratori, L.; Lohse, A.W.; Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ 2023, 380, e070201. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, M.A.; Shumbayawonda, E.; Dennis, A.; Ahmed, R.Z.; Rahim, M.N.; Ney, M.; Smith, L.; Kelly, M.; Banerjee, R.; Culver, E.L. Quantitative magnetic resonance imaging to aid clinical decision making in autoimmune hepatitis. eClinicalMedicine 2022, 46, 101325. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, D.; Carbone, M. Prognostic models and autoimmune liver diseases. Best Pract. Res. Clin. Gastroenterol. 2023, 67, 101878. [Google Scholar] [CrossRef]
- Johannes, H.; Malte, W.; Shaima, A.; Marcial, S.; Sören, W.; Anastasiya, P.; Christina, W.; Christoph, S.; Willhelm, L.A.; Till, K.; et al. Right and left liver lobe biopsies by minilaparoscopy reveal clinically significant sampling error in staging and grading of autoimmune hepatitis. Liver Int. 2023, 43, 1269–1276. [Google Scholar] [CrossRef]
- Ala, A.; Yin, J.L.; Cussen, C. A Snapshot of Digital Transformation in Hepatology and Rare Liver Disease Related Health Care: A UK Perspective. Mayo Clin. Proc. Digit. Health 2023, 1, 451–454. [Google Scholar] [CrossRef]
- FDA. Pediatric Research Equity Act, PREA. Available online: https://www.fda.gov/drugs/development-resources/pediatric-research-equity-act-prea (accessed on 7 October 2024).
- Gomes, N.B.N.; Torres, U.S.; Caiado, A.H.M.; Fucuta, P.S.; Ferraz, M.L.C.G.; D’ipolito, G. Diagnostic accuracy of an uncorrected native T1 mapping sequence for liver fibrosis and inflammation in autoimmune hepatitis: A prospective study using histopathology as reference standard. Radiol. Med. 2024. [Google Scholar] [CrossRef]
- Zhang, S.; Mak, L.Y.; Yuen, M.F.; Seto, W.K. Screening strategy for non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S103–S122. [Google Scholar] [CrossRef]


| Group | Study | Study Identifier | Location | Country | N | Median Age (Years) | Sex (Male) | Ethnicity | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|---|
| Paediatric | Kids4Life | NCT03198104 | Children’s Memorial Health Institute (IPCZD) | Poland | 21 | 15 (4) | 38% | Caucasian | 20.7 (3.8) |
| Southampton Hospital | United Kingdom | 27 | 11 (7) | 44% | Caucasian | 17.7 (3.1) | |||
| METCOG | Medical Research Council (MR/N029194/1) and CONACyT México (FONCICIT/37/2016) | Hospital Infantil de México Federico Gómez | Mexico | 53 | 8 (2) | 100% | Hispanic | 15.4 (2.1) | |
| Adult | UKBB | Access application 9914 | Multisite UK study | United Kingdom | 500 | 65 (14) | 44% | Mixed | 23.2 (2.3) |
| CoverScan | NCT04369807 | Multisite UK study | United Kingdom | 71 | 44 (20) | 31% | Mixed | 22.4 (3.8) |
| Sub-Group | Percentiles | ||||
|---|---|---|---|---|---|
| 2.5th | 25th | Median | 75th | 97.5th | |
| cT1 (ms) | |||||
| Paediatrics | 683 | 725 | 748 | 768 | 820 |
| Adults | 654 | 714 | 738 | 754 | 791 |
| PDFF (%) | |||||
| Paediatrics | 1 | 1.3 | 1.7 | 2.1 | 4.4 |
| Adults | 0.9 | 1.8 | 2.3 | 3 | 4.6 |
| cT1 (ms) | p-Value | PDFF (%) | p-Value | |
|---|---|---|---|---|
| Age groupwise comparison | ||||
| Paediatric | 748 (34) | <0.001 | 1.9 (0.9) | <0.001 |
| Adult | 732 (35) | 2.4 (0.9) | ||
| Sex groupwise comparison | ||||
| Paediatric | ||||
| Female | 755 (37) | 0.268 | 2.2 (1.3) | 0.452 |
| Male | 746 (32) | 1.7 (0.6) | ||
| Adult | ||||
| Female | 735 (35) | 0.054 | 2.3 (0.9) | <0.001 |
| Male | 729 (34) | 2.6 (0.9) | ||
| Metric | Intra-Class Correlation | Intra-Rater Lower Limit of Agreement | Intra-Rater Bias | Intra-Rater Upper Limit of Agreement | Inter-Rater Lower Limit of Agreement | Inter-Rater Bias | Inter-Rater Upper Limit of Agreement |
|---|---|---|---|---|---|---|---|
| cT1 | 0.99 (0.99 to 1.00) | −8 ms (−8 to −7 ms) | −1 ms (−2 to 0 ms) | 6 ms (5 to 7 ms) | −18 ms (−20 to −17 ms) | −6 ms (−7 to −5 ms) | 8 ms (4 to 11 ms) |
| PDFF | 0.99 (0.98 to 1.00) | −0.8% (−1 to −0.7%) | 0% (−0.1 to 0%) | 0.7% (0.7 to 0.7%) | −1.1% (−1.3 to −1%) | −0.2% (−0.3 to −0.1%) | 0.8% (0.7 to 0.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumbayawonda, E.; Beyer, C.; de Celis Alonso, B.; Hidalgo-Tobon, S.; López-Martínez, B.; Klunder-Klunder, M.; Miranda-Lora, A.L.; Thomas, E.L.; Bell, J.D.; Breen, D.J.; et al. Reference Range of Quantitative MRI Metrics Corrected T1 and Liver Fat Content in Children and Young Adults: Pooled Participant Analysis. Children 2024, 11, 1230. https://doi.org/10.3390/children11101230
Shumbayawonda E, Beyer C, de Celis Alonso B, Hidalgo-Tobon S, López-Martínez B, Klunder-Klunder M, Miranda-Lora AL, Thomas EL, Bell JD, Breen DJ, et al. Reference Range of Quantitative MRI Metrics Corrected T1 and Liver Fat Content in Children and Young Adults: Pooled Participant Analysis. Children. 2024; 11(10):1230. https://doi.org/10.3390/children11101230
Chicago/Turabian StyleShumbayawonda, Elizabeth, Cayden Beyer, Benito de Celis Alonso, Silvia Hidalgo-Tobon, Briceida López-Martínez, Miguel Klunder-Klunder, América Liliana Miranda-Lora, E. Louise Thomas, Jimmy D. Bell, David J. Breen, and et al. 2024. "Reference Range of Quantitative MRI Metrics Corrected T1 and Liver Fat Content in Children and Young Adults: Pooled Participant Analysis" Children 11, no. 10: 1230. https://doi.org/10.3390/children11101230
APA StyleShumbayawonda, E., Beyer, C., de Celis Alonso, B., Hidalgo-Tobon, S., López-Martínez, B., Klunder-Klunder, M., Miranda-Lora, A. L., Thomas, E. L., Bell, J. D., Breen, D. J., Janowski, K., Pronicki, M., Grajkowska, W., Wozniak, M., Jurkiewicz, E., Banerjee, R., Socha, P., & So, P.-W. (2024). Reference Range of Quantitative MRI Metrics Corrected T1 and Liver Fat Content in Children and Young Adults: Pooled Participant Analysis. Children, 11(10), 1230. https://doi.org/10.3390/children11101230








