Diagnostic Accuracy of Physical Examination and Pulse Oximetry for Critical Congenital Cardiac Disease Screening in Newborns
Abstract
:1. Introduction
2. Materials and Methods
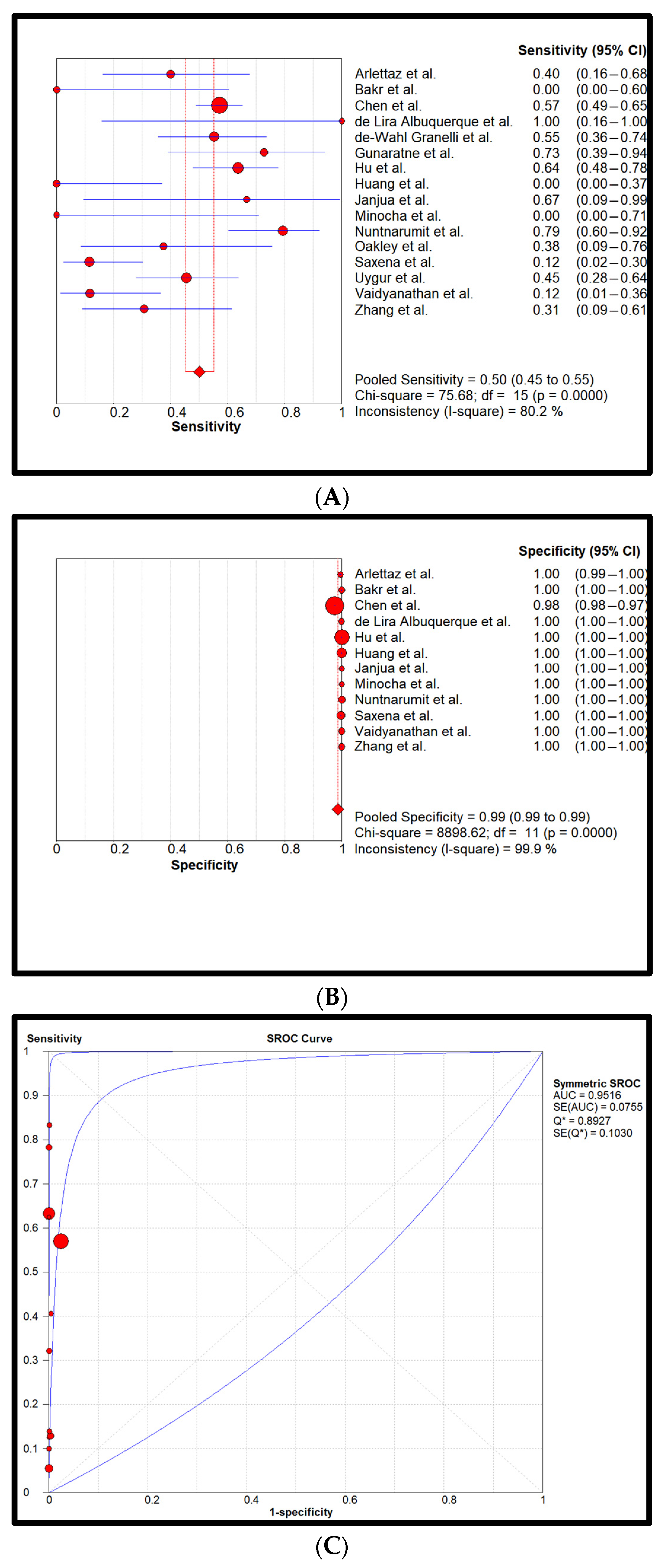
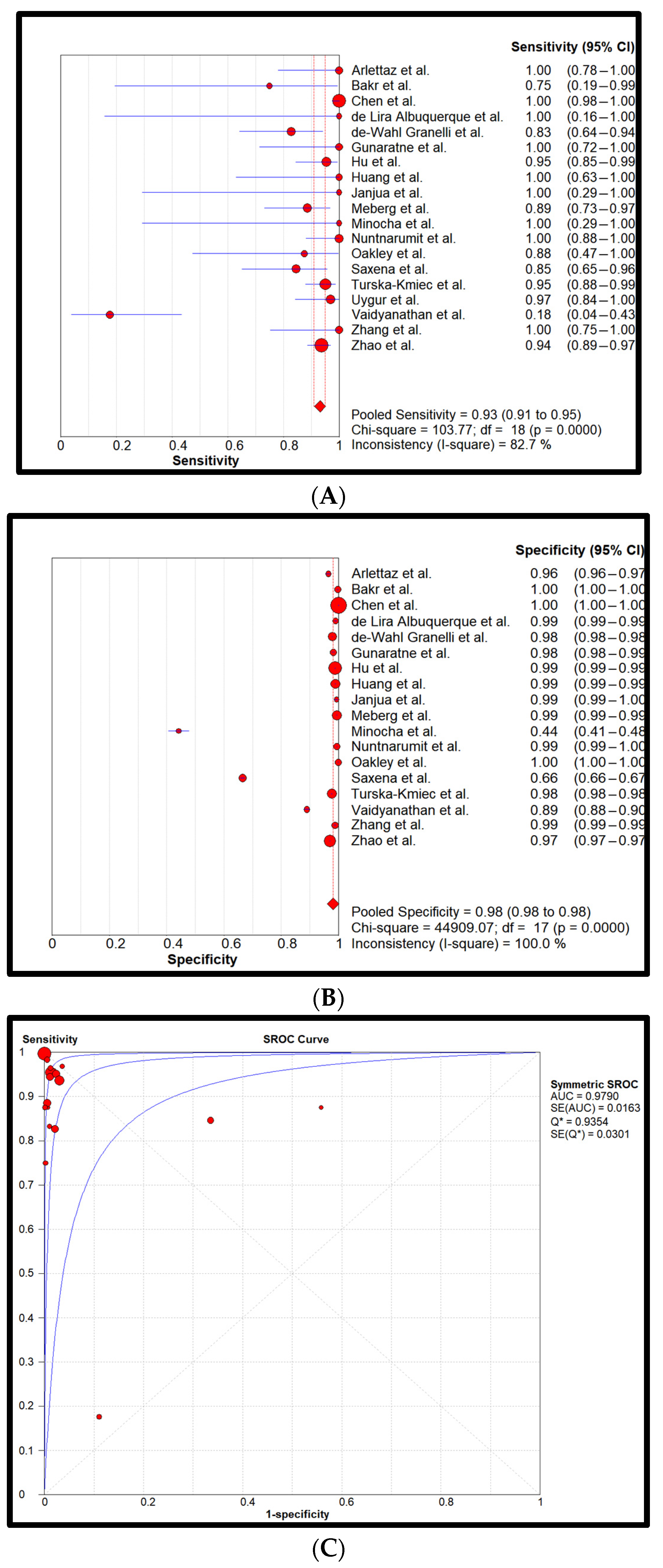
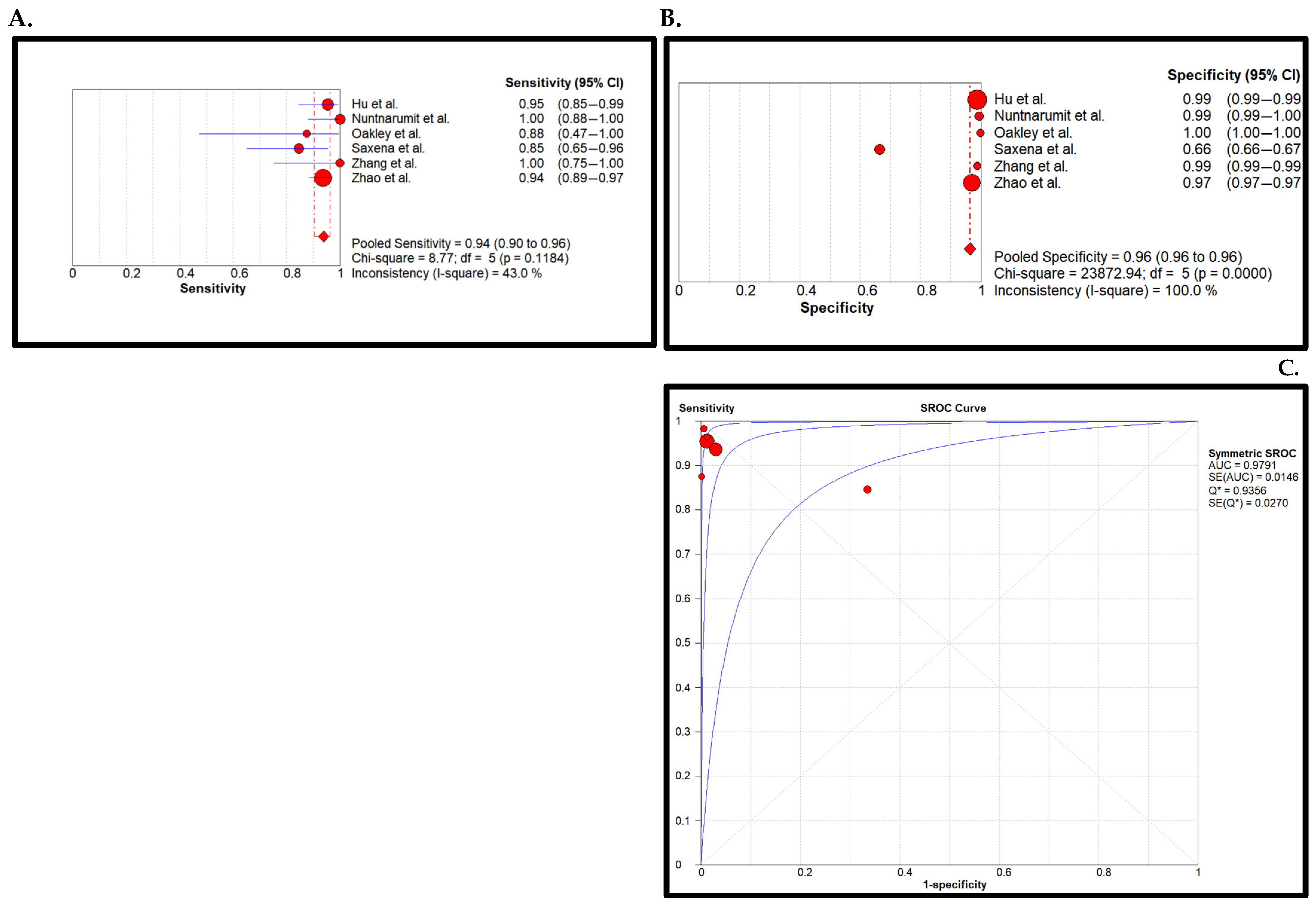
3. Results
4. Discussion
5. Conclusions
List of Abbreviations
| AC | Applicability Concern |
| AUC | Area Under the Curve |
| CHD | Congenital Heart Disease |
| CCHD | Critical Congenital Heart Disease |
| CI | Confidence Interval |
| DOR | Diagnostic Odds Ratio |
| FN | False Negatives |
| FP | False Positives |
| HIC | High-Income Countries |
| J.T | Jari Tristan |
| J.T.v.V. | Jari Tristan van Vliet |
| LMIC | Low Middle Income Countries |
| LR- | Negative Likelihood Ratio |
| LR+ | Positive Likelihood Ratio |
| M.G.S | Martijn G. Slieker |
| N.G.M. | Naizihijwa Gadi Majani |
| N.M | Naizihijwa Majani |
| NICU | Neonatal Intensive Care Unit |
| NPV | The average Negative Predictive Value |
| P.C | Pilly Chillo |
| PE | Physical Examination |
| PO | Pulse Oximetry |
| PPV | Positive Predictive Value |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QUADAS | Quality Assessment of primary Diagnostic Accuracy Studies |
| RoB | Risk of Bias |
| SROC | Summary Receiver Operating Curves |
| TN | True Negatives |
| TP | True Positives |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Overview of Included Studies
| Author | Year | Study Type | Study Group | Sample Size | CCHD (n) | Time of Screening | Location of PO Sensor | Cut-Off Values PO | PO Device | PE Components | Reference Standard | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arlettaz et al. [35] | 2006 | Prospective | All No resp. dist. No prematures | 3.262 | 15 | 6–12 h | Either foot | <95% | Nellcor NPB-40 | Murmur | Pre/Postnatal echocardiography | Zurich, Switzerland |
| Bakr et al. [36] | 2005 | Prospective | No NICU | 5.211 | 4 | 31,7 h | Right hand and either foot | <94% | Digioxi PO 920 | Murmur | Postnatal echocardiography | Taif, Saudi Arabia |
| Chen et al. [30] | 2023 | Prospective | All | 321.447 | 154 | 6–72 h | Right hand and either foot | <95% | Not specified | Murmur | Postnatal echocardiography | Hainan province, China |
| de Lira Albuquerque et al. [37] | 2015 | Prospective | All No Prematures | 4.027 | 2 | >24 h | Right hand and foot | <95% | PM 60 Mindray | Cyanosis, pulsations, and murmur | Postnatal echocardiography | Campina Grande, Brazil |
| de-Wahl Granelli et al. [29] | 2009 | Prospective | No NICU No prenatal diagnoses | 38.429 | 29 | 16 h before discharge | Right hand and either foot | <95% | Radical SET, version 4 | Murmur and femoral pulsations | Postnatal echocardiography | West Götaland province, Sweden |
| Gunaratne et al. [38] | 2021 | Prospective | No NICU No prematures No prenatal diagnoses No dysmorphic features | 5.435 | 11 | >24 h | Right hand and right foot | <95% | Radical7 Masimo SET Pulse | Cyanosis, femoral pulsations, and murmur | Postnatal echocardiography | Colombo, Sri Lanka |
