Preterm Birth and Kidney Health: From the Womb to the Rest of Life
Abstract
1. Introduction
2. Preterm Birth and Kidney Development
2.1. Preterm Birth
2.2. Normal Course of Kidney Development
2.3. Preterm Birth, Low Nephron Numbers, and CAKUT
3. Preterm Birth and Later-Life CKD: Evidence from Human Studies
4. Preterm Birth and Kidney Programming: Evidence from Animal Studies
4.1. Animal Models of Preterm Birth
4.2. Animal Models of Kidney Programming
4.3. Low Nephron Numbers
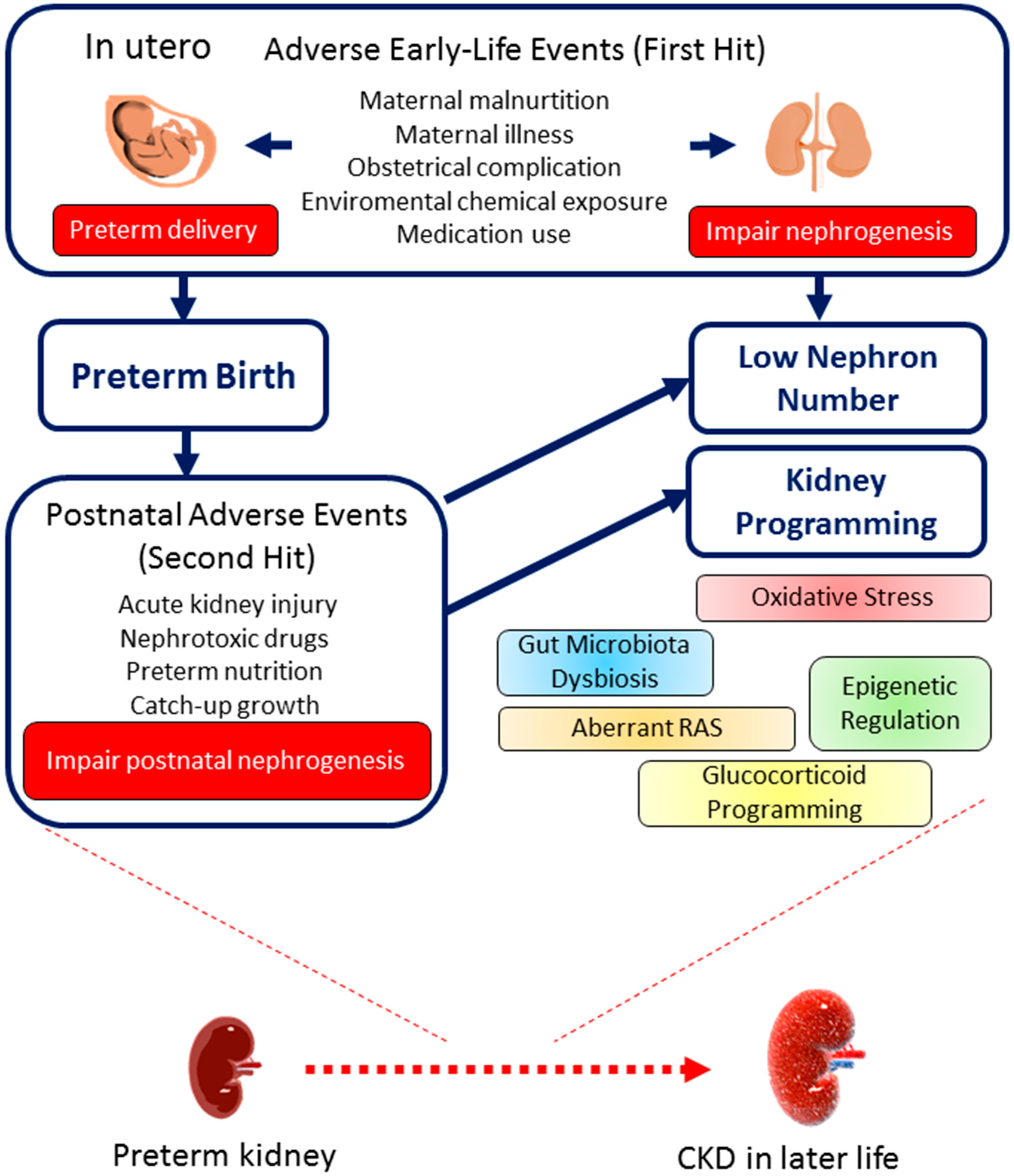
4.4. Prenatal Hits: Types of Maternal Insults
5. Hypothetical Mechanisms of Kidney Programming
5.1. Oxidative Stress
5.2. Aberrant RAS
5.3. Glucocorticoid Programming
5.4. Epigenetic Regulation
5.5. Gut Microbiota Dysbiosis
6. Postnatal Hits: What Preterm Infants May Face?
6.1. Acute Kidney Injury
6.2. Nephrotoxic Drugs
6.3. Preterm Nutrition and Catch-Up Growth
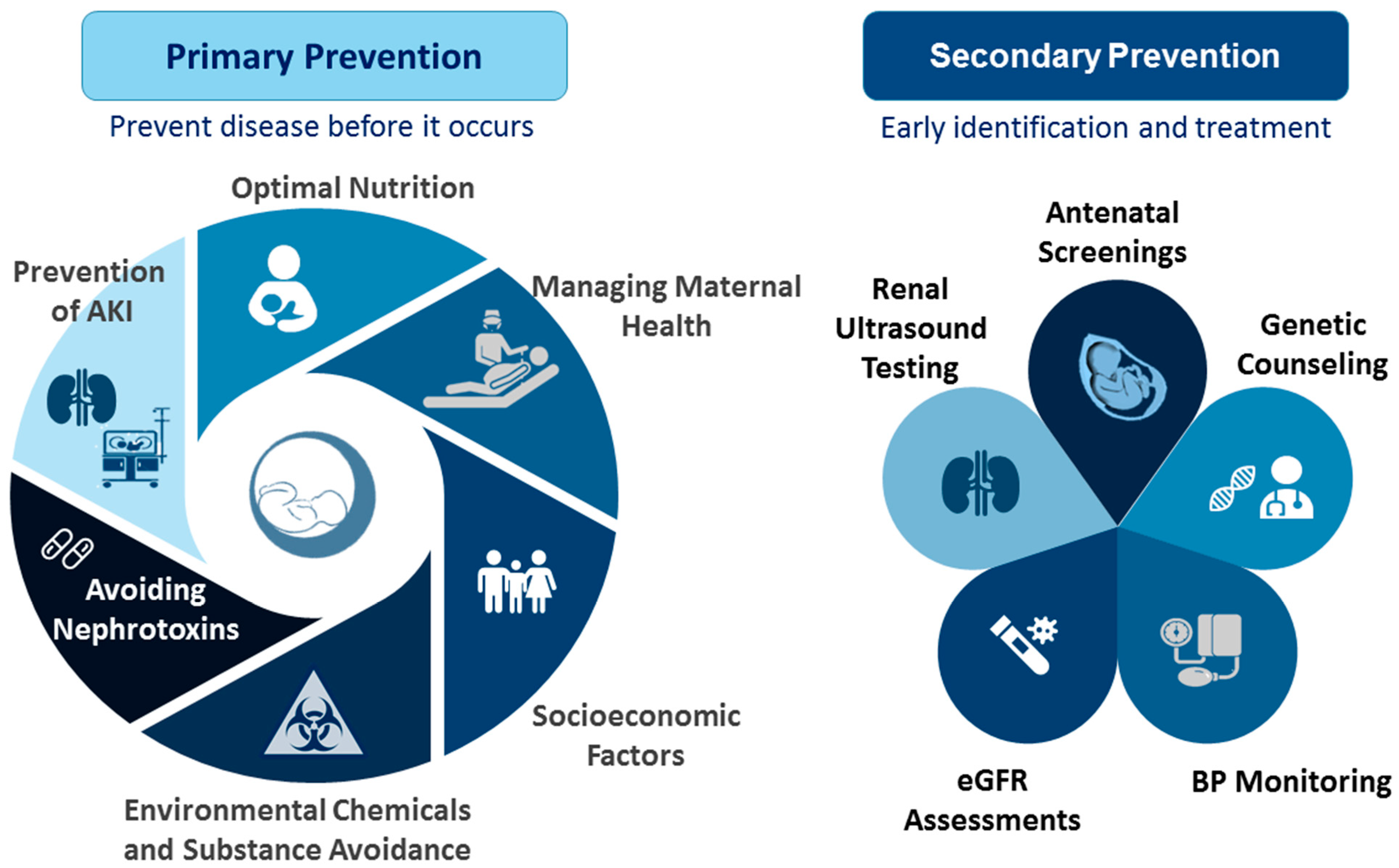
7. Recommended Actions: What Should We Do?
8. Strengths and Limitations
9. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, E.B.; Damião, R.; Moreira, D.A. Preterm birth prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 40–49. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Golding, J.; Kuh, D.; Wadsworth, M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989, 298, 564–567. [Google Scholar] [CrossRef]
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2014, 6, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Pravia, C.I.; Benny, M. Long-term consequences of prematurity. Cleve. Clin. J. Med. 2020, 87, 759–767. [Google Scholar] [CrossRef] [PubMed]
- GBD2017RiskFactor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. The First Thousand Days: Kidney Health and Beyond. Healthcare 2021, 9, 1332. [Google Scholar] [CrossRef]
- Chong, E.; Yosypiv, I.V. Developmental programming of hypertension and kidney disease. Int. J. Nephrol. 2012, 2012, 760580. [Google Scholar] [CrossRef]
- Ingelfinger, J.R.; Kalantar-Zadeh, K.; Schaefer, F. World Kidney Day Steering Committee. World Kidney Day 2016: Averting the legacy of kidney disease-focus on childhood. Pediatr. Nephrol. 2016, 31, 343–348. [Google Scholar] [CrossRef]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Developmental origins of chronic kidney disease: Should we focus on early life? Int. J. Mol. Sci. 2017, 18, 381. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Brenner, B.M. The clinical importance of nephron mass. J. Am. Soc. Nephrol. 2010, 21, 898–910. [Google Scholar] [CrossRef]
- Starr, M.C.; Hingorani, S.R. Prematurity and future kidney health: The growing risk of chronic kidney disease. Curr. Opin. Pediatr. 2018, 30, 228. [Google Scholar] [CrossRef]
- Nenov, V.D.; Taal, M.W.; Sakharova, O.V.; Brenner, B.M. Multi-hit nature of chronic renal disease. Curr. Opin. Nephrol. Hypertens. 2000, 9, 85–97. [Google Scholar] [CrossRef]
- Hack, M.; Flannery, D.J.; Schluchter, M.; Cartar, L.; Borawski, E.; Klein, N. Outcomes in young adulthood for very-low-birth-weight infants. N. Engl. J. Med. 2002, 346, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Shankaran, S. Short-and Long-Term Outcomes of Moderate and Late Preterm Infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar]
- Fernández de Gamarra-Oca, L.; Ojeda, N.; Gómez-Gastiasoro, A.; Peña, J.; Ibarretxe-Bilbao, N.; García-Guerrero, M.A.; Loureiro, B.; Zubiaurre-Elorza, L. Long-Term Neurodevelopmental Outcomes after Moderate and Late Preterm Birth: A Systematic Review. J. Pediatr. 2021, 237, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Carmody, J.B.; Charlton, J.R. Short-term gestation, long-term risk: Prematurity and chronic kidney disease. Pediatrics 2013, 131, 1168–1179. [Google Scholar] [CrossRef]
- Sangla, A.; Kandasamy, Y. Effects of prematurity on long-term renal health: A systematic review. BMJ Open 2021, 11, e047770. [Google Scholar] [CrossRef] [PubMed]
- Puddu, M.; Fanos, V.; Podda, F.; Zaffanello, M. The kidney from prenatal to adult life: Perinatal programming and reduction of number of nephrons during development. Am. J. Nephrol. 2009, 30, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Akalay, S.; Rayyan, M.; Fidlers, T.; van den Heuvel, L.; Levtchenko, E.; Arcolino, F.O. Impact of preterm birth on kidney health and development. Front. Med. 2024, 11, 1363097. [Google Scholar] [CrossRef]
- Engle, W.A. Age terminology during the perinatal period. Pediatrics 2004, 114, 1362–1364. [Google Scholar]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Mitrogiannis, I.; Evangelou, E.; Efthymiou, A.; Kanavos, T.; Birbas, E.; Makrydimas, G.; Papatheodorou, S. Risk factors for preterm birth: An umbrella review of meta-analyses of observational studies. BMC Med. 2023, 21, 494. [Google Scholar] [CrossRef]
