Abstract
Based on available data that include approximately 20 lines of evidence from studies in laboratory animal models, observations in humans, correlations in time, and pharmacological/toxicological considerations, it has been concluded without reasonable doubt and with no evidence to the contrary that exposure of susceptible babies and children to acetaminophen (paracetamol) induces many, if not most, cases of autism spectrum disorder (ASD). However, the relative number of cases of ASD that might be induced by acetaminophen has not yet been estimated. Here, we examine a variety of evidence, including the acetaminophen-induced reduction of social awareness in adults, the prevalence of ASD through time, and crude estimates of the relative number of ASD cases induced by acetaminophen during various periods of neurodevelopment. We conclude that the very early postpartum period poses the greatest risk for acetaminophen-induced ASD, and that nearly ubiquitous use of acetaminophen during early development could conceivably be responsible for the induction in the vast majority, perhaps 90% or more, of all cases of ASD. Despite over a decade of accumulating evidence that acetaminophen is harmful for neurodevelopment, numerous studies demonstrate that acetaminophen is frequently administered to children in excess of currently approved amounts and under conditions in which it provides no benefit. Further, studies have failed to demonstrate long-term benefits of acetaminophen for the pediatric population, leaving no valid rationale for continued use of the drug in that population given its risks to neurodevelopment.
1. Introduction
We have recently reviewed mounting evidence that acetaminophen (paracetamol) use in susceptible babies and children is associated with the development of autism spectrum disorder (ASD) and other neurodevelopmental disorders [1,2]. Based on approximately 20 lines of evidence, we previously concluded without reasonable doubt and with no evidence to the contrary that acetaminophen administration in susceptible babies and children is a causative agent for the induction of many, if not most, cases of ASD [1,2]. The conclusion is based on (a) studies in laboratory animal models [3,4,5,6,7,8], (b) understanding of the pharmacological mechanisms associated with acetaminophen toxicity [9], (c) connections between ASD, acetaminophen exposure, and human activities such as vaccination [10] and circumcision [11], (d) associations between acetaminophen administration and ASD during the later stages of pregnancy [12] and in early childhood [10,13], and (e) associations between acetaminophen use and ASD through time [9,14].
A current summary of evidence demonstrating that acetaminophen use in susceptible babies and children causes many, if not most, cases of ASD is shown in Table 1. Fourteen associations between acetaminophen use during early neurodevelopment and ASD are evident. Any one geographic or temporal association might be spurious, possibly unrelated to causality. For example, two independent studies, when taken together, show that the popularity of acetaminophen in two Scandinavian countries, Denmark and Finland, correlated with the prevalence of ASD in those countries (Figure 1). First, the sales of acetaminophen per unit population from 2006 through 2010 in Denmark were more than twofold greater than the sales of acetaminophen in Finland during the same time period [15]. Second, for children born in 2006, whose brain development might have been influenced by exposure to acetaminophen between 2006 and 2010, the prevalence of ASD in children born in Denmark was approximately 70% greater than the prevalence of ASD in children born in Finland [16]. Limitations to the conclusions that can be drawn from this previously unreported geographic association are evident. For example, total sales of acetaminophen do not necessarily reflect use of the drug during early development, when induction of ASD is possible, and therefore, this association does not necessarily reflect a direct association between pediatric use of acetaminophen and the prevalence of ASD. Nevertheless, when considered in light of other lines of evidence (Table 1), this association adds to the burden of evidence demonstrating without reasonable doubt that acetaminophen use in susceptible babies and children causes many, if not most, cases of ASD.

Table 1.
Current evidence leading to the conclusion that early exposure of individuals to acetaminophen (APAP) causes many, if not most, cases of ASD. Twenty-two lines of evidence, with 20 independent lines of evidence, are provided. Components of lines of evidence numbers 12 and 13 are derived from the same sources measuring temporal trends in ASD prevalence. Thus, although multiple sources corroborate those temporal trends, those two lines of evidence are not entirely independent. Lines of evidence 16 and 17 are derived from the same case-controlled study involving 81 individuals with ASD, and thus, those two sources are not independent.
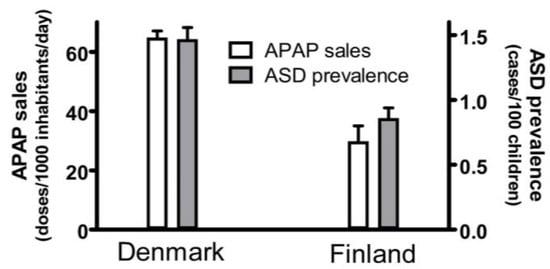
Figure 1.
