The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits
Abstract
1. Introduction
2. Evidence Supports Extensive Influence of Acetaminophen Exposure on Current Rates of ASD
3. Alternative Hypotheses
4. Periods of Sensitivity to Acetaminophen during Neurodevelopment
5. Improper Use of Acetaminophen to Treat Fevers Is Common
6. Overdoses of Acetaminophen in the Pediatric Population
7. Misguided but Currently Accepted Use of Acetaminophen
8. Changes in Practice Need to Be Made
- Category 1:
- Administration of acetaminophen in a manner that was never intended should be discontinued. This includes treatment of temperatures that do not technically constitute a fever and administration of the drug more frequently and at higher doses than recommended.
- Category 2:
- Administration of acetaminophen under conditions in which evidence demonstrates a lack of effectiveness should be discontinued. This includes the treatment of the pain of circumcision and perhaps the treatment of fevers to prevent febrile seizures.
- Category 3:
- Administration of acetaminophen under conditions in which no evidence demonstrates long-term benefits of treatment or in which evidence demonstrates a lack of long-term benefits should be discontinued. This includes the treatment of fevers and prophylactic treatments prior to labor and delivery.
- Category 4:
- Administration of acetaminophen that is no longer recommended by governing medical bodies should be discontinued. This includes the treatment of patients receiving vaccinations and will hopefully include many more reasons for administration in the future.
- Category 5:
- Administration of acetaminophen under conditions where evidence indicates that it is or may be beneficial should not be continued without disclosure of the drug’s long-term risks for neurodevelopment. All caregivers, including parents, should be made aware of evidence related to both benefits and risks so that they can make informed decisions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, E.; Jones Iii, J.P., 3rd; Bono-Lunn, D.; Kuchibhatla, M.; Palkar, A.; Cendejas Hernandez, J.; Sarafian, J.T.; Lawton, V.G.; Anderson, L.G.; Konsoula, Z.; et al. The safety of pediatric use of paracetamol (acetaminophen): A narrative review of direct and indirect evidence. Minerva Pediatr. 2022, 74, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jones, J.; Anderson, L.; Konsoula, Z.; Nevison, C.; Reissner, K.; Parker, W. Acetaminophen causes neurodevelopmental injury in susceptible babies and children: No valid rationale for controversy. Clin. Exp. Pediatr. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Herrington, J.A.; Guss Darwich, J.; Harshaw, C.; Brigande, A.M.; Leif, E.B.; Currie, P.J. Elevated ghrelin alters the behavioral effects of perinatal acetaminophen exposure in rats. Dev. Psychobiol. 2022, 64, e22252. [Google Scholar] [CrossRef] [PubMed]
- Harshaw, C.; Warner, A.G. Interleukin-1β-induced inflammation and acetaminophen during infancy: Distinct and interactive effects on social-emotional and repetitive behavior in C57BL/6J mice. Pharmacol. Biochem. Behav. 2022, 220, 173463. [Google Scholar] [CrossRef]
- Viberg, H.; Eriksson, P.; Gordh, T.; Fredriksson, A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicol. Sci. 2014, 138, 139–147. [Google Scholar] [CrossRef]
- Suda, N.; Hernandez, J.C.; Poulton, J.; Jones, J.P.; Konsoula, Z.; Smith, C.; Parker, W. Therapeutic doses of paracetamol with co-administration of cysteine and mannitol during early development result in long term behavioral changes in laboratory rats. PLoS ONE 2021, 16, e0253543. [Google Scholar] [CrossRef]
- Philippot, G.; Gordh, T.; Fredriksson, A.; Viberg, H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): Characterization of a critical period. J. Appl. Toxicol. 2017, 37, 1174–1181. [Google Scholar] [CrossRef]
- Dean, S.L.; Knutson, J.F.; Krebs-Kraft, D.L.; McCarthy, M.M. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur. J. Neurosci. 2012, 35, 1218–1229. [Google Scholar] [CrossRef]
- Parker, W.; Hornik, C.D.; Bilbo, S.; Holzknecht, Z.E.; Gentry, L.; Rao, R.; Lin, S.S.; Herbert, M.R.; Nevison, C.D. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J. Int. Med. Res. 2017, 45, 407–438. [Google Scholar] [CrossRef]
- Schultz, S.T.; Klonoff-Cohen, H.S.; Wingard, D.L.; Akshoomoff, N.A.; Macera, C.A.; Ji, M. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder. The results of a parent survey. Autism 2008, 12, 293–307. [Google Scholar] [CrossRef]
- Frisch, M.; Simonsen, J. Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year-old boys: National cohort study in Denmark. J. R. Soc. Med. 2015, 108, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Virk, J.; Olsen, J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism Res. Off. J. Int. Soc. Autism Res. 2016, 9, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Alemany, S.; Avella-García, C.; Liew, Z.; García-Esteban, R.; Inoue, K.; Cadman, T.; López-Vicente, M.; González, L.; Riaño Galán, I.; Andiarena, A.; et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur. J. Epidemiol. 2021, 36, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Rimland, B. The autism increase: Research needed on the vaccine connection. Autism Res. Rev. Int. 2000, 14, 3–6. [Google Scholar]
- Wastesson, J.W.; Martikainen, J.E.; Zoëga, H.; Schmidt, M.; Karlstad, Ø.; Pottegård, A. Trends in Use of Paracetamol in the Nordic Countries. Basic Clin. Pharmacol. Toxicol. 2018, 123, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Delobel-Ayoub, M.; Saemundsen, E.; Gissler, M.; Ego, A.; Moilanen, I.; Ebeling, H.; Rafnsson, V.; Klapouszczak, D.; Thorsteinsson, E.; Arnaldsdóttir, K.M.; et al. Prevalence of Autism Spectrum Disorder in 7–9-Year-Old Children in Denmark, Finland, France and Iceland: A Population-Based Registries Approach within the ASDEU Project. J. Autism Dev. Disord. 2020, 50, 949–959. [Google Scholar] [CrossRef]
- Philippot, G.; Hosseini, K.; Yakub, A.; Mhajar, Y.; Hamid, M.; Buratovic, S.; Fredriksson, R. Paracetamol (Acetaminophen) and its Effect on the Developing Mouse Brain. Front. Toxicol. 2022, 4, 867748. [Google Scholar] [CrossRef]