- Hinchliffe, S.A.; Sargent, P.H.; Howard, C.V.; Chan, Y.F.; van Velzen, D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab. Investig. 1991, 64, 777–784. [Google Scholar]
- Shah, M.M.; Sampogna, R.V.; Sakurai, H.; Bush, K.T.; Nigam, S.K. Branching morphogenesis and kidney disease. Development 2004, 131, 1449–1462. [Google Scholar] [CrossRef]
- Bertram, J.F.; Douglas-Denton, R.N.; Diouf, B.; Hughson, M.; Hoy, W.E. Human nephron number: Implications for health and disease. Pediatr. Nephrol. 2011, 26, 1529–1533. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Korngold, E.; Teele, R.L. Sonographic assessment of renal length in normal children. Am. J. Roentgenol. 1984, 142, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Filler, G.; Lopes, L.; Awuku, M. The importance of accurately assessing renal function in the neonate and infant. Adv. Clin. Chem. 2015, 71, 141–156. [Google Scholar] [PubMed]
- Liu, L.; Barajas, L. The rat renal nerves during development. Anat. Embryol. 1993, 188, 345–361. [Google Scholar] [CrossRef]
- Barajas, L.; Liu, L. The renal nerves in the newborn rat. Pediatr. Nephrol. 1993, 7, 657–666. [Google Scholar] [CrossRef]
- Tain, Y.L.; Luh, H.; Lin, C.Y.; Hsu, C.N. Incidence and Risks of Congenital Anomalies of Kidney and Urinary Tract in Newborns: A Population-Based Case-Control Study in Taiwan. Medicine 2016, 95, e2659. [Google Scholar] [CrossRef]
- Murugapoopathy, V.; Gupta, I.R. A primer on congenital anomalies of the kidneys and urinary tracts (CAKUT). Clin. J. Am. Soc. Nephrol. 2020, 15, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, G.; Tsuboi, N.; Shimizu, A.; Yokoo, T. Human nephron number, hypertension, and renal pathology. Anat. Rec. 2020, 303, 2537–2543. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Brenner, B.M. Birth weight, malnutrition and kidney-associated outcomes—A global concern. Nat. Rev. Nephrol. 2015, 11, 135–149. [Google Scholar] [CrossRef]
- Faa, G.; Gerosa, C.; Fanni, D.; Nemolato, S.; Locci, A.; Cabras, T.; Marinelli, V.; Puddu, M.; Zaffanello, M.; Monga, G.; et al. Marked interindividual variability in renal maturation of preterm infants: Lessons from autopsy. J. Matern. Fetal Neonatal Med. 2010, 23, 129–133. [Google Scholar] [CrossRef]
- Beeman, S.C.; Cullen-McEwen, L.A.; Puelles, G.; Zhang, M.; Wu, T.; Baldelomar, E.J.; Dowling, J.; Charlton, J.R.; Forbes, M.S.; Ng, A.; et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am. J. Physiol. Ren. Physiol. 2014, 306, F1381–F1390. [Google Scholar] [CrossRef]
- Vujic, A.; Kosutic, J.; Bogdanovic, R.; Prijic, S.; Milicic, B.; Igrutinovic, Z. Sonographic assessment of normal kidney dimensions in the first year of life—A study of 992 healthy infants. Pediatr. Nephrol. 2007, 22, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, D.M.; Lee, S.H.; Cho, Y.D. Usefulness of renal volume measurements obtained by a 3-dimensional sonographic transducer with matrix electronic arrays. J. Ultrasound Med. 2008, 27, 1673–1681. [Google Scholar] [CrossRef]
- Horie, A.; Abe, Y.; Koike, D.; Hirade, T.; Nariai, A.; Ito, T.; Katou, F. Long-term renal follow up of preterm neonates born before 35 weeks of gestation. Pediatr. Int. 2019, 61, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Kwinta, P.; Klimek, M.; Drozdz, D.; Grudzień, A.; Jagła, M.; Zasada, M.; Pietrzyk, J.J. Assessment of long-term renal complications in extremely low birth weight children. Pediatr. Nephrol. 2011, 26, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Vollsæter, M.; Halvorsen, T.; Markestad, T.; Øymar, K.; Ueland, P.M.; Meyer, K.; Midttun, Ø.; Bjørke-Monsen, A.L. Renal function and blood pressure in 11 year old children born extremely preterm or small for gestational age. PLoS ONE 2018, 13, e0205558. [Google Scholar] [CrossRef]
- Starzec, K.; Klimek, M.; Grudzień, A.; Jagła, M.; Kwinta, P. Longitudinal assessment of renal size and function in extremely low birth weight children at 7 and 11 years of age. Pediatr. Nephrol. 2016, 31, 2119–2126. [Google Scholar] [CrossRef]
- Raaijmakers, A.; Zhang, Z.Y.; Claessens, J.; Cauwenberghs, N.; van Tienoven, T.P.; Wei, F.F.; Jacobs, L.; Levtchenko, E.; Pauwels, S.; Kuznetsova, T.; et al. Does Extremely Low Birth Weight Predispose to Low-Renin Hypertension? Hypertension 2017, 69, 443–449. [Google Scholar] [CrossRef]
- Rodríguez-Soriano, J.; Aguirre, M.; Oliveros, R.; Vallo, A. Long-term renal follow-up of extremely low birth weight infants. Pediatr. Nephrol. 2005, 20, 579–584. [Google Scholar] [CrossRef]
- South, A.M.; Nixon, P.A.; Chappell, M.C.; Diz, D.I.; Russell, G.B.; Jensen, E.T.; Shaltout, H.A.; O’Shea, T.M.; Washburn, L.K. Renal function and blood pressure are altered in adolescents born preterm. Pediatr. Nephrol. 2019, 34, 137–144. [Google Scholar] [CrossRef]
- Sanderson, K.R.; Chang, E.; Bjornstad, E.; Hogan, S.L.; Hu, Y.; Askenazi, D.; Fry, R.C.; O’Shea, T.M. Albuminuria, Hypertension, and Reduced Kidney Volumes in Adolescents Born Extremely Premature. Front. Pediatr. 2020, 8, 230. [Google Scholar] [CrossRef]