The sales of acetaminophen (APAP) in Denmark and Finland correlated with the prevalence of autism spectrum disorder (ASD) in those countries. The sale of acetaminophen is limited to the sale of drugs containing acetaminophen as the only active ingredient and is measured in units of a “defined daily doses” (equal to 3 g of active ingredient) per 1000 inhabitants per day. The sales for each year from 2006 to 2010, as reported by Wastesson and colleagues [15], were averaged and the mean plotted. Sales for both Denmark and Finland showed upward trends every year, and the upper limit of the range (data from 2010) is shown by the error bars. The prevalence of ASD in Denmark and Finland was reported by Delobel-Ayoub and colleagues [16]. Data are shown for all children, including males and females, born in 2006, with prevalence measured at 9 years of age. The 95% confidence interval reported by Delobel-Ayoub and colleagues is indicated by the error bars.
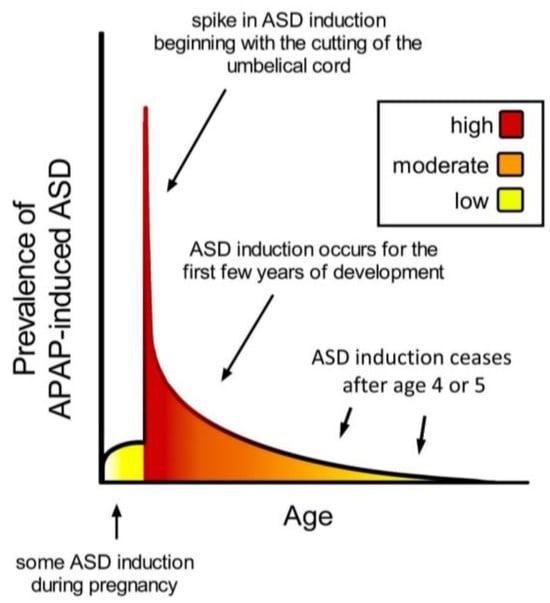
Figure 2.
Schematic diagram showing prevalence of acetaminophen (APAP)-induced ASD as a function of age. The neurodevelopmental window of sensitivity is broad, possibly bounded by the first trimester of pregnancy and the end of the 4th or 5th year of life. The shape of the curve is apparently determined by levels of exposure to acetaminophen, and sensitivity to acetaminophen, a function of oxidative stress and ability to metabolize acetaminophen [9]. ASD, autism spectrum disorder; APAP, acetaminophen (paracetamol).
This evidence for a causal relationship between acetaminophen exposure and ASD, when considered in aggregate, is overwhelming [1,2]. However, the question remains, exactly how much of the total burden of ASD is caused by exposure of susceptible babies and children to acetaminophen? The second question that will be addressed in this review is, do the risks of acetaminophen for neurodevelopment outweigh the benefits of the drug as it is currently used?
2. Evidence Supports Extensive Influence of Acetaminophen Exposure on Current Rates of ASD
Several observations are consistent with the view that the vast majority of ASD could be induced by acetaminophen. For example, the prevalence of ASD in the 1970s (1/2500), prior to the widespread use of acetaminophen in the pediatric population, was approximately 2% or less of the current prevalence (1/36). Further, a consistent hallmark of ASD is impairment in social awareness, and studies in adult humans indicate that acetaminophen specifically affects social awareness [53,54,67]. Thus, the view that ASD is a hallmark of acetaminophen-induced neurodevelopmental injury is consistent with all available information.
The association of ASD with numerous factors that cause oxidative stress, including but not limited to metabolic abnormalities, some genetic polymorphisms, infections, antibiotic use, and other medical conditions, is consistent with the fact that acetaminophen metabolism is more hazardous in the presence of oxidative stress, a condition associated with risk factors for ASD [9]. Thus, the view that the high prevalence of ASD can be largely accounted for by the currently ubiquitous use of acetaminophen provides a straightforward explanation for the numerous observed risk factors associated with ASD.
An estimation of the relative number of cases of ASD that are induced by acetaminophen might be obtained by considering studies that describe ASD induction during pregnancy, at birth, and later in postpartum development. A compilation of relevant information derived from such studies is shown in Table 2 and is consistent with the view that the vast majority of cases of ASD could conceivably be induced by acetaminophen. During pregnancy, studies of cohort data suggest that some cases of ASD are possibly caused by heavy use of acetaminophen [12,13]. However, based on an assessment of available data, we previously concluded [1] that the number of cases of ASD induced by acetaminophen exposure during pregnancy probably accounts for less than 20% and may account for 10% or less of the total cases of ASD. However, these cases add to the tally induced by postnatal exposure to acetaminophen.

Table 2.
Crude estimates of the relative fraction of total ASD induced by acetaminophen (APAP) at different times during neurodevelopment. The “early postnatal period” is defined for these purposes as the first 5 days postpartum, when many neonates would have been circumcised [68] prior to leaving the hospital [69]. The time when regression becomes observable is unclear [70], and therefore, the time between the peripartum period and when regression is observable is given as a range, between 2 and 12 months. A paucity of information exists for the period after the early postnatal period until regressive ASD would potentially be observable, between 2 and 12 months of age. ASD, autism spectrum disorder.