- Kanno, S.I.; Tomizawa, A.; Yomogida, S.; Hara, A. Glutathione peroxidase 3 is a protective factor against acetaminophen-induced hepatotoxicity in vivo and in vitro. Int. J. Mol. Med. 2017, 40, 748–754. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Wright, C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef]
- Posadas, I.; Santos, P.; Blanco, A.; Muñoz-Fernández, M.; Ceña, V. Acetaminophen induces apoptosis in rat cortical neurons. PLoS ONE 2010, 5, e15360. [Google Scholar] [CrossRef]
- Donovan, A.P.; Basson, M.A. The neuroanatomy of autism—A developmental perspective. J. Anat. 2017, 230, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Sokhadze, E.M.; Casanova, E.L.; Opris, I.; Abujadi, C.; Marcolin, M.A.; Li, X. Translational Neuroscience in Autism: From Neuropathology to Transcranial Magnetic Stimulation Therapies. Psychiatr. Clin. N. Am. 2020, 43, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zielke, H.R.; Yeh, D.; Yang, P. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism Res. Off. J. Int. Soc. Autism Res. 2018, 11, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.N.; Zhang, H.; Shu, Y.; Chen, S.; Hu, Y.Y.; Zhou, M. The neonatal levels of TSB, NSE and CK-BB in autism spectrum disorder from Southern China. Transl. Neurosci. 2016, 7, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Stancioiu, F.; Bogdan, R.; Dumitrescu, R. Neuron-Specific Enolase (NSE) as a Biomarker for Autistic Spectrum Disease (ASD). Life 2023, 13, 1736. [Google Scholar] [CrossRef]
- Anvik, J.O. Acetaminophen toxicosis in a cat. Can. Vet. J. = La Rev. Vet. Can. 1984, 25, 445–447. [Google Scholar]
- Savides, M.C.; Oehme, F.W.; Nash, S.L.; Leipold, H.W. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicol. Appl. Pharmacol. 1984, 74, 26–34. [Google Scholar] [CrossRef]
- Court, M.H. Feline drug metabolism and disposition: Pharmacokinetic evidence for species differences and molecular mechanisms. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1039–1054. [Google Scholar] [CrossRef]
- Lautz, L.S.; Jeddi, M.Z.; Girolami, F.; Nebbia, C.; Dorne, J. Metabolism and pharmacokinetics of pharmaceuticals in cats (Felix sylvestris catus) and implications for the risk assessment of feed additives and contaminants. Toxicol. Lett. 2021, 338, 114–127. [Google Scholar] [CrossRef]
- Miller, R.P.; Roberts, R.J.; Fischer, L.J. Acetaminophen elimination kinetics in neonates, children, and adults. Clin. Pharmacol. Ther. 1976, 19, 284–294. [Google Scholar] [CrossRef]
- Cook, S.F.; Stockmann, C.; Samiee-Zafarghandy, S.; King, A.D.; Deutsch, N.; Williams, E.F.; Wilkins, D.G.; Sherwin, C.M.; van den Anker, J.N. Neonatal Maturation of Paracetamol (Acetaminophen) Glucuronidation, Sulfation, and Oxidation Based on a Parent-Metabolite Population Pharmacokinetic Model. Clin. Pharmacokinet 2016, 55, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Cendejas-Hernandez, J.; Sarafian, J.; Lawton, V.; Palkar, A.; Anderson, L.; Lariviere, V.; Parker, W. Paracetamol (Acetaminophen) Use in Infants and Children was Never Shown to be Safe for Neurodevelopment: A Systematic Review with Citation Tracking. Eur. J. Pediatr. 2022, 181, 1835–1857. [Google Scholar] [CrossRef]
- Hall, C.; Smith, M. Increased cGMP Enforcement Has Gone International: South Korean Action against Johnson & Johnson Serves as Warning. White Collar Watch 2013; June. Available online: https://www.jdsupra.com/legalnews/increased-cgmp-enforcement-has-gone-inte-53643/ (accessed on 13 December 2023).
- Kim, Y.S.; Leventhal, B.L.; Koh, Y.J.; Fombonne, E.; Laska, E.; Lim, E.C.; Cheon, K.A.; Kim, S.J.; Kim, Y.K.; Lee, H.; et al. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry 2011, 168, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Baird, G. 2.64% of South Korean children aged 7 to 12 have autism spectrum disorders. Evid. Based Ment. Health 2012, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Raz, R.; Weisskopf, M.G.; Davidovitch, M.; Pinto, O.; Levine, H. Differences in autism spectrum disorders incidence by sub-populations in Israel 1992–2009: A total population study. J. Autism Dev. Disord. 2015, 45, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Levaot, Y.; Meiri, G.; Dinstein, I.; Menashe, I.; Shoham-Vardi, I. Autism Prevalence and Severity in Bedouin-Arab and Jewish Communities in Southern Israel. Community Ment. Health J. 2019, 55, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J. A history of drug advertising: The evolving roles of consumers and consumer protection. Milbank Q. 2006, 84, 659–699. [Google Scholar] [CrossRef]
- Tovo-Rodrigues, L.; Schneider, B.C.; Martins-Silva, T.; Del-Ponte, B.; Loret de Mola, C.; Schuler-Faccini, L.; Vianna, F.S.L.; Munhoz, T.N.; Entiauspe, L.; Silveira, M.F.; et al. Is intrauterine exposure to acetaminophen associated with emotional and hyperactivity problems during childhood? Findings from the 2004 Pelotas birth cohort. BMC Psychiatry 2018, 18, 368. [Google Scholar] [CrossRef]
- Vlenterie, R.; Wood, M.E.; Brandlistuen, R.E.; Roeleveld, N.; van Gelder, M.M.; Nordeng, H. Neurodevelopmental problems at 18 months among children exposed to paracetamol in utero: A propensity score matched cohort study. Int. J. Epidemiol. 2016, 45, 1998–2008. [Google Scholar] [CrossRef]
- Liew, Z.; Ritz, B.; Virk, J.; Arah, O.A.; Olsen, J. Prenatal Use of Acetaminophen and Child IQ: A Danish Cohort Study. Epidemiology 2016, 27, 912–918. [Google Scholar] [CrossRef]