- Keijzer-Veen, M.G.; Schrevel, M.; Finken, M.J.; Dekker, F.W.; Nauta, J.; Hille, E.T.; Frölich, M.; van der Heijden, B.J. Dutch POPS-19 Collaborative Study Group. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J. Am. Soc. Nephrol. 2005, 16, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; Winkleby, M.A.; Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: National cohort study. BMJ 2019, 365, l1346. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Salonen, M.K.; Kajantie, E.; Osmond, C. Prenatal growth and CKD in older adults: Longitudinal findings from the Helsinki birth cohort study, 1924–1944. Am. J. Kidney Dis. 2018, 71, 20–26. [Google Scholar] [CrossRef]
- Gjerde, A.; Lillas, B.S.; Marti, H.P.; Reisaeter, A.V.; Vikse, B.E. Intrauterine growth restriction, preterm birth and risk of end-stage renal disease during the first 50 years of life. Nephrol. Dial. Transplant. 2020, 35, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Manuel, C.R.; Ashby, C.R.; Reznik, S.E. Discrepancies in Animal Models of Preterm Birth. Curr. Pharm. Des. 2017, 23, 6142–6148. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Mrinalini, C. Animal models of preterm birth. Trends Endocrinol. Metab. 2004, 15, 479–487. [Google Scholar] [CrossRef]
- Green, E.S.; Arck, P.C. Pathogenesis of preterm birth: Bidirectional inflammation in mother and fetus. Semin. Immunopathol. 2020, 42, 413–429. [Google Scholar] [CrossRef]
- Valenzuela, I.; Kinoshita, M.; van der Merwe, J.; Maršál, K.; Deprest, J. Prenatal interventions for fetal growth restriction in animal models: A systematic review. Placenta 2022, 126, 90–113. [Google Scholar] [CrossRef]
- Aljunaidy, M.M.; Morton, J.S.; Kirschenman, R.; Phillips, T.; Case, C.P.; Cooke, C.M.; Davidge, S.T. Maternal treatment with a placental-targeted antioxidant (MitoQ) impacts offspring cardiovascular function in a rat model of prenatal hypoxia. Pharmacol. Res. 2018, 134, 332–342. [Google Scholar] [CrossRef]
- Lin, F.; Yu, X.; Zhang, X.; Guo, Y.; Huang, Y.; Zhou, J.; Zeng, P.; Ye, D.; Huang, Y. A synthetic analog of lipoxin A4 partially alleviates dexamethasone-induced fetal growth restriction in rats. Placenta 2013, 34, 941–948. [Google Scholar] [CrossRef]
- Al Ghafli, M.H.M.; Padmanabhan, R.; Kataya, H.H.; Berg, B. Effects of alpha-lipoic acid supplementation on maternal diabetes-induced growth retardation and congenital anomalies in rat fetuses. Mol. Cell. Biochem. 2004, 261, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Gorbatova, D.M.; Nemova, E.P.; Solomina, A.S.; Durnev, A.D.; Seredenin, S.B. Prenatal effects of peat combustion products and afobazole correction thereof in the rat progeny. Bull. Exp. Biol. Med. 2015, 158, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Turgut, N.H.; Temiz, T.K.; Bagcivan, I.; Turgut, B.; Gulturk, S.; Karadas, B. The effect of sildenafil on the altered thoracic aorta smooth muscle responses in rat pre-eclampsia model. Eur. J. Pharmacol. 2008, 589, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, A.P.; Nowak, P.C.; Tran, N.T.; Winer, N.; Darmaun, D. L-citrulline supplementation enhances fetal growth and protein synthesis in rats with intrauterine growth restriction. J. Nutr. 2016, 146, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.W.; Wakatsuki, K.A.; Hamada, F.; Shinohara, K.; Hayashi, Y.; Imamura, R.; Fukaya, T. Melatonin preserves fetal growth in rats by protecting against ischemia/reperfusion-induced oxidative/nitrosative mitochondrial damage in the placenta. J. Pineal Res. 2008, 45, 271–276. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hu, X.G.; Zhou, Y.; Yu, Z.; Fu, L.; Zhang, G.B.; Bo, Q.L.; Wang, H.; Zhang, C.; Xu, D.X. Obeticholic Acid Protects against Lipopolysaccharide-Induced Fetal Death and Intrauterine Growth Restriction through Its Anti-Inflammatory Activity. J. Immunol. 2016, 197, 4762–4770. [Google Scholar] [CrossRef]
- Koleganova, N.; Piecha, G.; Ritz, E.; Becker, L.E.; Müller, A.; Weckbach, M.; Nyengaard, J.R.; Schirmacher, P.; Gross-Weissmann, M.L. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am. J. Physiol. Renal Physiol. 2011, 301, F344–F354. [Google Scholar] [CrossRef]
- Luzardo, R.; Silva, P.A.; Einicker-Lamas, M.; Ortiz-Costa, S.; do Carmo Mda, G.; Vieira-Filho, L.D.; Paixão, A.D.; Lara, L.S.; Vieyra, A. Metabolic programming during lactation stimulates renal Na+ transport in the adult offspring due to an early impact on local angiotensin II pathways. PLoS ONE 2011, 6, e21232. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal l-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chen, C.C.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Melatonin attenuates prenatal dexamethasone-induced blood pressure increase in a rat model. J. Am. Soc. Hypertens. 2014, 8, 216–226. [Google Scholar] [CrossRef]