Induction of ASD by acetaminophen near the time of birth apparently contributes extensively to the total cases of ASD induced by acetaminophen. A study in the US found striking associations between ASD and acetaminophen in cord blood [43]. When children were divided into three groups based on their cord blood acetaminophen, the relative prevalence of ASD was 1.0 (baseline), 2.14, and 3.62, positively correlated with acetaminophen levels. Based on these data, one could crudely estimate that cord blood acetaminophen accounts for a 125% increase in ASD, or almost 56% of all cases of ASD {[(1.0 + 2.14 + 3.62)/(1 + 1 + 1)] = 2.2533}. This crude estimate does not consider a number of issues, including potentially confounding factors such as the reason for administration of acetaminophen. However, a 100% (twofold) increase in infantile ASD is associated with circumcision [11], a minor medical procedure often associated with acetaminophen use shortly after birth, supporting the view that the very early postpartum period is a time of very high risk for acetaminophen-induced ASD. As discussed previously [1], a twofold increase in infantile ASD at the time of circumcision could account for 15% to 20% of all cases of autism in a country such as the United States, which has a male circumcision rate of approximately 60% [71]. Finally, the case-controlled study by Schultz [10] found a 20-fold greater prevalence of regressive ASD when acetaminophen was used between 12 and 18 months of age, suggesting that the vast majority of regressive ASD, reflecting approximately 30% of all cases [70], may be due to acetaminophen exposure. The Schultz study included 81 children with ASD and 79 neurotypical controls, but the confidence errors were substantial, leaving uncertainty regarding the exact number of cases of regressive ASD induced by acetaminophen. However, the view that the vast majority of regressive autism might be induced by acetaminophen is supported by surveys of parents with children who have ASD. Between 30% and 50% of parents surveyed in the USA attribute their child’s ASD at least in part to vaccination [51,52], a procedure often accompanied by administration of acetaminophen.
Given the above considerations, the assertion that eliminating exposure to acetaminophen during neurodevelopment would result in a greater than 90% decrease in the prevalence of ASD seems plausible. It could be argued that acetaminophen cannot possibly cause all cases of ASD since ASD was discovered in the 1940s, whereas acetaminophen was not used until the 1950s. However, phenacetin and acetanilide, both of which are converted into acetaminophen by the human body [72,73], were introduced in Germany in the 1880s and widely used as analgesics [73,74] until they were determined to be excessively toxic and their use was banned in most countries in the 1970s and 1980s [73,75]. With this in mind, it is possible that some of the earliest known cases of ASD reported in the 1940s in Germany [76] and in the USA [77] were in fact induced by the metabolite acetaminophen as a result of exposure to phenacetin or acetanilide.
The idea that acetaminophen induces the vast majority of cases of ASD is attractive from a scientific perspective, providing a straightforward explanation for numerous observations in the field, some of which are summarized above. The idea is also appealing from a public health perspective, as it provides a means for rapidly reducing the burden of ASD, which now affects one in every 36 US children [78]. However, the uncertainty (confidence intervals) associated with the Schultz study of regressive autism [10] and with the study of cord blood acetaminophen [43] are very large, and thus, the relative amount of ASD induced by acetaminophen cannot be accurately determined from these studies. Further, studies of large cohorts are complicated by several factors, including poor documentation of over-the-counter acetaminophen use [2], precluding the reliability of the quantitative estimates obtained in such studies [1]. Thus, although the idea that acetaminophen induces the vast majority of cases of ASD is attractive, the idea cannot be embraced with confidence at the present time. However, our perspective is that the induction in most cases of ASD by acetaminophen exposure in susceptible babies and children is likely, and the induction in the vast majority of cases, perhaps more than 90%, is plausible. Without the ability to objectively test the null hypothesis, the historical record provides rationale for this conclusion.
3. Alternative Hypotheses
It could be argued that the reason for giving acetaminophen, for example, an infection-induced fever, is a confounding factor. In this hypothetical scenario, the reason for giving acetaminophen is either the cause of ASD induction itself or an indicator of the presence of ASD. However, this explanation does not account for several observations [1,2], including the increasing prevalence of ASD over time and the association of ASD with the circumcision procedure. Further, if infection-induced fevers were the cause of ASD, then it is expected that pandemics associated with unhygienic conditions in the past would have induced pandemics of ASD. Finally, as highlighted above, clinical indications for acetaminophen use generally cause oxidative stress, meaning that the clinical indications precipitating the administration of acetaminophen are not potential confounding factors, but rather are cofactors in the induction of injury.