| Hu et al. [27] | 2017 | Prospective | No NICU No prenatal diagnoses | 167.190 | 230 | 26 h | Right hand and either foot | <95% | RAD-5v Masimo | Murmur | Postnatal echocardiography | Shanghai, China |
| Huang et al. [39] | 2022 | Retrospective | All | 44.147 | 8 | 20 h | Right hand and either foot | <95% | Masimo | Murmur | Postnatal echocardiography | Linyi, China |
| Janjua et al. [26] | 2022 | Prospective | No NICU No prematures No dysmorphic features | 1.082 | 3 | <24 h | Either foot | <95% | (L&T Multipara monitor Planet 40 Nellcor oximax SpO2 module | Blood pressure, femoral pulsations, murmur, hepatomegaly, oedema, dysmorphism, colour, respiratory rate, and capillary refilling time | Postnatal echocardiography | Dubai, UAE |
| Meberg et al. [56] | 2008 | Prospective | No NICU No prenatal diagnoses | 50.008 | 35 | <48 h | Either foot | <95% | RAD-5v Masimo | Murmur, Cyanosis, and HF-symptoms | Postnatal echocardiography | Norway (14 hospitals) |
| Minocha et al. [32] | 2018 | Retrospective | No NICU No prenatal diagnoses No dysmorphic features | 777 | 3 | <72 h | Right hand and either foot | <95% | Unknown | Murmur | Postnatal echocardiography | Miami, USA |
| Nuntnarumit et al. [40] | 2018 | Prospective | No NICU No prematures No prenatal diagnoses No dysmorphic features | 10.603 | 29 | 48 h | Right hand and either foot | <95% | Masimo Radical 7 | Murmur, cyanosis, femoral pulsations, and blood pressure | Postnatal echocardiography | Bangkok & Nakhon Ratchasima, Thailand |
| Oakley et al. [41] | 2015 | Prospective | No NICU No prematures No prenatal diagnoses | 6.329 | 8 | 28 h | Either foot | <95% | Nellcor NPB 40 | Cyanosis, murmur, and femoral pulsations | Postnatal echocardiography | Newport, UK |
| Saxena et al. [34] | 2015 | Prospective | All | 19.009 | 26 | <48 h | Either foot | <95% | Mindray PM-60 | Cyanosis, murmur, and respiratory distress | Postnatal echocardiography | New Delhi, India |
| Taksande et al. [42] | 2017 | Prospective | All No prenatal diagnoses | 4.926 | 9 | <12 h | Right hand an either foot | <95% | Massimo SET | Cyanosis, femoral pulsations, precordial pulsations, murmur, tachypnoea, and chest retractions | Postnatal echocardiography | Warda, India |
| Turska kmieć et al. [43] | 2012 | Prospective | All | 52.993 | 82 | 7.3 h | Either foot | <95% | Novametrix, Nellcor and Masimo | Murmur, cyanosis, tachypnoea, and femoral pulsations | Pre/Postnatal echocardiography | Mazovia province, Poland |
| Uygur et al. [33] | 2019 | Prospective | All No prematures | 3.175 | 33 | 24–48 h | Left foot | <95% | Masimo Radical SET | Murmur, cyanosis, femoral pulsations | Pre/Postnatal echocardiography | Izmir, Turkey |
| Vaidyanathan et al. [31] | 2022 | Prospective | All | 5.487 | 17 | 48 h | Either foot | <94% | Nellcor Oximax N-65 | Murmur, cyanosis, and precordial pulsations | Postnatal echocardiography | Kochi, India |
| Zhang et al. [44] | 2021 | Prospective | All | 8.305 | 13 | 24–72 h | Right hand and either foot | <95% | Masimo RAD-5 | Murmur | Postnatal echocardiography | Jinjiang, China |