- Lisle, S.J.; Lewis, R.M.; Petry, C.J.; Ozanne, S.E.; Hales, C.N.; Forhead, A.J. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br. J. Nutr. 2003, 90, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Paixão, A.D.; Maciel, C.R.; Teles, M.B.; Figueiredo-Silva, J. Regional Brazilian diet-induced low birth weight is correlated with changes in renal hemodynamics and glomerular morphometry in adult age. Biol. Neonate 2001, 80, 239–246. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Merlet-Bénichou, C.; Gilbert, T.; Muffat-Joly, M.; Lelièvre-Pégorier, M.; Leroy, B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr. Nephrol. 1994, 8, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.Q.; Zhang, H.G.; Yuan, Z.B.; Yang, D.L.; Hao, L.Y.; Li, X.H. Prenatal exposure to lipopolysaccharide alters the intrarenal renin-angiotensin system and renal damage in offspring rats. Hypertens. Res. 2010, 33, 76–82. [Google Scholar] [CrossRef]
- Gray, S.P.; Denton, K.M.; Cullen-McEwen, L.; Bertram, J.F.; Moritz, K.M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 2010, 21, 1891–1902. [Google Scholar] [CrossRef]
- Wei, Z.; Song, L.; Wei, J.; Chen, T.; Chen, J.; Lin, Y.; Xia, W.; Xu, B.; Li, X.; Chen, X.; et al. Maternal exposure to di-(2-ethylhexyl)phthalate alters kidney development through the renin-angiotensin system in offspring. Toxicol. Lett. 2012, 212, 212–221. [Google Scholar] [CrossRef]
- Celsi, G.; Kistner, A.; Aizman, R.; Eklöf, A.C.; Ceccatelli, S.; de Santiago, A.; Jacobson, S.H. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr. Res. 1998, 44, 317–322. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Quan, A.; Weinberg, A.; Baum, M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001, 59, 1663–1669. [Google Scholar] [CrossRef]
- Woods, L.L.; Morgan, T.K.; Resko, J.A. Castration fails to prevent prenatally programmed hypertension in male rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1111–R1116. [Google Scholar] [CrossRef]
- Slabiak-Blaz, N.; Adamczak, M.; Gut, N.; Grajoszek, A.; Nyengaard, J.R.; Ritz, E.; Wiecek, A. Administration of cyclosporine a in pregnant rats—The effect on blood pressure and on the glomerular number in their offspring. Kidney Blood Press. Res. 2015, 40, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Lee, C.T.; Lin, Y.J.; Tsai, C.C. N-Acetylcysteine prevents programmed hypertension in male rat offspring born to suramin-treated mothers. Biol. Reprod. 2016, 95, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.D.; Walton, S.L.; Gazzard, S.E.; van der Wolde, J.; Mathias, P.C.F.; Moritz, K.M.; Cullen-McEwen, L.A.; Bertram, J.F. Maternal hypoxia developmentally programs low podocyte endowment in male, but not female offspring. Anat. Rec. 2020, 303, 2668–2678. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef]
- Solhaug, M.J.; Bolger, P.M.; AJose, P. The developing kidney and environmental toxins. Pediatrics 2004, 113, 1084–1091. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Adverse Impact of Environmental Chemicals on Developmental Origins of Kidney Disease and Hypertension. Front. Endocrinol. 2021, 12, 745716. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Tain, Y.L. Maternal exposure to bisphenol A combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018, 19, 2459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Chen, L.; Wang, X.J.; Jiang, Q.H.; Bei, X.Y.; Sun, W.L.; Xia, S.J.; Jiang, J.T. Maternal exposure to di-n-butyl phthalate (DBP) induces renal fibrosis in adult rat offspring. Oncotarget 2017, 8, 31101–31111. [Google Scholar] [CrossRef]
- Sukjamnong, S.; Chan, Y.L.; Zakarya, R.; Nguyen, L.T.; Anwer, A.G.; Zaky, A.A.; Santiyanont, R.; Oliver, B.G.; Goldys, E.; Pollock, C.A.; et al. MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci. Rep. 2018, 8, 6631. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.F.; Bueters, R.R.; Huigen, M.C.; Russel, F.G.; Masereeuw, R.; van den Heuvel, L.P. Effect of drugs on renal development. Clin. J. Am. Soc. Nephrol. 2011, 6, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Li, L.C.; Kuo, H.C.; Hsu, C.N. Gestational Exposure to Maternal Systemic Glucocorticoids and Childhood Risk of CKD. Am. J. Kidney Dis. 2024, 84, 215–223.e1. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Tain, Y.L. Postnatal dexamethasone-induced programmed hypertension is related to the regulation of melatonin and its receptors. Steroids 2016, 108, 1–6. [Google Scholar] [CrossRef]
- Akiyama, S.; Hamdeh, S.; Murakami, N.; Cotter, T.G.; Suzuki, H.; Tsuchiya, K. Pregnancy and neonatal outcomes in women receiving calcineurin inhibitors: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2022, 88, 3950–3961. [Google Scholar] [CrossRef]