Environmental factors that cause oxidative stress are expected to enhance acetaminophen-induced neurodevelopmental injury due to their impact on drug metabolism [9]. Thus, the previously noted [9] association of a wide range of oxidative stress-inducing factors with ASD is not surprising. In addition, based on the available evidence outlined in the Introduction (see Table 1), these oxidative stress-inducing factors are not expected to induce ASD in the absence of acetaminophen administration. A notable exception under specific circumstances may be some pesticides. Pesticides in general result in increased oxidative stress [79] and are generally associated with a moderately increased risk of ASD [80]. As such, pesticides fit into a broad category of oxidative stress-inducing factors that negatively impact acetaminophen metabolism and are associated with ASD [9]. However, some pesticides differ from most oxidative stress inducing factors in that they are metabolized by the liver into either acetaminophen [81,82], biologically active metabolites of acetaminophen [83,84], or a variety of compounds structurally related to those metabolites [85]. This suggests the possibility that exposure to some pesticides could induce ASD directly due to the presence of acetaminophen or related metabolites without therapeutic administration of the drug. However, pesticide contaminants in food tend to be less than 1 mg/kg [85,86], and a hundred-fold or more dilution in the human body following consumption and digestion would place the levels of these chemicals hundreds of times lower than the therapeutic dose of acetaminophen, which is approximately 15 mg/kg body weight. Thus, it is expected that typical exposures to pesticides in contaminated food might impose a very minor risk in comparison to therapeutic usage of acetaminophen, and such exposure would not typically, by itself, induce ASD. That being said, heavy exposure during the production, distribution, or use of bulk pesticides could conceivably provide sufficient exposure to acetaminophen or related metabolites for ASD induction in the absence of therapeutic use of the drug. Such a scenario may account for the observation that children of mothers who worked as farmers in Vietnam had an almost fivefold greater prevalence of ASD as compared to children of mothers who were government staff (OR = 4.72, 95% CI 2.03–10.97) [87].
It could be argued that no dramatic change in the prevalence of ASD has occurred over the past 40 years and that much of the perceived change is due to a variety of factors unrelated to neurodevelopment. Proposed factors include changing diagnostic criteria, increasing awareness, and funding/resource-driven increases in diagnosis. However, data pointing at the role of acetaminophen in the induction of ASD undermine this assertion. For example, the much greater prevalence of ASD associated with circumcision [11] and cord blood acetaminophen [43] is difficult to explain if no real increase in the prevalence of ASD exists. While various changes in social and clinical factors undoubtedly caused increases in the measured prevalence of ASD at the time that those factors emerged, careful analysis reveals that such factors do not account for 40 years of steadily increasing prevalence [88]. Further, recent changes in diagnostic criteria tend to reduce, not increase, the diagnosis of ASD: Version Five of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), published in 2013, is generally considered to provide more, not less, restrictive diagnostic criteria than version four of the manual (DSM-IV) [89], which was published in 1994. Equally important is the consistent ratio through time of severe to mild impairment in individuals with ASD, indicating that changes in prevalence are not due to medical professionals more effectively identifying relatively mild cases. In addition, if there has been little or no real change in the prevalence of ASD in the past century, it is difficult to explain why the condition was not identified until the 1940s and why the measured prevalence did not substantially increase until the 1980s and, particularly, the 1990s. In contrast, if acetaminophen exposure in susceptible babies and children is indeed responsible for a significant portion of ASD, then these trends in time are fully expected.
Because of its association with inflammation [9,90,91] and its currently increasing prevalence, ASD fits into a category of now-prevalent medical conditions that emerged following the second industrial revolution [92,93]. Those conditions, generally associated with increased inflammation, include allergies, autoimmune diseases, some neuropsychiatric disorders, and a variety of cardiovascular problems [94,95,96]. Rare or even absent in ancestral human populations, these conditions emerged as a result of environment and/or lifestyle changes [94,95,96]. Thus, the dramatically increased prevalence of ASD fits into an established pattern among numerous medical conditions, and its dramatically increased prevalence can be taken as an indication of environment and/or lifestyle changes, not as an indicator that a common condition was previously mistaken as rare. Indeed, dozens of rare, mutation-induced diseases have been identified over the past two centuries [97], and the prevalence of those diseases has remained rare.