- Liew, Z.; Bach, C.C.; Asarnow, R.F.; Ritz, B.; Olsen, J. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int. J. Epidemiol. 2016, 45, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Azuine, R.E.; Zhang, Y.; Hou, W.; Hong, X.; Wang, G.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure with Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry 2020, 77, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Avella-Garcia, C.B.; Julvez, J.; Fortuny, J.; Rebordosa, C.; Garcia-Esteban, R.; Galan, I.R.; Tardon, A.; Rodriguez-Bernal, C.L.; Iniguez, C.; Andiarena, A.; et al. Acetaminophen use in pregnancy and neurodevelopment: Attention function and autism spectrum symptoms. Int. J. Epidemiol. 2016, 45, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, E.; Handal, M.; Selmer, R.; Brandlistuen, R.E.; Skurtveit, S. Language competence and communication skills in 3-year-old children after prenatal exposure to analgesic opioids. Pharmacoepidemiol. Drug Saf. 2017, 26, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Rebordosa, C.; Lee, P.C.; Olsen, J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014, 168, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ystrom, E.; Gustavson, K.; Brandlistuen, R.E.; Knudsen, G.P.; Magnus, P.; Susser, E.; Davey Smith, G.; Stoltenberg, C.; Suren, P.; Haberg, S.E.; et al. Prenatal Exposure to Acetaminophen and Risk of ADHD. Pediatrics 2017, 140, e20163840. [Google Scholar] [CrossRef]
- Thompson, J.M.; Waldie, K.E.; Wall, C.R.; Murphy, R.; Mitchell, E.A. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS ONE 2014, 9, e108210. [Google Scholar] [CrossRef]
- Stergiakouli, E.; Thapar, A.; Davey Smith, G. Association of Acetaminophen Use During Pregnancy with Behavioral Problems in Childhood: Evidence against Confounding. JAMA Pediatr. 2016, 170, 964–970. [Google Scholar] [CrossRef]
- Brandlistuen, R.E.; Ystrom, E.; Nulman, I.; Koren, G.; Nordeng, H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int. J. Epidemiol. 2013, 42, 1702–1713. [Google Scholar] [CrossRef]
- Freed, G.L.; Clark, S.J.; Butchart, A.T.; Singer, D.C.; Davis, M.M. Parental vaccine safety concerns in 2009. Pediatrics 2010, 125, 654–659. [Google Scholar] [CrossRef]
- Bazzano, A.; Zeldin, A.; Schuster, E.; Barrett, C.; Lehrer, D. Vaccine-related beliefs and practices of parents of children with autism spectrum disorders. Am. J. Intellect. Dev. Disabil. 2012, 117, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.D.; Krajbich, I.; Way, B.M. Acetaminophen influences social and economic trust. Sci. Rep. 2019, 9, 4060. [Google Scholar] [CrossRef]
- Dewall, C.N.; Macdonald, G.; Webster, G.D.; Masten, C.L.; Baumeister, R.F.; Powell, C.; Combs, D.; Schurtz, D.R.; Stillman, T.F.; Tice, D.M.; et al. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychol. Sci. 2010, 21, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Durso, G.R.O.; Luttrell, A.; Way, B.M. Over-the-Counter Relief from Pains and Pleasures Alike: Acetaminophen Blunts Evaluation Sensitivity to Both Negative and Positive Stimuli. Psychol. Sci. 2015, 26, 750–758. [Google Scholar] [CrossRef]
- Randles, D.; Kam, J.W.Y.; Heine, S.J.; Inzlicht, M.; Handy, T.C. Acetaminophen attenuates error evaluation in cortex. Soc. Cogn. Affect. Neurosci. 2016, 11, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Hutabarat, R.M.; Unadkat, J.D.; Kushmerick, P.; Aitken, M.L.; Slattery, J.T.; Smith, A.L. Disposition of drugs in cystic fibrosis. III Acetaminophen. Clin. Pharmacol. Ther. 1991, 50, 695–701. [Google Scholar] [CrossRef]
- Kearns, G.L. Hepatic drug metabolism in cystic fibrosis: Recent developments and future directions. Ann. Pharmacother. 1993, 27, 74–79. [Google Scholar] [CrossRef]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation deficit in “low-functioning” autistic children: A pilot study. Biol. Psychiatry 1999, 46, 420–424. [Google Scholar] [CrossRef]
- Guengerich, F.P. A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol. Res. 2021, 37, 1–23. [Google Scholar] [CrossRef]
- Albano, E.; Rundgren, M.; Harvison, P.J.; Nelson, S.D.; Moldéus, P. Mechanisms of N-acetyl-p-benzoquinone imine cytotoxicity. Mol. Pharmacol. 1985, 28, 306–311. [Google Scholar] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. I Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. IV Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973, 187, 211–217. [Google Scholar] [PubMed]
- Du, K.; Farhood, A.; Jaeschke, H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch. Toxicol. 2017, 91, 761–773. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Elwell, C.; Johnson, M.H. Mitochondrial Dysfunction in Autism Spectrum Disorders. Autism—Open Access 2016, 6, 1000190. [Google Scholar] [CrossRef]
- Mischkowski, D.; Crocker, J.; Way, B.M. A Social Analgesic? Acetaminophen (Paracetamol) Reduces Positive Empathy. Front. Psychol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Many, B.T.; Rizeq, Y.K.; Vacek, J.; Cheon, E.C.; Johnson, E.; Hu, Y.Y.; Raval, M.V.; Abdullah, F.; Goldstein, S.D. A contemporary snapshot of circumcision in US children’s hospitals. J. Pediatr. Surg. 2020, 55, 1134–1138. [Google Scholar] [CrossRef]
- Campbell, O.M.; Cegolon, L.; Macleod, D.; Benova, L. Length of Stay after Childbirth in 92 Countries and Associated Factors in 30 Low- and Middle-Income Countries: Compilation of Reported Data and a Cross-sectional Analysis from Nationally Representative Surveys. PLoS Med. 2016, 13, e1001972. [Google Scholar] [CrossRef]
- Tan, C.; Frewer, V.; Cox, G.; Williams, K.; Ure, A. Prevalence and Age of Onset of Regression in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analytical Update. Autism Res. Off. J. Int. Soc. Autism Res. 2021, 14, 582–598. [Google Scholar] [CrossRef]
- Owings, M.; Uddin, S.; Williams, S. Trends in Circumcision for Male Newborns in U.S. Hospitals: 1979–2010. In Centers for Disease Control and Prevention; Statistics NCfH: Hyattsville, MD, USA, 2013; pp. 1–5. [Google Scholar]
- Clissold, S.P. Paracetamol and phenacetin. Drugs 1986, 32 (Suppl. S4), 46–59. [Google Scholar] [CrossRef]
- Mahmud, S.; Rosen, N. History of NSAID Use in the Treatment of Headaches Pre and Post-industrial Revolution in the United States: The Rise and Fall of Antipyrine, Salicylic Acid, and Acetanilide. Curr. Pain Headache Rep. 2019, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Morris, D., Haddy, A., Eds.; Analgesic contents of patent medicines of the early 20th century: Bromo-Seltzer and Antikamnia. In Proceedings of the 264th ACS National Meeting, Chicago, IL, USA, 21–25 August 2022. [Google Scholar]
- Ninan, B.; Wertheimer, A. Withdrawing Drugs in the U.S. Versus Other Countries. Inov. Pharm. 2012, 3, 87. [Google Scholar] [CrossRef][Green Version]