- Quan, A. Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum. Dev. 2006, 82, 23–28. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Chronic Kidney Disease and Gut Microbiota: What Is Their Connection in Early Life? Int. J. Mol. Sci. 2022, 23, 3954. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide. 2016, 53, 6–12. [Google Scholar] [CrossRef]
- Jun, M.; Venkataraman, V.; Razavian, M.; Cooper, B.; Zoungas, S.; Ninomiya, T.; Webster, A.C.; Perkovic, V. Antioxidants for chronic kidney disease. Cochrane Database Syst. Rev. 2012, 10, CD008176. [Google Scholar] [CrossRef] [PubMed]
- Yosypiv, I.V. Renin-angiotensin system in ureteric bud branching morphogenesis: Insights into the mechanisms. Pediatr. Nephrol. 2011, 26, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.C.; Langley-Evans, S.C. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin. Sci. 2000, 98, 269–275. [Google Scholar] [CrossRef]
- Manning, J.; Vehaskari, V.M. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R80–R84. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 506–513. [Google Scholar] [CrossRef]
- Hsu, C.N.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y.; Tain, Y.L. Aliskiren administration during early postnatal life sex-specifically alleviates hypertension programmed by maternal high fructose consumption. Front. Physiol. 2016, 7, 299. [Google Scholar] [CrossRef]
- Pringle, K.G.; Zakar, T.; Lumbers, E.R. The intrauterine renin-angiotensin system: Sex-specific effects on the prevalence of spontaneous preterm birth. Clin. Exp. Pharmacol. Physiol. 2017, 44, 605–610. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Targeting the Renin-Angiotensin-Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021, 22, 2298. [Google Scholar] [CrossRef]
- Singh, R.R.; Moritz, K.M.; Bertram, J.F.; Cullen-McEwen, L.A. Effects of dexamethasone exposure on rat metanephric development: In vitro and in vivo studies. Am. J. Physiol. Renal Physiol. 2007, 293, F548–F554. [Google Scholar] [CrossRef]
- Singh, R.R.; Cuffe, J.S.; Moritz, K.M. Short-and long-term effects of exposure to natural and synthetic glucocorticoids during development. Clin. Exp. Pharmacol. Physiol. 2012, 39, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 2: Mechanisms. Nat. Rev. Endocrinol. 2014, 10, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Chang, H.Y.; Tain, Y.L. Prenatal dexamethasone-induced programmed hypertension and renal programming. Life Sci. 2015, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro de Andrade Ramos, B.; da Silva, M.G. The Burden of Genetic and Epigenetic Traits in Prematurity. Reprod. Sci. 2018, 25, 471–479. [Google Scholar] [CrossRef]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y.; Lee, C.T. Transcriptome analysis in rat kidneys: Importance of genes involved in programmed hypertension. Int. J. Mol. Sci. 2015, 16, 4744–4758. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y.; Huang, L.T. Renal Transcriptome analysis of programmed hypertension induced by maternal nutritional insults. Int. J. Mol. Sci. 2015, 16, 17826–17837. [Google Scholar] [CrossRef]
- Song, R.; Van Buren, T.; Yosypiv, I.V. Histone deacetylases are critical regulators of the renin-angiotensin system during ureteric bud branching morphogenesis. Pediatr. Res. 2010, 67, 573–578. [Google Scholar] [CrossRef]
- Wu, T.H.; Kuo, H.C.; Lin, I.C.; Chien, S.J.; Huang, L.T.; Tain, Y.L. Melatonin prevents neonatal dexamethasone induced programmed hypertension: Histone deacetylase inhibition. J. Steroid Biochem. Mol. Biol. 2014, 144, 253–259. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chan, J.Y.; Hsu, C.N. Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life. Nutrients 2016, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, A.A.; Lindsay, K.L.; Alberdi, G.; McAuliffe, F.M.; Gibney, E.R. Nutrition During Pregnancy Impacts Offspring’s Epigenetic Status-Evidence from Human and Animal Studies. Nutr. Metab. Insights 2016, 8, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ilicic, M.; Zakar, T.; Gregson, A.; Hussein, W.M.; Smith, R.; Paul, J.W. Histone Deacetylase Inhibitors: Providing New Insights and Therapeutic Avenues for Unlocking Human Birth. Reprod. Sci. 2022, 29, 3134–3146. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; De La Cochetiere, M.-F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Mercer, E.M.; Arrieta, M.C. Probiotics to improve the gut microbiome in premature infants: Are we there yet? Gut Microbes 2023, 15, 2201160. [Google Scholar] [CrossRef]
- Chirico, V.; Lacquaniti, A.; Tripodi, F.; Conti, G.; Marseglia, L.; Monardo, P.; Gitto, E.; Chimenz, R. Acute Kidney Injury in Neonatal Intensive Care Unit: Epidemiology, Diagnosis and Risk Factors. J. Clin. Med. 2024, 13, 3446. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Pei, J.; Jiang, X.; Tang, J. Acute kidney injury in premature and low birth weight neonates: A systematic review and meta-analysis. Pediatr. Nephrol. 2022, 37, 275–287. [Google Scholar] [CrossRef]