4. Periods of Sensitivity to Acetaminophen during Neurodevelopment
The timing of acetaminophen-mediated induction of ASD during brain development is of considerable interest. Based on an analysis of cohort data by Liew and colleagues [12], the neurodevelopmental window sensitive to acetaminophen may begin very early during pregnancy, perhaps in the first trimester (Figure 2). However, the time of birth is evidently the most critical period in terms of sensitivity to acetaminophen-induced neurodevelopmental injury (Figure 2). The view that newborns are exquisitely sensitive to acetaminophen is consistent with the 2-fold greater prevalence of infantile ASD associated with circumcision [11] and the 3.6-fold greater prevalence of ASD in the third of children with the highest levels of acetaminophen in their cord blood compared to the third with the lowest levels [43]. As discussed previously [2], the well-established age-dependent pharmacology of acetaminophen [98,99] dictates that, by unit weight, the mother/fetus dyad is far more capable of metabolizing and detoxifying acetaminophen than the newborn. The dramatic differences in weight-adjusted capacity to metabolize and detoxify acetaminophen between pregnant women and newborns [2] are expected to adversely affect the developing brain of susceptible newborns when the umbilical cord is cut in the presence of acetaminophen. Based on pharmacokinetic considerations, the first 10 days of life, in particular, should be the most sensitive to acetaminophen, when the glucuronidation process, important for the detoxification of acetaminophen [2], is not yet functional [100]. Following the perinatal period, the work by Schultz [10] discussed above and the observations from parents discussed previously [9] indicate that acetaminophen induces many, if not most, cases of regressive ASD. Based on a meta-analysis by Tan and colleagues [70], regression can occur in children as old as 4 or 5 years, but the time distribution of regression is skewed toward earlier ages, with half of all cases of regression probably occurring before approximately 1.5 years of age. Thus, as shown in Figure 2, the neurodevelopmental window for the induction of ASD by acetaminophen is broad, with the prevalence of induction apparently dictated by the level of drug exposure and by susceptibility.
The current presumption in the medical community that acetaminophen is safe for pediatric use is based on dozens of studies in babies and children that incorrectly assumed that the toxic effects of the drug in adults would be the same for babies and children [32]. However, given that the therapeutic target of acetaminophen involves brain function, pediatric use of acetaminophen should have been preceded by extensive tests using animal models for neurodevelopment [1]. Preclinical toxicity screens using laboratory animals often fail to detect drug toxicity in humans [101]. However, neurodevelopment is a conserved process across mammalian species, and laboratory animals provide a very good model for examining brain sensitivity in the perinatal period [102]. Indeed, the toxicity of acetaminophen for the developing brain is readily detected using perinatal laboratory animal models [1,3,4,5,6,7,8]. Given the severe adverse effects of acetaminophen on neurodevelopment observed in laboratory animals by several independent laboratories using a variety of experimental designs, we conclude that the standard safeguards involving preclinical testing that protect the population from exposure to drugs with a poor benefit-to-risk ratio were not employed when acetaminophen was introduced into widespread pediatric use in the 1980s.
Based on available evidence (Table 1), we are confident that many cases of ASD, and perhaps the vast majority, are caused by exposure of susceptible individuals to acetaminophen during early stages of neurodevelopment. With this in mind, we urgently encourage regulatory and policy-making bodies to restrict the pediatric use of acetaminophen and stress the importance of consumer education to inform individuals of current knowledge regarding the impact of acetaminophen on the developing brain. The fact that consumers lack education regarding the dangers of acetaminophen for neurodevelopment is evident from numerous studies outlined below showing widespread frivolous use of the drug for lowering body temperatures that are not actually high enough to be classified as a fever [103,104,105,106,107] and misuse of the drug either by giving too high of a dose or administering the drug too frequently [105,106,107,108,109,110,111,112,113,114].
5. Improper Use of Acetaminophen to Treat Fevers Is Common
Existing studies suggest that acetaminophen is not administered to children in a manner that weighs the drug’s evident benefits against its risks, resulting in an overaggressive administration of antipyretics in children. Part of this problem involves misconceptions regarding what temperature constitutes a fever. According to the definition first described by Carl R.A. Wunderlich more than a century ago [115] and still widely accepted today [116], a fever in humans constitutes a temperature greater than or equal to 38.0 °C (100.4 °F). However, a temperature of 38.3 °C (100.9 °F) is a more appropriate cutoff for a fever [117], with many healthy infants having a normal temperature of 38.1 °C or 38.2 °C, especially during the summer months [118]. Nevertheless, approximately half of parents consider a temperature of less than 38 °C (100.4 °F) to be a fever [119], and among surveyed pediatric emergency nurses, 46% also stated that a temperature less than 38 °C is considered a fever [120]. These findings indicate that many parents and health care workers do not know how to accurately define a fever.
Fever in a variety of circumstances, including brain injury, is associated with worsened outcomes and can lead to damage to specific organs, including the kidneys and the liver [121]. However, fevers associated with infection constitute a critical component of the immune response to infection and are beneficial [122,123]. Evans and colleagues, for example, assert that an increase of 1 to 4 °C in core body temperature is associated with “improved survival and resolution of many infections” [124]. With this in mind, antipyretic treatments are, in fact, immunosuppressive. Further, even within the higher range of 40 °C to 42 °C, there is no evidence to suggest that typical fevers in children without brain injury present an increased risk for adverse health outcomes such as brain damage [116,122,123].