| Zhao et al. [28] | 2014 | Prospective | All No prenatal diagnoses | 120.707 | 146 | 43 h | Right hand and either foot | <95% | Masimo RAD-5v | Tachypnoea, cyanosis, murmur and (facial) malformations | Postnatal echocardiography | Ten provinces, China |
| Total | 20 studies | 872,549 | 857 |
Appendix B. Risk of Bias Analysis
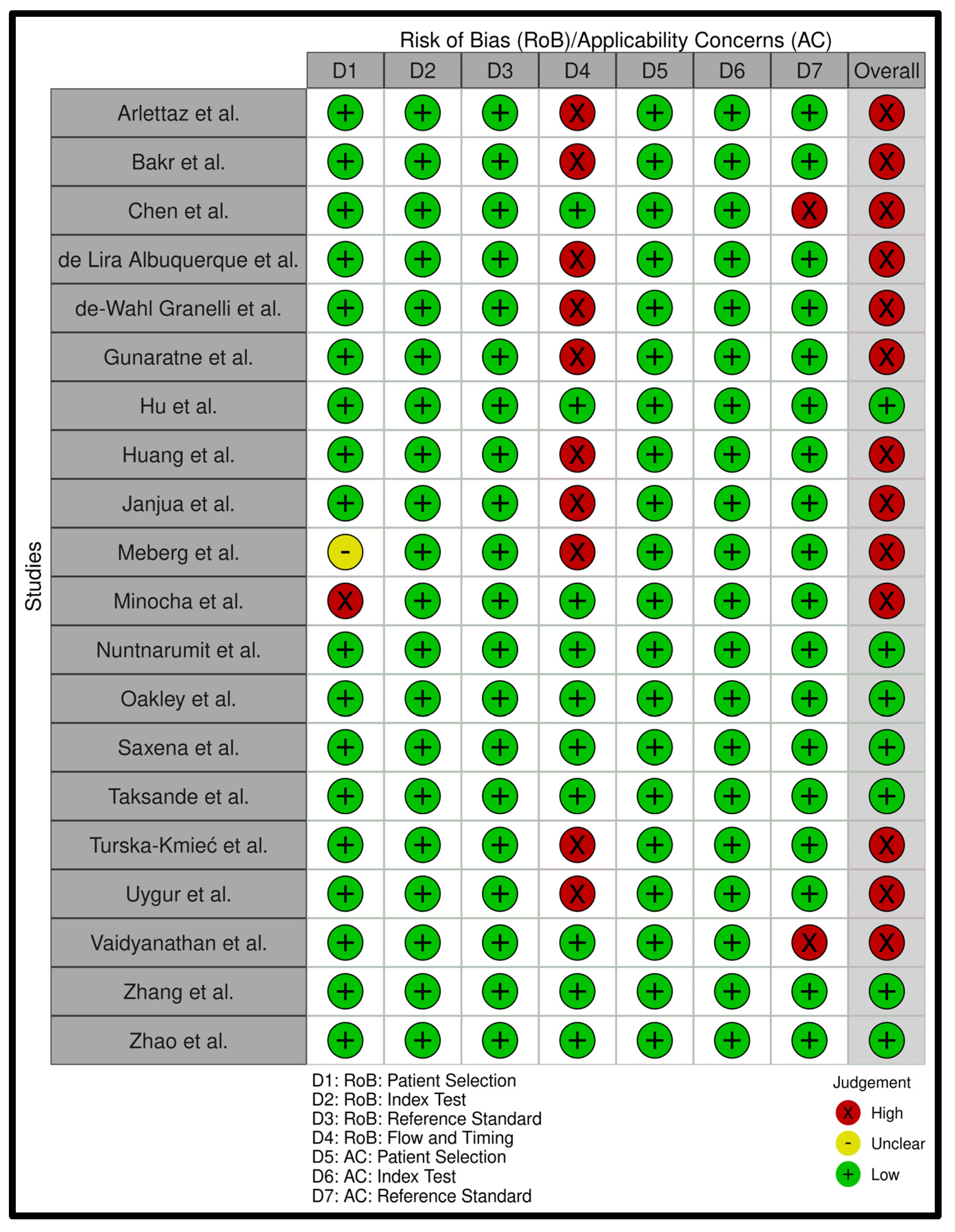
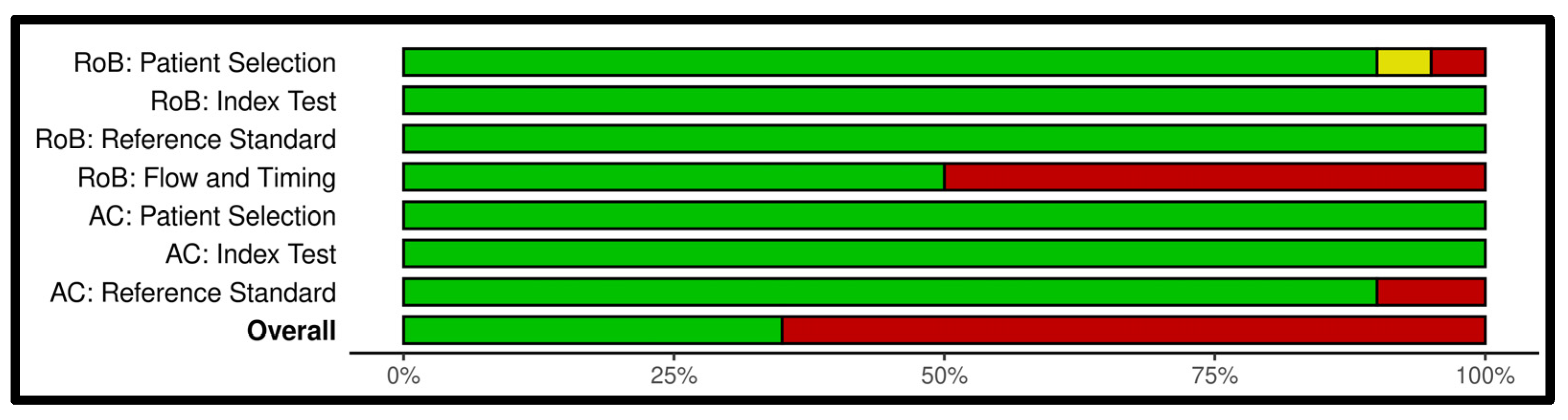
Appendix C. Search Strategies for All Databases
| Subject | Search Terms |
|---|---|
| Critical congenital heart disease | (‘Cardiovascular Malformation’/exp OR ‘Cardiovascular Abnormalit*’:ti,ab,kw OR ‘Congenital Heart Disease*’:ti,ab,kw OR ‘Congenital Heart Defect*’:ti,ab,kw OR ‘CCHD*’:ti,ab,kw OR ‘CHD’:ti,ab,kw OR ‘CHDs’:ti,ab,kw) |
| AND | |
| Physical examination | (‘Physical Examination’/exp OR ‘Cyanosis’/exp OR ‘Blood Pressure Measurement’/exp OR ‘Physical Examination’:ti,ab,kw OR ‘Cyanosis’:ti,ab,kw OR ‘Inspection’:ti,ab,kw OR ‘Auscultation’:ti,ab,kw OR ‘Murmur*’:ti,ab,kw OR ‘Assessment’:ti,ab,kw OR ‘Vital Signs’:ti,ab,kw OR ‘Blood Pressure’:ti,ab,kw OR ‘Heart Rate’:ti,ab,kw OR ‘Respiratory Rate’:ti,ab,kw OR ‘Evaluation’:ti,ab,kw OR ‘Colour’:ti,ab,kw OR ‘Color’:ti,ab,kw OR ‘Palpation’:ti,ab,kw OR ‘Capillary refill’:ti,ab,kw |
| OR | |
| Clinical scores | ‘Clinical Decision Rule’/exp OR ‘Health Status Indicator’/exp OR ‘Clinical Decision Rule*’:ti,ab,kw OR ‘Health Status Indicator*’:ti,ab,kw OR ‘Clinical score*’:ti,ab,kw OR ‘Nadas’:ti,ab,kw OR ‘C-CHEWS’:ti,ab,kw OR ‘Apgar’:ti,ab,kw) |
| AND | |
| Pulse oximetry | (‘Oxygen Saturation’/exp OR ‘Oximetry’/exp OR ‘Oxygen Saturation*’:ti,ab,kw OR ‘Oximetr*’:ti,ab,kw OR ‘Oxymetr*’:ti,ab,kw OR ‘POx’:ti,ab,kw OR ‘Pulse-Oximetr*’:ti,ab,kw OR ‘Pulse-Oxymetr*’:ti,ab,kw) |
| AND | |
| Reference standard | (‘Echography’/exp OR ‘Ultrasonography’:ti,ab,kw OR ‘Echocardiography’:ti,ab,kw OR ‘Ultrasound’:ti,ab,kw OR ‘US’:ti,ab,kw OR ‘Echo’:ti,ab,kw OR ‘Doppler’:ti,ab,kw OR ‘Prenatal Diagnosis’/exp OR ‘Diagnos*’:ti,ab,kw OR ‘Reference standard’:ti,ab,kw OR ‘Ultrasonic’:ti,ab,kw OR ‘Golden standard’:ti,ab,kw) |
| NOT | |
| (‘conference abstract’/it) |
| Subject | Search Terms |
|---|---|
| Critical congenital heart disease | (“Cardiovascular Abnormalities”[Title/Abstract] OR “Cardiovascular Abnormalit*”[Title/Abstract] OR “Congenital Heart Disease*”[Title/Abstract] OR “Congenital Heart Defect*”[Title/Abstract] OR “CCHD*”[Title/Abstract] OR “CHD”[Title/Abstract] OR “CHDs”[Title/Abstract]) |
| AND | |
| Physical examination | (“Physical Examination”[Title/Abstract] OR “Cyanosis”[Title/Abstract] OR “Blood Pressure Determination”[Title/Abstract] OR “Physical Examination”[Title/Abstract] OR “Cyanosis”[Title/Abstract] OR “Inspection”[Title/Abstract] OR “Auscultation”[Title/Abstract] OR “Murmur*”[Title/Abstract] OR “Assessment”[Title/Abstract] OR “Vital Signs”[Title/Abstract] OR “Blood Pressure”[Title/Abstract] OR “Heart Rate”[Title/Abstract] OR “Respiratory Rate”[Title/Abstract] OR “Evaluation”[Title/Abstract] OR “Colour”[Title/Abstract] OR “Color”[Title/Abstract] OR “Palpation”[Title/Abstract] OR “Capillary refill”[Title/Abstract] |
| OR | |