- Cai, C.; Qiu, G.; Hong, W.; Shen, Y.; Gong, X. Clinical effect and safety of continuous renal replacement therapy in the treatment of neonatal sepsis-related acute kidney injury. BMC Nephrol. 2020, 21, 286. [Google Scholar] [CrossRef]
- Muk, T.; Jiang, P.P.; Stensballe, A.; Skovgaard, K.; Sangild, P.T.; Nguyen, D.N. Prenatal Endotoxin Exposure Induces Fetal and Neonatal Renal Inflammation via Innate and Th1 Immune Activation in Preterm Pigs. Front. Immunol. 2020, 11, 565484. [Google Scholar] [CrossRef] [PubMed]
- McCurnin, D.; Seidner, S.; Chang, L.Y.; Waleh, N.; Ikegami, M.; Petershack, J.; Yoder, B.; Giavedoni, L.; Albertine, K.H.; Dahl, M.J.; et al. Ibuprofen-induced patent ductus arteriosus closure: Physiologic, histologic, and biochemical effects on the premature lung. Pediatrics 2008, 121, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.W.; Groeneveld, A.B.; Slutsky, A.S.; Plötz, F.B. Mechanical ventilation and acute renal failure. Crit. Care Med. 2005, 33, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, A.D.; Griffin, R.L.; Vincent, K.; Askenazi, D.J.; Segar, J.L.; Kupferman, J.C.; Rastogi, S.; Selewski, D.T.; Steflik, H.J. Incidence, Risk Factors, and Outcomes Associated with Recurrent Neonatal Acute Kidney Injury in the AWAKEN Study. JAMA 2024, 7, e2355307. [Google Scholar] [CrossRef]
- Adegboyega, O.O.; Singh, Y.; Bhutada, A.; Kupferman, J.C.; Rastogi, S. Recurrent acute kidney injury in preterm neonates is common and associated with worse outcomes and higher mortality. Pediatr. Res. 2022, 92, 284–290. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Ng, K.H.; Mammen, C. The path to chronic kidney disease following acute kidney injury: A neonatal perspective. Pediatr. Nephrol. 2017, 32, 227–241. [Google Scholar] [CrossRef]
- Steflik, H.J.; Charlton, J.R.; Briley, M.; Selewski, D.T.; Gist, K.M.; Hanna, M.H.; Askenazi, D.; Griffin, R. Neonatal Kidney Collaborative*. Neonatal Nephrotoxic Medication Exposure and Early Acute Kidney Injury: Results from the AWAKEN Study. J. Perinatol. 2023, 43, 1029–1037. [Google Scholar] [CrossRef]
- Murphy, H.J.; Thomas, B.; Van Wyk, B.; Tierney, S.B.; Selewski, D.T.; Jetton, J.G. Nephrotoxic medications and acute kidney injury risk factors in the neonatal intensive care unit: Clinical challenges for neonatologists and nephrologists. Pediatr. Nephrol. 2020, 35, 2077–2088. [Google Scholar] [CrossRef]
- Mohamed, T.H.; Abdi, H.H.; Magers, J.; Prusakov, P.; Slaughter, J.L. Nephrotoxic medications and associated acute kidney injury in hospitalized neonates. J. Nephrol. 2022, 35, 1679–1687. [Google Scholar] [CrossRef]
- Slater, M.B.; Gruneir, A.; Rochon, P.A.; Howard, A.W.; Koren, G.; Parshuram, C.S. Identifying high-risk medications associated with acute kidney injury in critically ill patients: A pharmacoepidemiologic evaluation. Paediatr. Drugs 2017, 19, 59–67. [Google Scholar] [CrossRef]
- Hanna, M.H.; Askenazi, D.J.; Selewski, D.T. Drug-induced acute kidney injury in neonates. Curr. Opin. Pediatr. 2016, 28, 180–187. [Google Scholar] [CrossRef] [PubMed]
- De Cock, R.F.; Allegaert, K.; Schreuder, M.F.; Sherwin, C.M.; de Hoog, M.; van den Anker, J.N.; Danhof, M.; Knibbe, C.A. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin. Pharmacokinet. 2012, 51, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.W.; Koo, S.H.; Lee, J.H.; Hwang, J.H.; Kim, S.S.; Lee, E.K.; Chang, W.; Chang, Y.S.; Park, W.S. A comparison of AmBisome to amphotericin B for treatment of systemic candidiasis in very low birth weight infants. Yonsei Med. J. 2007, 48, 619–626. [Google Scholar] [CrossRef]
- Allegaert, K. The impact of ibuprofen or indomethacin on renal drug clearance in neonates. J. Matern. Fetal Neonatal Med. 2009, 22, 88–91. [Google Scholar] [CrossRef]
- Gouyon, J.B.; Guignard, J.P. Management of acute renal failure in newborns. Pediatr. Nephrol. 2000, 14, 1037–1044. [Google Scholar] [CrossRef]
- Guignard, J.P.; Iacobelli, S. Use of diuretics in the neonatal period. Pediatr. Nephrol. 2021, 36, 2687–2695. [Google Scholar] [CrossRef]
- Stoops, C.; Stone, S.; Evans, E.; Dill, L.; Henderson, T.; Griffin, R.; Goldstein, S.L.; Coghill, C.; Askenazi, D.J. Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): Reduction of Nephrotoxic Medication-Associated Acute Kidney Injury in the Neonatal Intensive Care Unit. J. Pediatr. 2019, 215, 223–228.e6. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, S.; Germani, D.; Branca, F. Low birthweight and adult insulin resistance: The “catch-up growth” hypothesis. Arch. Dis. Child. Fetal Neonatal. 1999, 81, F71–F73. [Google Scholar] [CrossRef]