Several investigators have reported “fever phobia”—exaggerated concerns about fever in children and its complications (e.g., seizures, brain damage, etc.) [106,116,119,122,125]. Ninety-one percent of caregivers believe fevers can have harmful effects, with 21% of caregivers listing brain damage and 14% listing death [119]. Further, sixty percent of pediatricians state that temperatures of 104 °F (40 °C) or greater can cause seizures, brain damage, or death [104]. However, as stated by the American Academy of Pediatrics, to their knowledge, a child dying from a simple febrile seizure “has never been reported” [126]. This is despite the fact that 2–5% of all children experience febrile seizures. Fever in children causes disproportionate anxiety even among health care professionals; for example, among pediatric emergency nurses, 38% state that temperatures less than 40 °C could cause serious complications [120].
Consequentially, antipyretics are administered by caretakers [106,116,119,125] and pediatric health care professionals [104,120], even when there is minimal fever or no fever. A survey of 340 caregivers in two hospital-based pediatric clinics in Maryland found that 25% of caregivers gave antipyretics for temperatures under 37.8 °C (less than 100 °F) [119]. Another survey of 230 caregivers of children in a Pediatric Emergency Department in Virginia reported that 63.9% considered a temperature of less than 37.8 °C to be the minimum temperature for antipyretics [106]. A survey of caregivers of 201 children in Israel estimated that 65.2% of caregivers indicated that they would administer antipyretics for temperatures lower than 38 °C [105]. Further, among pediatricians in Massachusetts, 72% reported they always or often recommended treatment to reduce fever (including acetaminophen), and 89% stated they did so at temperatures between 38.3 °C and 38.9 °C [104]. Finally, an Italian study found that a surprising 74% of all administrations of acetaminophen for fever were given to treat fevers less than 38.4 °C. The authors conclude that “preventive action should be taken regarding the use of acetaminophen as antipyretic drug in children in order to reduce the fever phobia and self-prescription…” [103]. Thus, with the possible exception of a study of 402 parents in Palestine that found that only 1.5% would give antipyretics for temperatures less than 38 °C [125], numerous studies point toward a wide-spread fever phobia, with many parents and even health care workers overtreating fevers.
6. Overdoses of Acetaminophen in the Pediatric Population
Acetaminophen has a relatively low “therapeutic index”—the difference in the amount required for a therapeutic effect and the amount that is toxic is relatively small. A low therapeutic index, coupled with wide availability and apparently wide use, poses safety concerns with respect to dosing [127]. Research indicates that some caregivers administer incorrect doses to children, with some studies demonstrating a supratherapeutic dosage being given [105,109,110,113,114] and other studies finding administration at intervals that are too frequent [105,106,108,109,110,111,112]. Additionally, the observation that more than one medication containing acetaminophen is being given to children at the same time, resulting in overexposure to the drug, is also problematic [128].
A variety of evidence indicates that overdoses of acetaminophen in the pediatric population are common. A study of caregivers to 200 children in Turkey, for example, found that 8.4% of the patients received doses of acetaminophen that exceeded the recommended maximum dose [113]. Further, a study of another 200 patients aged 10 years or younger at the pediatric emergency department at Jacobi Medical Center in New York found that 15% of 124 patients receiving acetaminophen were given overdoses [109]. The authors also noted that a combined 51% of caregivers incorrectly stated that the dosage should be based on either the age of the child or the height of the fever; caregivers who correctly stated that the dosage should be based on their child’s weight were significantly less likely to give the wrong dosage (relative risk (RR) = 0.71, p < 0.03, 95% CI = 0.52–0.97) [109]. In another Turkish study, 12.1% of parents overdosed their child with acetaminophen [114]. A study in Saudi Arabia estimated that 27% of children aged 14 or younger who had been given acetaminophen for fever prior to visiting the emergency department were given a supratherapeutic dose of acetaminophen [110]. Similar results were found in an Italian study, with 24% of children visiting a primary care center for fever having received an overdose of acetaminophen [103]. In one of the most dramatic examples of overdosing, among 201 caregivers surveyed in Israel, 34.8% reported administering higher-than-recommended doses of acetaminophen [105].
In addition to overdose of acetaminophen via administration of too much drug, as described above, studies from around the world point toward all-too-common administration of a greater number of doses within a given time frame than is recommended. For example, an Australian survey of 401 parents found that 3.8% reported intervals of administration to their children that were too short—medication was administered at intervals shorter than the accepted minimum of 4 h [108]. A similar study conducted in New York found that 4% of caregivers administered acetaminophen to children too frequently [109]. Furthermore, a survey in Baltimore found that, among 340 caregivers, 14% gave acetaminophen to children every 3 h or less [119]. In another study, this one in Virginia, 8% of 230 caregivers administered the drug to children too frequently [106]. A Saudi Arabian study found that 14% of caregivers administered acetaminophen to children too frequently [110]. Twenty-seven percent of caregivers surveyed in Abu Dhabi, United Arab Emirates, reported giving their child acetaminophen more frequently than every 4 h [111]. Among 201 children in Israel, 19.9% were given acetaminophen every 1–3 h if their fever persisted [105]. Further, a retrospective study showed that 52% of pediatric patients with hepatotoxicity had received adult preparations of acetaminophen [112].