- Asperger, H. Die “Autistischen Psychopathen” im Kindesalter. Arch. Für Psychiatr. Und Nervenkrankh. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020; Morbidity and Mortality Weekly Report Surveillance Summaries; CDC: Washington, DC, USA, 2002. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-the Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Chen, D.; Xu, Y.; Lan, L.; Zhao, S.; Liu, Q.; Snijders, A.M.; Xia, Y. Maternal exposure to pesticides and autism or attention-deficit/hyperactivity disorders in offspring: A meta-analysis. Chemosphere 2023, 313, 137459. [Google Scholar] [CrossRef] [PubMed]
- Modick, H.; Schütze, A.; Pälmke, C.; Weiss, T.; Brüning, T.; Koch, H.M. Rapid determination of N-acetyl-4-aminophenol (paracetamol) in urine by tandem mass spectrometry coupled with on-line clean-up by two dimensional turbulent flow/reversed phase liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 925, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, G.; Weiss, T.; Modick, H.; Käfferlein, H.U.; Brüning, T.; Koch, H.M. N-Acetyl-4-aminophenol (paracetamol), N-acetyl-2-aminophenol and acetanilide in urine samples from the general population, individuals exposed to aniline and paracetamol users. Int. J. Hyg. Environ. Health 2014, 217, 592–599. [Google Scholar] [CrossRef]
- Eiche, A.; Bexell, G.; Sandelin, K. Genotoxicity of p-aminophenol in somatic and germ line cells of Drosophila melanogaster. Mutat. Res. 1990, 240, 87–92. [Google Scholar] [CrossRef]
- Hogestatt, E.D.; Jonsson, B.A.; Ermund, A.; Andersson, D.A.; Bjork, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef]
- Agency UEP. Title 40—Protection of Environment. Chapter I—Environmental Protection Agency (continued). Subchapter E—Pesticide Programs. Part 180—Tolerances and Exemptions for Pesticide Chemical Residues in Food; 2014. Available online: https://www.govinfo.gov/app/details/CFR-2016-title40-vol26/CFR-2016-title40-vol26-part180 (accessed on 13 December 2023).
- El-Sheikh, E.A.; Ramadan, M.M.; El-Sobki, A.E.; Shalaby, A.A.; McCoy, M.R.; Hamed, I.A.; Ashour, M.B.; Hammock, B.D. Pesticide Residues in Vegetables and Fruits from Farmer Markets and Associated Dietary Risks. Molecules 2022, 27, 8072. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.M.; Le, T.V.; Chu, T.T.Q.; Le, B.N.; Duong, M.D.; Thanh, N.M.; Tac Pham, V.; Minas, H.; Bui, T.T.H. Prevalence of autism spectrum disorders and their relation to selected socio-demographic factors among children aged 18–30 months in northern Vietnam, 2017. Int. J. Ment. Health Syst. 2019, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Nevison, C.; Blaxill, M.; Zahorodny, W. California Autism Prevalence Trends from 1931 to 2014 and Comparison to National ASD Data from IDEA and ADDM. J. Autism Dev. Disord. 2018, 48, 4103–4117. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, L.D.; Rice, C.E.; Barger, B.; Soke, G.N.; Lee, L.C.; Moody, E.; Edmondson-Pretzel, R.; Levy, S.E. DSM-5 criteria for autism spectrum disorder maximizes diagnostic sensitivity and specificity in preschool children. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Feldon, J.; Dammann, O. Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res. 2011, 69 Pt 2, 26R–33R. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Jones, J.P.; Parker, W. Is autism a member of a family of diseases resulting from genetic/cultural mismatches? Implications for treatment and prevention. Autism Res. Treat. 2012, 2012, 910946. [Google Scholar] [CrossRef]
- Becker, K.G. Autism, asthma, inflammation, and the hygiene hypothesis. Med. Hypotheses 2007, 69, 731–740. [Google Scholar] [CrossRef]
- Bickler, S.W.; DeMaio, A. Western diseases: Current concepts and implications for pediatric surgery research and practice. Pediatr. Surg. Int. 2008, 24, 251–255. [Google Scholar] [CrossRef]
- Parker, W.; Patel, E.; Jirků-Pomajbíková, K.; Laman, J.D. COVID-19 morbidity in lower versus higher income populations underscores the need to restore lost biodiversity of eukaryotic symbionts. iScience 2023, 26, 106167. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, F.; Willighagen, E.L.; Kutmon, M.; van Hoften, M.; Curfs, L.M.G.; Evelo, C.T. A resource to explore the discovery of rare diseases and their causative genes. Sci. Data 2021, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.O.; Robson, R.A.; Birkett, D.J. Paracetamol metabolism in pregnancy. Br. J. Clin. Pharmacol. 1986, 22, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cresteil, T. Onset of xenobiotic metabolism in children: Toxicological implications. Food Addit. Contam. 1998, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, K.; Wright, I.M.; Schneider, J.J.; Jones, A.L.; Martin, J.H. Pharmacokinetics in neonatal prescribing: Evidence base, paradigms and the future. Br. J. Clin. Pharmacol. 2015, 80, 1281–1288. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.D.; Charvet, C.J.; Clancy, B.; Darlington, R.B.; Finlay, B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 2013, 33, 7368–7383. [Google Scholar] [CrossRef]
- Lubrano, R.; Paoli, S.; Bonci, M.; Di Ruzza, L.; Cecchetti, C.; Falsaperla, R.; Pavone, P.; Matin, N.; Vitaliti, G.; Gentile, I. Acetaminophen administration in pediatric age: An observational prospective cross-sectional study. Ital. J. Pediatr. 2016, 42, 20. [Google Scholar] [CrossRef][Green Version]
- May, A.; Bauchner, H. Fever Phobia: The Pediatrician’s Contribution. Pediatrics 1992, 90, 851–854. [Google Scholar] [CrossRef]
- Bilenko, N.; Tessler, H.; Okbe, R.; Gorodischer, R. Determinants of antipyretic misuse in children up to 5 years of age: A cross-sectional study. Clin. Ther. 2006, 28, 783–793. [Google Scholar] [CrossRef]
- Poirier, M.P.; Collins, E.P.; McGuire, E. Fever phobia: A survey of caregivers of children seen in a pediatric emergency department. Clin. Pediatr. 2010, 49, 530–534. [Google Scholar] [CrossRef] [PubMed]
- ProPublica. Use Only as Directed2013 June 28, 2018. Available online: https://www.propublica.org/article/tylenol-mcneil-fda-use-only-as-directed (accessed on 20 September 2013).