- McLeod, G.; Farrent, S.; Gilroy, M.; Page, D.; Oliver, C.J.; Richmond, F.; Cormack, B.E. Variation in neonatal nutrition practice and implications: A survey of Australia and New Zealand neonatal units. Front. Nutr. 2021, 8, 8. [Google Scholar] [CrossRef]
- Meiliana, M.; Alexander, T.; Bloomfield, F.H.; Cormack, B.E.; Harding, J.E.; Walsh, O.; Lin, L. Nutrition guidelines for preterm infants: A systematic review. JPEN J. Parenter. Enteral. Nutr. 2024, 48, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Georgieff, M.K. Committee on nutrition. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: A systematic review and meta-analysis. J. Pediatr. 2019, 210, 69–80. [Google Scholar] [CrossRef]
- Embleton, N.D.; Korada, M.; Wood, C.L.; Pearce, M.S.; Swamy, R.; Cheetham, T.D. Catch-Up growth and metabolic outcomes in adolescents born preterm. Arch. Dis. Child 2016, 101, 1026–1031. [Google Scholar] [CrossRef]
- Cauzzo, C.; Chiavaroli, V.; Di Valerio, S.; Chiarelli, F. Birth size, growth trajectory and later cardio-metabolic risk. Front. Endocrinol. 2023, 14, 1187261. [Google Scholar] [CrossRef]
- Liu, C.; Tian, J.; Jose, M.D.; Dwyer, T.; Venn, A.J. BMI Trajectories from Childhood to Midlife are Associated with Subclinical Kidney Damage in Midlife. Obesity 2021, 29, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; RAngaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Garcia-Garcia, G.; Lui, S.-F.; Andreoli, S.; Fung, W.W.-S.; Hradsky, A.; Kumaraswami, L.; Liakopoulos, V.; Rakhimova, Z.; Saadi, G.; et al. Kidney health for everyone everywhere—From prevention to detection and equitable access to care. Pediatr. Nephrol. 2020, 35, 1801–1810. [Google Scholar] [CrossRef]
- Beluska-Turkan, K.; Korczak, R.; Hartell, B.; Moskal, K.; Maukonen, J.; Alexander, D.E.; Salem, N.; Harkness, L.; Ayad, W.; Szaro, J.; et al. Nutritional gaps and supplementation in the first 1000 days. Nutrients 2019, 11, 2891. [Google Scholar] [CrossRef]
- Boies, E.G.; Vaucher, Y.E. ABM Clinical Protocol #10: Breastfeeding the Late Preterm (34-36 6/7 Weeks of Gestation) and Early Term Infants (37-38 6/7 Weeks of Gestation), Second Revision 2016. Breastfeed Med. 2016, 11, 494–500. [Google Scholar] [PubMed]
- World Health Organization: Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 18 September 2024).
- Chang, H.H.; Larson, J.; Blencowe, H.; Spong, C.Y.; Howson, C.P.; Cairns-Smith, S.; Lackritz, E.; Lee, S.K.; Mason, E.; Serazin, A.C.; et al. Born Too Soon preterm prevention analysis group. Preventing preterm births: Analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 2013, 381, 223–234. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Marchant, A.; Way, S.S. Vaccination strategies to enhance immunity in neonates. Science 2020, 368, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422D. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.J.; Bihari, D.J. Preventing renal failure in the critically ill. There are no magic bullets—Just high quality intensive care. BMJ 2001, 322, 1437–1439. [Google Scholar] [PubMed]
- Jetton, J.G.; Askenazi, D.J. Acute kidney injury in the neonate. Clin. Perinatol. 2014, 41, 487–502. [Google Scholar] [CrossRef]
- Low Birth Weight and Nephron Number Working Group. The Impact of Kidney Development on the Life Course: A Consensus Document for Action. Nephron 2017, 136, 3–49. [Google Scholar] [CrossRef]
- Htay, H.; Alrukhaimi, M.; Ashuntantang, G.E.; Bello, A.K.; Bellorin-Font, E.; Gharbi, M.B.; Braam, B.; Feehally, J.; Harris, D.C.; Jha, V.; et al. Global access of patients with kidney disease to health technologies and medications: Findings from the Global Kidney Health Atlas project. Kidney Int. Suppl. 2018, 8, 64–73. [Google Scholar] [CrossRef]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury—Pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Puelles, V.G.; Bertram, J.F. Counting glomeruli and podocytes: Rationale and methodologies. Curr. Opin. Nephrol. Hypertens. 2015, 24, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Developmental origins of kidney disease: Why oxidative stress matters? Antioxidants 2021, 10, 33. [Google Scholar] [CrossRef]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal taurine supplementation prevents metabolic and cardiovascular effects of maternal diabetes in adult rat offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar] [PubMed]
- Torrens, C.; Brawley, L.; Anthony, F.W.; Dance, C.S.; Dunn, R.; Jackson, A.A.; Poston, L.; Hanson, M.A. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension 2006, 47, 982–987. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Hrenak, J.; Paulis, L.; Repova, K.; Aziriova, S.; Nagtegaal, E.J.; Reiter, R.J.; Simko, F. Melatonin and renal protection: Novel perspectives from animal experiments and human studies (review). Curr. Pharm. Des. 2015, 21, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Tsiani, E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients 2019, 11, 1624. [Google Scholar] [CrossRef]