| Clinical scores | “Clinical Decision Rules”[Title/Abstract] OR “Health Status Indicators”[Title/Abstract] OR “Clinical Decision Rule*”[Title/Abstract] OR “Health Status Indicator*”[Title/Abstract] OR “Clinical score*”[Title/Abstract] OR “Nadas”[Title/Abstract] OR “C-CHEWS”[Title/Abstract] OR “Apgar”[Title/Abstract]) |
| AND | |
| Pulse oximetry | (“Oxygen Saturation”[Title/Abstract] OR “Oximetry”[Title/Abstract] OR “Oxygen Saturation*”[Title/Abstract] OR “Oximetr*”[Title/Abstract] OR “Oxymetr*”[Title/Abstract] OR “POx”[Title/Abstract] OR “Pulse-Oximetr*”[Title/Abstract] OR “Pulse-Oxymetr*”[Title/Abstract]) |
| AND | |
| Reference standard | (“Ultrasonography”[Title/Abstract] OR “Ultrasonography”[Title/Abstract] OR “Echocardiography”[Title/Abstract] OR “Ultrasound”[Title/Abstract] OR “US”[Title/Abstract] OR “Echo”[Title/Abstract] OR “Doppler”[Title/Abstract] OR “Prenatal Diagnosis”[Title/Abstract] OR “Diagnos*”[Title/Abstract] OR “Reference standard”[Title/Abstract] OR “Ultrasonic”[Title/Abstract] OR “Golden standard”[Title/Abstract]) |
| Subject | Search Terms |
|---|---|
| Critical congenital heart disease | (“Cardiovascular Malformation” OR “Cardiovascular Abnormalit*” OR “Congenital Heart Disease*” OR “Congenital Heart Defect*” OR “CCHD*” OR “CHD” OR “CHDs”) |
| AND | |
| Physical examination | (“Cyanosis” OR “Blood Pressure Measurement” OR “Physical Examination” OR “Cyanosis” OR “Inspection” OR “Auscultation” OR “Murmur*” OR “Assessment” OR “Vital Signs” OR “Blood Pressure” OR “Heart Rate” OR “Respiratory Rate” OR “Evaluation” OR “Colour” OR “Color” OR “Palpation” OR “Capillary refill” |
| OR | |
| Clinical scores | “Clinical Decision Rule*” OR “Health Status Indicator*” OR “Clinical score*” OR “Nadas” OR “C-CHEWS” OR “Apgar”) |
| AND | |
| Pulse oximetry | (“Oxygen Saturation*” OR “Oximetr*” OR “Oxymetr*” OR “POx” OR “Pulse-Oximetr*” OR “Pulse-Oxymetr*”) |
| AND | |
| Reference standard | (“Echography” OR “Ultrasonography” OR “Echocardiography” OR “Ultrasound” OR “US” OR “Echo” OR “Doppler” OR “Prenatal Diagnosis” OR “Diagnos*” OR “Reference standard” OR “Ultrasonic” OR “Golden standard”) |
References
- Oster, M.E.; Lee, K.A.; Honein, M.A.; Riehle-Colarusso, T.; Shin, M.; Correa, A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 2013, 131, e1502–e1508. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Correa, A.; Erickson, J.D. Racial and temporal variations in the prevalence of heart defects. Pediatrics 2001, 107, E32. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J. Pediatr. 2008, 153, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef]
- Taksande, A.; Jameel, P.Z. Critical Congenital Heart Disease in Neonates: A Review Article. Curr. Pediatr. Rev. 2021, 17, 120–126. [Google Scholar] [CrossRef]
- Martin, G.R.; Schwartz, B.N.; Hom, L.A.; Donofrio, M.T. Lessons Learned from Infants with Late Detection of Critical Congenital Heart Disease. Pediatr. Cardiol. 2022, 43, 580–585. [Google Scholar] [CrossRef]
- Peterson, C.; Ailes, E.; Riehle-Colarusso, T.; Oster, M.E.; Olney, R.S.; Cassell, C.H.; Fixler, D.E.; Carmichael, S.L.; Shaw, G.M.; Gilboa, S.M. Late detection of critical congenital heart disease among US infants: Estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014, 168, 361–370. [Google Scholar] [CrossRef]