- Walsh, A.; Edwards, H.; Fraser, J. Over-the-counter medication use for childhood fever: A cross-sectional study of Australian parents. J. Paediatr. Child Health 2007, 43, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Lacher, B.; Crain, E.F. Acetaminophen and ibuprofen dosing by parents. Pediatr. Emerg. Care 2000, 16, 394–397. [Google Scholar] [CrossRef]
- Alomar, M.; Alenazi, F.; Alruwaili, N. Accuracy of acetaminophen dosing in children by caregivers in Saudi Arabia. Ann. Saudi Med. 2011, 31, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.G.; Grunfeld, A.F. ‘Fever phobia’ in the emergency department: A survey of children’s caregivers. Eur. J. Emerg. Med. Off. J. Eur. Soc. Emerg. Med. 2006, 13, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Heubi, J.E.; Barbacci, M.B.; Zimmerman, H.J. Therapeutic misadventures with acetaminophen: Hepatoxicity after multiple doses in children. J. Pediatr. 1998, 132, 22–27. [Google Scholar] [CrossRef]
- Arikan, Z.; Teksam, O.; Kara, A.; Kale, G. Determining causes and frequency of misdosing of antipyretics in patients presenting with fever to pediatric emergency. Turk. Arch. Pediatr. 2012, 47, 114–118. [Google Scholar]
- Yavuz, E.; Yayla, E.; Cebeci, S.E.; Kirimli, E.; Gumustakim, R.S.; Cakir, L.; Dogan, S. Parental beliefs and practices regarding childhood fever in Turkish primary care. Niger. J. Clin. Pract. 2017, 20, 93–98. [Google Scholar] [CrossRef]
- Mackowiak, P.A.; Worden, G. Carl Reinhold August Wunderlich and the evolution of clinical thermometry. Clin. Infect. Dis. 1994, 18, 458–467. [Google Scholar] [CrossRef]
- Schmitt, B.D. Fever phobia: Misconceptions of parents about fevers. Am. J. Dis. Child 1980, 134, 176–181. [Google Scholar] [CrossRef]
- Kothari, V.M.; Karnad, D.R. New onset fever in the intensive care unit. J. Assoc. Phys. India 2005, 53, 949–953. [Google Scholar]
- Herzog, L.W.; Coyne, L.J. What is fever? Normal temperature in infants less than 3 months old. Clin. Pediatr. 1993, 32, 142–146. [Google Scholar] [CrossRef]
- Crocetti, M.; Moghbeli, N.; Serwint, J. Fever phobia revisited: Have parental misconceptions about fever changed in 20 years? Pediatrics 2001, 107, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.P.; Davis, P.H.; Gonzalez-del Rey, J.A.; Monroe, K.W. Pediatric emergency department nurses’ perspectives on fever in children. Pediatr. Emerg. Care 2000, 16, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.J.; Hanna-Jumma, S.; Carraretto, M.; Forni, L. The pathophysiological basis and consequences of fever. Crit. Care 2016, 20, 200. [Google Scholar] [CrossRef]
- Sullivan, J.E.; Farrar, H.C. Fever and Antipyretic Use in Children. Pediatrics 2011, 127, 580–587. [Google Scholar] [CrossRef]
- El-Radhi, A.S.M. Fever management: Evidence vs current practice. Clin. Pediatr. 2012, 1, 29–33. [Google Scholar]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Al-Jabi, S.W.; Sweileh, W.M.; Nabulsi, M.M.; Tubaila, M.F.; Awang, R.; Sawalha, A.F. Beliefs and practices regarding childhood fever among parents: A cross-sectional study from Palestine. BMC Pediatr. 2013, 13, 66. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; American Academy of Pediatrics. Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures. Febrile Seizures: Clinical Practice Guideline for the Long-term Management of the Child with Simple Febrile Seizures. Pediatrics 2008, 121, 1281–1286. [Google Scholar] [CrossRef]
- James, L.; Sullivan, J.E.; Roberts, D. The proper use of acetaminophen. Paediatr. Child Health 2011, 16, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Administration USFaD. Consumer Updates—Don’t Double Up. on Acetaminophen; FDA: Silver Spring, MD, USA, 2013. [Google Scholar]
- Greene, J.W.; Craft, L.; Ghishan, F. Acetaminophen poisoning in infancy. Am. J. Dis. Child 1983, 137, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Okasora, K.; Tanabe, T.; Ogino, M.; Yamazaki, S.; Oba, C.; Syabana, K.; Nomura, S.; Shirasu, A.; Inoue, K.; et al. Acetaminophen and Febrile Seizure Recurrences during the Same Fever Episode. Pediatrics 2018, 142, e20181009. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Suto, M.; Tsuji, M.; Sasaki, H.; Takehara, K.; Ishiguro, A.; Kubota, M. Use of antipyretics for preventing febrile seizure recurrence in children: A systematic review and meta-analysis. Eur. J. Pediatr. 2021, 180, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Guo, N.W.; Huang, C.C.; Wang, S.T.; Tsai, J.J. Neurocognitive attention and behavior outcome of school-age children with a history of febrile convulsions: A population study. Epilepsia 2000, 41, 412–420. [Google Scholar] [CrossRef]