Another possible facet of acetaminophen overdose is administering different medications that contain acetaminophen. A survey conducted by Princeton Survey Research Associates International in 2013 found that 35% ± 6.7% of the parents amongst the 1003 adults surveyed said it was safe to administer the maximum dosage of Children’s Tylenol® in combination with Children’s Tylenol Plus Multi-Symptom Cold® to a child [107]. Considering that both products contain acetaminophen, this could lead to a dose of acetaminophen that is toxic for children [127,128,129].
The problems with misuse of acetaminophen described above are numerous and include administration of a dose that exceeds the maximum recommended dose, too frequent administration of the drug, simultaneous administration of more than one formulation containing the drug, and administration of the drug for invalid reasons. Given the frequency of these problems, as described above, it is apparent that the risks of administering acetaminophen are not seriously considered by many caregivers. One potential indicator of the casual indifference toward exposure of the developing brain to acetaminophen is the treatment of infants during circumcision without regard for the amount of acetaminophen that might be carried over from their mother following birth. Given the apparent importance of cord blood acetaminophen and acetaminophen administration during circumcision in the induction of ASD, described above, this potential facet of acetaminophen overdose should not be overlooked. This potential method of exceeding the recommended dose of acetaminophen adds to the other problems described above, all of which violate the currently accepted standards and reflect widespread disregard for neurodevelopmental risks associated with pediatric use of acetaminophen.
7. Misguided but Currently Accepted Use of Acetaminophen
Some immediate changes in currently accepted uses of acetaminophen are warranted because the accepted practice is not justified based on available evidence. Although acetaminophen, when administered rectally in a hospital setting, may prevent some repeated febrile seizures during a single febrile episode [130], most controlled studies find that acetaminophen does not prevent febrile seizures in pediatric patients [131]. This conclusion indicates that acetaminophen is generally not helpful under circumstances that are often assumed to be dire and for which the drug is often assumed to be essential. However, a steering committee for the American Academy of Pediatrics points out that “with the exception of a high rate of recurrence, no long-term adverse effects of simple febrile seizures have been identified” [126]. Thus, the view that a simple febrile seizure poses a significant risk is an assumption that is contradicted by studies that have attempted but failed to identify any such risk [132,133,134].
For three decades now, the effect of acetaminophen on febrile seizures has been questioned. Schnaiderman and colleagues at Tel Aviv University concluded in 1993 that “prophylactic administration of acetaminophen in children with febrile seizures is not effective in the prevention of fever, the reduction of its degree, or in preventing the early recurrence of febrile seizures” [135]. In their study, Schnaiderman evaluated 104 children, with an average age of 1.9 years, who were admitted to the hospital for a febrile seizure. Prevention of a subsequent febrile seizure was attempted by administration of acetaminophen every 4 h for four days or until the body temperature returned to normal for 12–18 h. As a control group, children were treated with acetaminophen “as needed” when their body temperature reached 37.9 °C, which is approaching the minimum temperature defined as a fever, 38.0 °C (100.4° F). This approach resulted in substantially greater amounts of acetaminophen being administered to the group given prophylaxis, but without a significant effect on outcome. However, Schnaiderman did not use a placebo group and acknowledged that lower levels of acetaminophen may have resulted in more febrile seizures. Nevertheless, Schnaiderman was able to conclude confidently that additional acetaminophen would not have been helpful. This conclusion is important, as it points toward the futility of administering excess acetaminophen in an attempt to bring down fevers.
After the Schnaiderman study, Uhari and colleagues at the University of Oulu, using a placebo group, conducted a study designed to test the potential for acetaminophen to prevent febrile seizures. In 1995, they concluded that treating fevers 40 °C or higher with acetaminophen had no effect on the occurrence of febrile seizures [136]. Uhari’s study involved a two-year follow-up time with 153 children who had previously experienced a febrile seizure, and the average age was approximately 1.7 years at the beginning of the study. Over a decade later, evaluating a different cohort of children, investigators at the University of Oulu confirmed the results from their earlier study, concluding that antipyretic agents, including acetaminophen, “are ineffective for the prevention of recurrences of febrile seizures and for the lowering of body temperature in patients with a febrile episode that leads to a recurrent febrile seizure” [137]. This conclusion from the University of Oulu was subsequently supported by others [122,138,139,140], including a steering committee for the American Academy of Pediatrics [126]. However, Murata and colleagues recently reported that rectal administration of acetaminophen in a hospital setting can prevent approximately 60% of febrile seizure recurrences within the same febrile episode when compared to children who were not treated (no placebo) [130]. Unfortunately, Murata concluded that the drug is “safe” under these circumstances, not taking into account the significant risk the drug poses to neurodevelopment [1,2] and the apparent non-risk of febrile seizures [126].