- Bakker, M.K.; Bergman, J.E.H.; Krikov, S.; Amar, E.; Cocchi, G.; Cragan, J.; Walle, H.E.K.d.; Gatt, M.; Groisman, B.; Liu, S.; et al. Prenatal diagnosis and prevalence of critical congenital heart defects: An international retrospective cohort study. BMJ Open 2019, 9, e028139. [Google Scholar] [CrossRef]
- Moshi, F.V.; Mbotwa, C.H. Determinants for choice of home birth over health facility birth among women of reproductive age in Tanzania: An analysis of data from the 2015–16 Tanzania demographic and health survey and malaria indicator survey. BMC Pregnancy Childbirth 2020, 20, 561. [Google Scholar] [CrossRef]
- Zheleva, B.; Nair, S.M.; Dobrzycka, A.; Saarinen, A. Considerations for Newborn Screening for Critical Congenital Heart Disease in Low- and Middle-Income Countries. Int. J. Neonatal Screen. 2020, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Narayen, I.C.; Blom, N.A.; van Geloven, N.; Blankman, E.I.; Broek, A.J.v.D.; Bruijn, M.; Clur, S.-A.B.; Dungen, F.A.v.D.; Havers, H.M.; van Laerhoven, H.; et al. Accuracy of Pulse Oximetry Screening for Critical Congenital Heart Defects after Home Birth and Early Postnatal Discharge. J. Pediatr. 2018, 197, 29–35.e1. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Liu, D.; Liu, G.; Wang, H. A Meta-Analysis about the Screening Role of Pulse Oximetry for Congenital Heart Disease. Biomed. Res. Int. 2017, 2017, 2123918. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Brown, K.; Zamora, J.; Khan, K.S.; Ewer, A.K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: A systematic review and meta-analysis. Lancet 2012, 379, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Bello, H.C.; Trujillo, D.L.; Moreno, G.A.; Torres, M.T.; Restrepo, A.T.; Fonseca, A.; Reyes, N.S.; Chamorro, C.L.; Verano, R.J. Oximetry and neonatal examination for the detection of critical congenital heart disease: A systematic review and meta-analysis. F1000Research 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Bellsham-Revell, D.H. The Critically Ill Neonate: Cardiac Cause of Collapse paediatricfoam.com Paediatric Foam. 2018. Available online: https://www.paediatricfoam.com/2017/09/the-critically-ill-neonate-cardiac-causes-of-collapse/ (accessed on 22 December 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Q.; Ma, X.; Yan, W.; Ge, X.; Jia, B.; Liu, F.; Wu, L.; Ye, M.; Huang, G. Pulse oximetry could significantly enhance the early detection of critical congenital heart disease in neonatal intensive care units. Acta Paediatr. 2016, 105, e499–e505. [Google Scholar] [CrossRef]
- Alan, C.; Korkmaz, L. The importance and effectiveness of cardiac screening in early diagnosis of critical congenital heart diseases. Ann. Med. Res. 2021, 28, 1917–1921. [Google Scholar] [CrossRef]
- Gálvez-Cancino, F. Sensibilidad y especificidad del soplo y la cianosis para la detección de cardiopatía congénita en la etapa neonatal. Rev. Mex. De Pediatr. 2017, 84, 189–195. [Google Scholar]