- Vieira-Filho, L.D.; Cabral, E.V.; Santos, F.T.; Coimbra, T.M.; Paixão, A.D. Alpha-tocopherol prevents intrauterine undernutrition-induced oligonephronia in rats. Pediatr. Nephrol. 2011, 26, 2019–2029. [Google Scholar] [CrossRef]
- Costa, M.R.; Pires, K.M.; Nalbones-Barbosa, M.N.; Dos Santos Valença, S.; Resende, Â.C.; de Moura, R.S. Grape skin extract-derived polyphenols modify programming-induced renal endowment in prenatal protein-restricted male mouse offspring. Eur. J. Nutr. 2016, 55, 1455–1464. [Google Scholar] [CrossRef]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supple mentation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef]
- Moore, T.A.; Ahmad, I.M.; Zimmerman, M.C. Oxidative Stress and Preterm Birth: An Integrative Review. Biol. Res. Nurs. 2018, 20, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Study Population | Sample Size | Age at Measure (Years) | Adverse Kidney Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Horie et al. | Japan | GA < 35 weeks | 168 | 2 | Low eGFR | [43] |
| Kwinta et al. | Poland | Median GA 27 weeks | 78 | 6–7 | Low kidney volume and high cystatin C | [44] |
| Vollsæter et al. | Norway | GA < 28 weeks | 57 | 11 | Low eGFR | [45] |
| Starzec et al. | Poland | GA < 28 weeks | 64 | 11 | Low kidney volume and high cystatin C | [46] |
| Raaijmakers et al. | Belgium | GA < 34 weeks | 93 | 11 | Low eGFR and kidney length, and high systolic and diastolic BP | [47] |
| Rodríguez-Soriano et al. | Spain | GA < 35 weeks | 40 | 6–12 | Low eGFR | [48] |
| South et al. | USA | GA < 37 weeks | 96 | 14 | Low eGFR and high systolic and diastolic BP | [49] |
| Sanderson et al. | USA | GA < 28 weeks | 42 | 15 | Low kidney volume, microalbuminuria, and elevated BP | [50] |
| Keijzer-Veen et al. | Netherlands | GA < 32 weeks | 442 | 19 | Low eGFR and microalbuminuria | [51] |
| Crump et al. | Sweden | GA < 28 weeks | 4,186,615 | 43 | CKD | [52] |
| Eriksson et al. | Finland | GA < 34 weeks | 20,431 | 86 or death | CKD | [53] |
| Experimental Model | Reduced Nephron Number | Age at Evaluation (Weeks) | Kidney Outcomes | Ref. |
|---|---|---|---|---|
| Maternal nutrition | ||||
| Low sodium diet (0.07%) during gestation and breastfeeding | Yes | 1 | ↑ BP at 5 mo | [67] |
| High sodium diet (3%) during gestation and breastfeeding | Yes | 1 | Glomerular hypertrophy, ↑ BP at 5 mo | [67] |
| Low protein diet (8% protein) during lactation | Yes | 8 | ↑ BP at 5 mo | [68] |
| 50% caloric restriction during gestation and breastfeeding | Yes | 12 | ↔ GFR, glomerular hypertrophy, ↑ BP, tubulointerstitial injury | [69] |
| Low protein diet (8.5% protein) during gestation | Yes | 22 | ↔ GFR, ↑ BP | [70] |
| Iron restriction diet (3 mg/kg diet) from 1 wk before mating and through pregnancy | Yes | 72 | Glomerular hypertrophy, ↑ BP | [71] |
| Multi-deficient diet during gestation | Yes | 12 | ↑ GFR, glomerular hypertrophy | [72] |
| Maternal illness and obstetrical complication | ||||
| Streptozotocin (STZ)-induced diabetes during gestation | Yes | 12 | ↔ GFR, ↑ BP, tubulointerstitial injury | [73] |
| Partial ligation of uterine ligation | Yes | 2 | ↓ GFR, glomerular hypertrophy | [74] |
| Lipopolysaccharide (0.79 mg/kg/day) i.p. at gestational day 8, 10, and 12 | Yes | 7 | ↓ GFR | [75] |
| Environmental chemical exposure | ||||
| Ethanol (1 g/kg/day) at gestational day 13.5 and 14.5 | Yes | 4 | ↓ GFR at 6 mo | [76] |
| DEHP exposure (0.25 or 6.25 mg/kg/day) during pregnancy | Yes | 21 | ↓ GFR, ↑ BP | [77] |
| Medication use | ||||
| Dexamethasone (0.1 mg/kg/day) during gestation | Yes | 8 | ↓ GFR, glomerular hypertrophy | [78] |
| Dexamethasone (0.2 mg/kg/day) at gestational day 15 and 16 or 17 and 18 | Yes | 8 | ↔ GFR, unchanged glomerular morphology | [79] |
| Dexamethasone (0.1 mg/kg/day) from gestational day 16 to 22. | Yes | 16 | ↑ BP | [80] |
| Cyclosporine (3.3 mg/kg/day) from gestational day 10 to postnatal day 7 | Yes | 12 | ↔ GFR, glomerular hypertrophy | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hsu, C.-N. Preterm Birth and Kidney Health: From the Womb to the Rest of Life. Children 2024, 11, 1213. https://doi.org/10.3390/children11101213
Tain Y-L, Hsu C-N. Preterm Birth and Kidney Health: From the Womb to the Rest of Life. Children. 2024; 11(10):1213. https://doi.org/10.3390/children11101213
Chicago/Turabian StyleTain, You-Lin, and Chien-Ning Hsu. 2024. "Preterm Birth and Kidney Health: From the Womb to the Rest of Life" Children 11, no. 10: 1213. https://doi.org/10.3390/children11101213
APA StyleTain, Y.-L., & Hsu, C.-N. (2024). Preterm Birth and Kidney Health: From the Womb to the Rest of Life. Children, 11(10), 1213. https://doi.org/10.3390/children11101213