- Ellenberg, J.H.; Nelson, K.B. Febrile Seizures and Later Intellectual Performance. Arch. Neurol. 1978, 35, 17–21. [Google Scholar] [CrossRef]
- Verity, C.M.; Butler, N.R.; Golding, J. Febrile convulsions in a national cohort followed up from birth. II—Medical history and intellectual ability at 5 years of age. Br. Med. J. (Clin. Res. Ed) 1985, 290, 1311–1315. [Google Scholar] [CrossRef]
- Schnaiderman, D.; Lahat, E.; Sheefer, T.; Aladjem, M. Antipyretic effectiveness of acetaminophen in febrile seizures: Ongoing prophylaxis versus sporadic usage. Eur. J. Pediatr. 1993, 152, 747–749. [Google Scholar] [CrossRef]
- Uhari, M.; Rantala, H.; Vainionpää, L.; Kurttila, R. Effect of acetaminophen and of low intermittent doses of diazepam on prevention of recurrences of febrile seizures. J. Pediatr. 1995, 126, 991–995. [Google Scholar] [CrossRef]
- Strengell, T.; Uhari, M.; Tarkka, R.; Uusimaa, J.; Alen, R.; Lautala, P.; Rantala, H. Antipyretic agents for preventing recurrences of febrile seizures: Randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2009, 163, 799–804. [Google Scholar] [CrossRef]
- Duffner, P.K. Anti-pyretic agents are ineffective in the prevention of febrile seizures. J. Pediatr. 2010, 156, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Monfries, N.; Goldman, R.D. Prophylactic antipyretics for prevention of febrile seizures following vaccination. Can. Fam. Physician 2017, 63, 128–130. [Google Scholar] [PubMed]
- El-Radhi, A.S.; Barry, W. Do antipyretics prevent febrile convulsions? Arch. Dis. Child 2003, 88, 641–642. [Google Scholar] [CrossRef][Green Version]
- Howard, C.R.; Howard, F.M.; Weitzman, M.L. Acetaminophen analgesia in neonatal circumcision: The effect on pain. Pediatrics 1994, 93, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.; Moody, M.A.; Walter, E.B. Effect of antipyretic analgesics on immune responses to vaccination. Hum. Vaccines Immunother. 2016, 12, 2391–2402. [Google Scholar] [CrossRef]
- WHO. Reducing pain at the time of vaccination: WHO position paper, September 2015-Recommendations. Vaccine 2016, 34, 3629–3630. [Google Scholar] [CrossRef] [PubMed]
- Homme, J.H.; Fischer, P.R. Randomised controlled trial: Prophylactic paracetamol at the time of infant vaccination reduces the risk of fever but also reduces antibody response. Evid.—Based Med. 2010, 15, 50–51. [Google Scholar] [CrossRef]
- Young, P.; Saxena, M.; Bellomo, R.; Freebairn, R.; Hammond, N.; van Haren, F.; Holliday, M.; Henderson, S.; Mackle, D.; McArthur, C.; et al. Acetaminophen for Fever in Critically Ill Patients with Suspected Infection. N. Engl. J. Med. 2015, 373, 2215–2224. [Google Scholar] [CrossRef]
- Wrotek, S.; LeGrand, E.K.; Dzialuk, A.; Alcock, J. Let fever do its job: The meaning of fever in the pandemic era. Evol. Med. Public Health 2021, 9, 26–35. [Google Scholar] [CrossRef]
- Thibault, C.; Pelletier, É.; Nguyen, C.; Trottier, E.D.; Doré-Bergeron, M.J.; DeKoven, K.; Roy, A.M.; Piché, N.; Delisle, J.F.; Morin, C.; et al. The Three W’s of Acetaminophen In Children: Who, Why, and Which Administration Mode. J. Pediatr. Pharmacol. Ther. 2023, 28, 20–28. [Google Scholar] [CrossRef]
- Simmons, K.; Ortiz, R.; Kossowsky, J.; Krummenacher, P.; Grillon, C.; Pine, D.; Colloca, L. Pain and placebo in pediatrics: A comprehensive review of laboratory and clinical findings. Pain 2014, 155, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Freo, U.; Ruocco, C.; Valerio, A.; Scagnol, I.; Nisoli, E. Paracetamol: A Review of Guideline Recommendations. J. Clin. Med. 2021, 10, 3420. [Google Scholar] [CrossRef] [PubMed]
- Towheed, T.E.; Maxwell, L.; Judd, M.G.; Catton, M.; Hochberg, M.C.; Wells, G. Acetaminophen for osteoarthritis. Cochrane Database Syst. Rev. 2006, 2006, Cd004257. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Ashoorion, V.; Gmora, S.; Anvari, M.; Hong, D. Intravenous Acetaminophen versus Placebo in Post-bariatric Surgery Multimodal Pain Management: A Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2019, 29, 1420–1428. [Google Scholar] [CrossRef]