- Song, J.; Huang, X.; Zhao, S.; Chen, J.; Chen, R.; Wu, G.; Xu, Z. Diagnostic value of pulse oximetry combined with cardiac auscultation in screening congenital heart disease in neonates. J. Int. Med. Res. 2021, 49, 3000605211016137. [Google Scholar] [CrossRef] [PubMed]
- Zuppa, A.A.; Riccardi, R.; Catenazzi, P.; D’Andrea, V.; Cavani, M.; D’Antuono, A.; Iafisco, A.; Romagnoli, C. Clinical examination and pulse oximetry as screening for congenital heart disease in low-risk newborn. J. Matern. Fetal Neonatal Med. 2015, 28, 7–11. [Google Scholar] [CrossRef]
- Janjua, D.; Singh, J.; Agrawal, A. Pulse oximetry as a screening test for congenital heart disease in newborns. J. Mother. Child. 2022, 26, 1–9. [Google Scholar]
- Hu, X.-J.; Ma, X.-J.; Zhao, Q.-M.; Yan, W.-L.; Ge, X.-L.; Jia, B.; Liu, F.; Wu, L.; Ye, M.; Liang, X.-C.; et al. Pulse Oximetry and Auscultation for Congenital Heart Disease Detection. Pediatrics 2017, 140, e20171154. [Google Scholar] [CrossRef]
- Zhao, Q.-M.; Ma, X.-J.; Ge, X.-L.; Liu, F.; Yan, W.-L.; Wu, L.; Ye, M.; Liang, X.-C.; Zhang, J.; Gao, Y.; et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: A prospective study. Lancet 2014, 384, 747–754. [Google Scholar] [CrossRef]
- de-Wahl Granelli, A.; Wennergren, M.; Sandberg, K.; Mellander, M.; Bejlum, C.; Inganas, L.; Eriksson, M.; Segerdahl, N.; Agren, A.; Ekman-Joelsson, B.-M.; et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: A Swedish prospective screening study in 39,821 newborns. BMJ 2009, 338, a3037. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Zhang, D.F.; Wang, Y.Z.; Zhang, X.Y. Appropriate Technology for Screening, Diagnosis, and Evaluation of Neonatal Congenital Heart Disease in the Southernmost Region of China. Iran. J. Pediatr. 2023, 33, e132589. [Google Scholar] [CrossRef]
- Vaidyanathan, B.; Sathish, G.; Mohanan, S.T.; Sundaram, K.R.; Warrier, K.K.; Kumar, R.K. Clinical screening for Congenital heart disease at birth: A prospective study in a community hospital in Kerala. Indian. Pediatr. 2011, 48, 25–30. [Google Scholar] [CrossRef]
- Minocha, P.; Agarwal, A.; Jivani, N.; Swaminathan, S. Evaluation of Neonates With Suspected Congenital Heart Disease: A New Cost-Effective Algorithm. Clin. Pediatr. 2018, 57, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Uygur, O.; Koroglu, O.A.; Levent, E.; Tosyali, M.; Akisu, M.; Yalaz, M.; Kultursay, N. The value of peripheral perfusion index measurements for early detection of critical cardiac defects. Pediatr. Neonatol. 2019, 60, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Mehta, A.; Ramakrishnan, S.; Sharma, M.; Salhan, S.; Kalaivani, M.; Juneja, R. Pulse oximetry as a screening tool for detecting major congenital heart defects in Indian newborns. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F416–F421. [Google Scholar] [CrossRef] [PubMed]
- Arlettaz, R.; Bauschatz, A.S.; Mönkhoff, M.; Essers, B.; Bauersfeld, U. The contribution of pulse oximetry to the early detection of congenital heart disease in newborns. Eur. J. Pediatr. 2006, 165, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.F.; Habib, H.S. Combining pulse oximetry and clinical examination in screening for congenital heart disease. Pediatr. Cardiol. 2005, 26, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, F.; Maia, E.; Figueiredo, V.; Mourato, F.; Mattos, S. Clinical Examination and Pulse Oximetry to Detect Congenital Heart Disease. Int. J. Cardiovasc. Sci. 2015, 28, 148–151. [Google Scholar]
- Gunaratne, C.R.; Hewage, I.; Fonseka, A.; Thennakoon, S. Comparison of pulse oximetry screening versus routine clinical examination in detecting critical congenital heart disease in newborns. Sri Lanka J. Child. Health 2021, 50, 4–11. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, S.; Zhang, X.; Kong, L.; Wu, W.; Yue, S.; Tian, N.; Zhu, G.; Hu, A.; Xu, J.; et al. Large scale application of pulse oximeter and auscultation in screening of neonatal congenital heart disease. BMC Pediatr. 2022, 22, 483. [Google Scholar] [CrossRef]
- Nuntnarumit, P.; Thanomsingh, P.; Limrungsikul, A.; Wanitkun, S.; Sirisopikun, T.; Ausayapao, P. Pulse oximetry screening for critical congenital heart diseases at two different hospital settings in Thailand. J. Perinatol. 2018, 38, 181–184. [Google Scholar] [CrossRef]
- Oakley, J.L.; Soni, N.B.; Wilson, D.; Sen, S. Effectiveness of pulse-oximetry in addition to routine neonatal examination in detection of congenital heart disease in asymptomatic newborns. J. Matern. Fetal Neonatal Med. 2015, 28, 1736–1739. [Google Scholar] [CrossRef]
- Taksande, A.; Meshram, R.; Lohakare, A.; Purandare, S.; Biyani, U.; Vagha, J. An update work of pulse oximetry screening for detecting critical congenital heart disease in the newborn. Images Paediatr. Cardiol. 2017, 19, 12–18. [Google Scholar] [PubMed]
- Turska Kmieć, A.; Borszewska Kornacka, M.K.; Błaż, W.; Kawalec, W.; Zuk, M. Early screening for critical congenital heart defects in asymptomatic newborns in Mazovia province: Experience of the POLKARD pulse oximetry programme 2006–2008 in Poland. Kardiol. Pol. 2012, 70, 370–376. [Google Scholar] [PubMed]
- Zhang, Y.L.; Bai, H.T. A Study on Clinical Screening of Neonatal Congenital Heart Disease in Jinjiang City. Int. J. Gen. Med. 2021, 14, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, Y.; Ma, F.; Ge, X.; Gu, Q.; Huang, M.; Zhang, Y.; Sun, K.; Hu, X.; Yang, M.; et al. Impact of Newborn Screening Programme for Congenital Heart Disease in Shanghai: A five-year observational study in 801,831 newborns. Lancet Reg. Health West. Pac. 2023, 33, 100688. [Google Scholar] [CrossRef]
- Martin, G.R.; Ewer, A.K.; Gaviglio, A.; Hom, L.A.; Saarinen, A.; Sontag, M.; Burns, K.M.; Kemper, A.R.; Oster, M.E. Updated Strategies for Pulse Oximetry Screening for Critical Congenital Heart Disease. Pediatrics 2020, 146, e20191650. [Google Scholar] [CrossRef] [PubMed]
- Ewer, A.K.; Middleton, L.J.; Furmston, A.T.; Bhoyar, A.; Daniels, J.P.; Thangaratinam, S.; Deeks, J.J.; Khan, K.S. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): A test accuracy study. Lancet 2011, 378, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Stallings, E.B.; Isenburg, J.L.; Aggarwal, D.; Lupo, P.J.; Oster, M.E.; Shephard, H.; Liberman, R.F.; Kirby, R.S.; Nestoridi, E.; Hansen, B.; et al. Prevalence of critical congenital heart defects and selected co-occurring congenital anomalies, 2014–2018: A U.S. population-based study. Birth Defects Res. 2022, 114, 45–56. [Google Scholar] [CrossRef]