- Windorfer, A.; Vogel, C. Investigations concerning serum concentration and temperature following oral application of a new paracetamol preparation (author’s transl). Klin. Padiatr. 1976, 188, 430–434. [Google Scholar]
- FDA. FDA Clarifies Results of Recent Advisory Committee Meeting on Oral Phenylephrine; US Government—FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
| Summary of Evidence | Nature of Evidence |
|---|---|
| 1. Early life exposure to APAP at doses similar to or even less than doses received by human babies and children results in long-term, profound modification of brain function in both laboratory mice and rats [5,6,7,8,17], by definition a severe adverse event that should have precluded any clinical testing of APAP in babies and small children. | Multiple, independent laboratory animal studies demonstrate that APAP is not safe for neurodevelopment. |
| 2. In laboratory rats, APAP affects the developing male brain more than the female brain [8]. In laboratory mice, males are more susceptible to APAP-mediated liver injury than are females [18]. ASD is more prevalent in males than in females [19]. | Laboratory animal studies of APAP-mediated injury reflect the sex distribution of ASD in humans. |
| 3. APAP causes apoptosis-mediated death of cortical neurons in adult laboratory rats at concentrations lower than it causes liver failure [20]. Affected cortical neurons are implicated in ASD [21,22], and individuals with ASD have increased levels of biomarkers for neuronal apoptosis [23,24,25]. | A laboratory animal study of APAP-mediated brain injury reflects biomarkers of injury in humans with ASD. |
| 4. Adult cats are susceptible to APAP-mediated injury due to the lack of a robust glucuronidation-dependent capacity for metabolism [26,27,28,29]. Human neonates similarly lack a robust glucuronidation-dependent pathway [30,31]. | Metabolic status causing sensitivity to APAP-mediated injury in an animal model reflects the metabolic status of human neonates. |
| 5. APAP use in babies and children was assumed to be safe during the 1970s despite the fact that it targets brain function and was never shown to be safe for neurodevelopment [32]. | Demonstration that the current safeguards for drug approval were bypassed for pediatric use of APAP. |
| 6. Circumcision of males, often performed using APAP as an analgesic, is associated with a twofold increase in the risk for early-onset (infantile) ASD [11]. | Temporal association with neonatal APAP use and ASD. |
| 7. APAP-containing products used by South Korean children were repeatedly found to contain amounts of the drug, exceeding the package label [33], and an exceptionally high prevalence of ASD was identified in South Korea [34,35]. | Temporal association of accidental, excess APAP administration and ASD. |
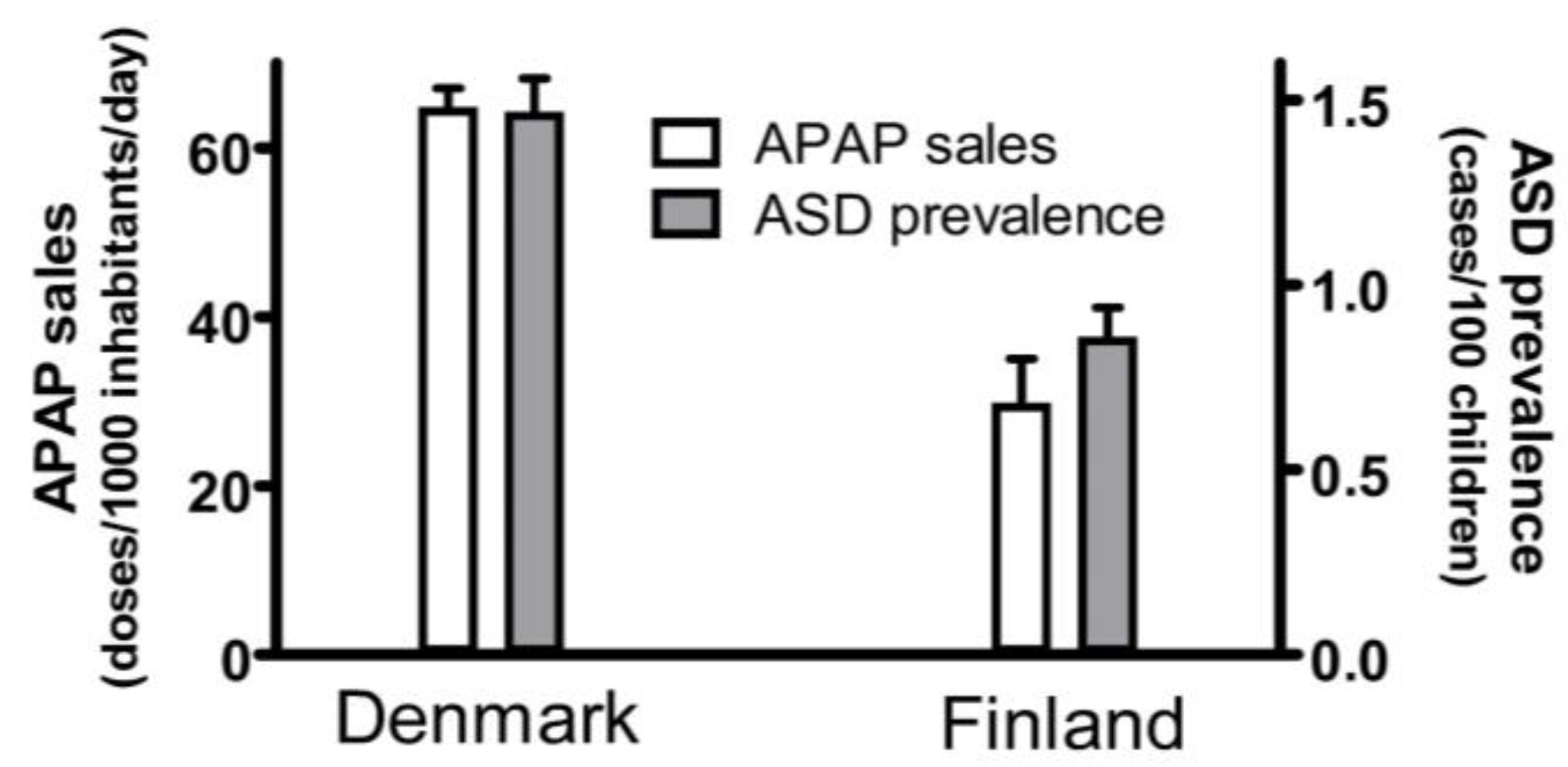
| 8. The popularity of APAP use and the prevalence of ASD was substantially higher in Denmark than in Finland in the mid-2000s (Figure 1). | Geographic association between APAP use and ASD. |
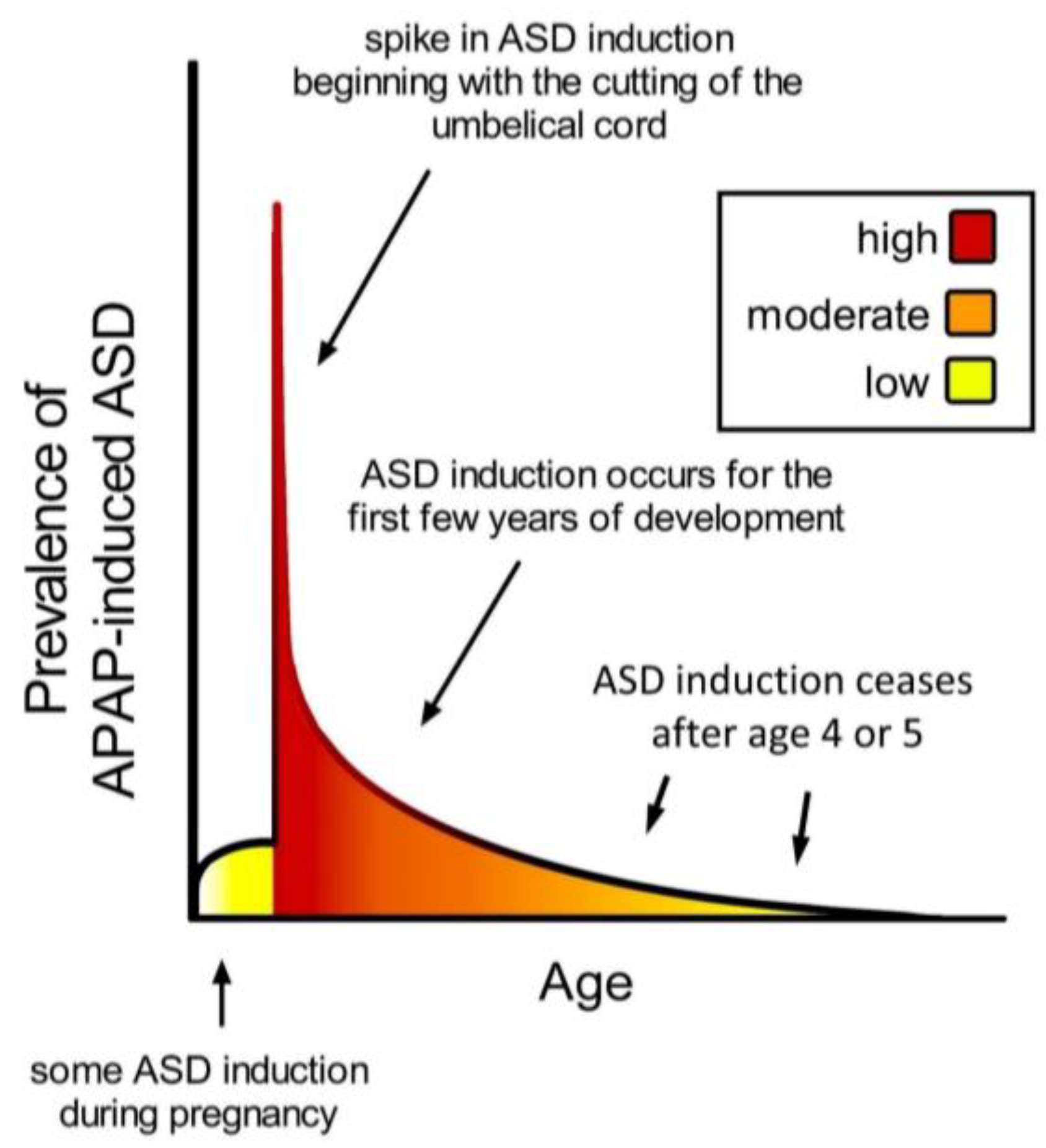
| 9. Ultra-Orthodox Jews [36] and Arabs [36,37] in Israel have a reported prevalence of ASD less than half of that of other Israelis. Traditional circumcision practices employed by Ultra-Orthodox Jews do not utilize APAP, and circumcision practices in Arab communities take place outside of the neurodevelopmental window sensitive to ASD induction (Figure 2). | Temporal association between neonatal use of APAP and ASD. |
| 10. Analysis of 61,430 babies in the Danish National Birth Cohort found an odds ratio (OR) of 1.3 (CI 1.02–1.66) for ASD associated with postnatal APAP exposure [13]. The approach used in the analysis is expected to dramatically underestimate the real odds ratio [1]. | Association with postpartum APAP use and ASD from one epidemiologic study. |
| 11. The ratio of regressive to infantile ASD rose at the same time as pediatric APAP use rose [14] after aspirin was associated with Reye’s syndrome [9]. | Temporal association between the pediatric use of APAP and the qualitative nature of ASD. |
| 12. The incidence of ASD began to increase in the early 1980s, coinciding with the increase in APAP use after aspirin was associated with Reye’s syndrome [9]. | Temporal association between pediatric use of APAP and the prevalence of ASD in the early 1980s. |