- Patton, C.; Hey, E. How effectively can clinical examination pick up congenital heart disease at birth? Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F263–F267. [Google Scholar] [CrossRef]
- Danford, D.A.; Martin, A.B.; Fletcher, S.E.; Gumbiner, C.H. Echocardiographic yield in children when innocent murmur seems likely but doubts linger. Pediatr. Cardiol. 2002, 23, 410–414. [Google Scholar] [CrossRef]
- Frank, J.E.; Jacobe, K.M. Evaluation and management of heart murmurs in children. Am. Fam. Physician 2011, 84, 793–800. [Google Scholar]
- Izhar, F.M.; Abqari, S.; Shahab, T.; Ali, S.M. Clinical score to detect congenital heart defects: Concept of second screening. Ann. Pediatr. Cardiol. 2020, 13, 281–288. [Google Scholar] [PubMed]
- Rahmawati, R.; Adriansyah, R.; Trisnawati, Y.; Harahap, J.; Sianturi, P.; Pasaribu, A.P. Accuracy of NADAS criteria to establish diagnosis in children with suspected congenital heart disease. Paediatr. Indones. 2023, 63, 267–273. [Google Scholar] [CrossRef]
- James, M.; Poornima, K.N.; Ninan, P.J. Evaluation of children with cardiac murmur using Nadas criteria. Int. J. Contemp. Pediatr. 2018, 5, 363–367. [Google Scholar] [CrossRef]
- Jiang, S.L.; Zhan, Y.J.; Yan, P.; Yue, Y.; Tang, J. Pulse Oximetry and Perfusion Index Screening for Congenital Heart Defects: A Systematic Review and Meta-analysis. Am. J. Perinatol. 2022, 40, 1611–1617. [Google Scholar] [CrossRef]
- Meberg, A.; Brügmann-Pieper, S.; Due, R., Jr.; Eskedal, L.; Fagerli, I.; Farstad, T.; Frøisland, D.H.; Sannes, C.H.; Johansen, O.J.; Keljalic, J.; et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. J. Pediatr. 2008, 152, 761–765. [Google Scholar] [CrossRef]


| PE | PO | PE Only | PO Only | PE AND PO | PE OR PO |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 1 | 0 | 1 |
| 1 | 0 | 1 | 0 | 0 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Vliet, J.T.; Majani, N.G.; Chillo, P.; Slieker, M.G. Diagnostic Accuracy of Physical Examination and Pulse Oximetry for Critical Congenital Cardiac Disease Screening in Newborns. Children 2024, 11, 47. https://doi.org/10.3390/children11010047
van Vliet JT, Majani NG, Chillo P, Slieker MG. Diagnostic Accuracy of Physical Examination and Pulse Oximetry for Critical Congenital Cardiac Disease Screening in Newborns. Children. 2024; 11(1):47. https://doi.org/10.3390/children11010047
Chicago/Turabian Stylevan Vliet, Jari T., Naizihijwa G. Majani, Pilly Chillo, and Martijn G. Slieker. 2024. "Diagnostic Accuracy of Physical Examination and Pulse Oximetry for Critical Congenital Cardiac Disease Screening in Newborns" Children 11, no. 1: 47. https://doi.org/10.3390/children11010047
APA Stylevan Vliet, J. T., Majani, N. G., Chillo, P., & Slieker, M. G. (2024). Diagnostic Accuracy of Physical Examination and Pulse Oximetry for Critical Congenital Cardiac Disease Screening in Newborns. Children, 11(1), 47. https://doi.org/10.3390/children11010047