| 13. The incidence of ASD has steadily increased [9] as direct-to-consumer advertising [38] and perhaps other factors have driven up the use of pharmaceutical products. | Temporal association between the use of APAP and the prevalence of ASD post-1990. |
| 14. Maternal use of APAP during pregnancy is associated with long-term effects that include lower IQ, increased ASD, and increased ADHD in their children [12,13,39,40,41,42,43,44,45,46,47,48,49,50]. | Association with prepartum APAP use and neurodevelopmental problems from numerous epidemiologic studies, some with controls for indication. |
| 15. Levels of APAP in cord blood are associated with ASD [43]. | Association with APAP use during the peripartum period and the prevalence of ASD. |
| 16. APAP given alongside the MMR vaccine but not the MMR vaccination alone was associated with ASD [10]. | Dramatically enhanced risk of ASD associated with the use of APAP with vaccination found in a case-controlled study involving 81 children with ASD. |
| 17. APAP use during early childhood is associated with a dramatic increase in regressive ASD [10]. | Dramatically enhanced risk of regressive ASD associated with APAP use found in a case-controlled study involving 81 children with ASD. |
| 18. Many parents believe that their children’s ASD was induced by a vaccine [51,52]. APAP is frequently used with vaccinations, although vaccinations alone do not cause ASD. | Association between APAP use and ASD inadvertently and consistently made by a substantial fraction of parents of children with ASD. |
| 19. APAP use in adults temporarily blunts social trust [53] and awareness [54], emotional responses to external stimuli [55], and the ability to identify errors [56], indicating that the drug targets regions of the brain affected in patients with ASD. | The transient effects of APAP in adult humans are reflected in the symptoms of ASD. |
| 20. Cystic fibrosis is associated with unusually efficient (effective) metabolism of APAP [57,58], and evidence suggests that the prevalence of ASD is very low in patients with cystic fibrosis [9]. | Resistance to APAP-mediated injury is apparently associated with a very low prevalence of ASD. |
| 21. Genetic and immune factors associated with an increased risk of ASD have a detrimental effect on the body’s ability to metabolize APAP [9,59,60]. | Plausible mechanism: risk factors for ASD and for adverse reactions to APAP are equivalent. |
| 22. APAP is known to be highly toxic in the presence of oxidative stress [61] via a mechanism that involves the formation of the toxic metabolite NAPQI [62,63,64] and concomitant mitochondrial damage [65]. Oxidative stress [9] and possibly mitochondrial dysfunction [66] also play a role in ASD. | Plausible mechanism: production of toxic metabolites from APAP under conditions involved with ASD pathology is established. |
| Time Period (Age) | Crude Estimates of Relative Fraction of Total ASD Induced by APAP | Source of Data/Information for Crude Estimate |
|---|---|---|
| Prenatal | 10–20% | Cohort studies |
| Early postnatal period (birth to 5 days) | 50–60% | Association of ASD with cord blood acetaminophen [43], supported by association of ASD with circumcision [11] |
| 5 days until 2–12 months (regression not observable) | No information available | Not applicable |
| 2–12 months until 4 or 5 years (regression observable) | 20–30% | Small case control study [10], supported by observations of parents [51,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, W.; Anderson, L.G.; Jones, J.P.; Anderson, R.; Williamson, L.; Bono-Lunn, D.; Konsoula, Z. The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits. Children 2024, 11, 44. https://doi.org/10.3390/children11010044
Parker W, Anderson LG, Jones JP, Anderson R, Williamson L, Bono-Lunn D, Konsoula Z. The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits. Children. 2024; 11(1):44. https://doi.org/10.3390/children11010044
Chicago/Turabian StyleParker, William, Lauren G. Anderson, John P. Jones, Rachel Anderson, Lauren Williamson, Dillan Bono-Lunn, and Zacharoula Konsoula. 2024. "The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits" Children 11, no. 1: 44. https://doi.org/10.3390/children11010044
APA StyleParker, W., Anderson, L. G., Jones, J. P., Anderson, R., Williamson, L., Bono-Lunn, D., & Konsoula, Z. (2024). The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits. Children, 11(1), 44. https://doi.org/10.3390/children11010044