In addition to the discontinued use of acetaminophen for the treatment of fevers in the pediatric population, acetaminophen should no longer be used to treat pain and discomfort in cases where it is proven to be ineffective for such treatment. For example, a controlled study demonstrated that acetaminophen does not block the pain of circumcision [141]. Further, despite the fact that the use of acetaminophen before and after vaccination is common [142], the World Health Organization does not recommend routine acetaminophen use with vaccination [143] because it may dampen the intended effects of the vaccines [144] and/or because it has not proven to be effective for management of the pain and discomfort that can accompany vaccination.
The lack of a beneficial effect of acetaminophen on some important outcomes is probably not unique to the pediatric population. A randomized, controlled clinical trial showed that treatment of infection-associated fevers with acetaminophen in hospitalized adults did not improve mortality rates or the need for intensive care [145]. The patients in this study experienced approximately 16% mortality during the 90-day study period, and were, on average, undoubtedly less healthy than the average individual with a fever who is not hospitalized. This study therefore indicates that, even under dire circumstances, treatment of infection-associated fevers in adults is not warranted. Furthermore, fevers are an important and effective component of the immune response against infection [116,122,123,124,146]. As such, any effort to block the benefits of a fever should be justified by evidence that the effort carries benefits that outweigh the risks. Thus, current evidence indicates that the benefits of reducing fevers with acetaminophen are unproven at best and possibly non-existent, whereas the risks of the treatment in the pediatric population regarding neurodevelopment are definitely considerable.
8. Changes in Practice Need to Be Made
Recommended changes to established obstetric and pediatric practices can be classified into five categories based on current recommendations from governing medical organizations and on current evidence regarding benefits of treatment. Importantly, changes in four of the five categories are expected to have minimal or no negative impacts on medical management of patients.
- Category 1:
- Administration of acetaminophen in a manner that was never intended should be discontinued. This includes treatment of temperatures that do not technically constitute a fever and administration of the drug more frequently and at higher doses than recommended.
- Category 2:
- Administration of acetaminophen under conditions in which evidence demonstrates a lack of effectiveness should be discontinued. This includes the treatment of the pain of circumcision and perhaps the treatment of fevers to prevent febrile seizures.
- Category 3:
- Administration of acetaminophen under conditions in which no evidence demonstrates long-term benefits of treatment or in which evidence demonstrates a lack of long-term benefits should be discontinued. This includes the treatment of fevers and prophylactic treatments prior to labor and delivery.
- Category 4:
- Administration of acetaminophen that is no longer recommended by governing medical bodies should be discontinued. This includes the treatment of patients receiving vaccinations and will hopefully include many more reasons for administration in the future.
- Category 5:
- Administration of acetaminophen under conditions where evidence indicates that it is or may be beneficial should not be continued without disclosure of the drug’s long-term risks for neurodevelopment. All caregivers, including parents, should be made aware of evidence related to both benefits and risks so that they can make informed decisions.
Some of the above-recommended changes to pediatric practice should have immediate beneficial effects with little to no decrease in medical care. Most obviously, discontinuation of the misadministration of acetaminophen (Category 1) is expected to prove useful and may be facilitated by public education regarding the risks of the drug regarding neurodevelopment. Public education, while likely necessary to accomplish the suggested Category 1 changes, may simultaneously facilitate the recommended Category 5 changes. As another example, given the apparently heightened sensitivity of the brain to acetaminophen-mediated injury during the neonatal period (Figure 2), eliminating the use of acetaminophen for circumcision (Category 2) and for the hepatitis B vaccine (Categories 3 and 4), two situations for which acetaminophen is often administered in the first hours of life, could make a significant difference in the prevalence of ASD and is expected to have minimal adverse consequences.
Perhaps the most difficult decisions by caregivers will need to be made for acetaminophen use in Category 5, where evidence indicates that acetaminophen is or perhaps might be helpful. Indications that fall under this category include pain management under some conditions. Studies, which are generally conducted with adults rather than children [147,148], show that acetaminophen mitigates pain under a range of conditions and that the drug has been widely accepted as an effective analgesic [149]. However, the value of pain management using acetaminophen is being questioned [149], with the benefits of the treatment being relatively minor in some scenarios. For example, a metanalysis of randomized controlled trials for the treatment of chronic arthritis pain showed that the relative percent improvement with treatment using acetaminophen was only 5% from baseline, with an absolute change of 4 points on a scale of 0 to 100 [150]. Similarly, in a metanalysis of randomized controlled trials for the treatment of post-bariatric surgical pain, the authors concluded that the use of acetaminophen after bariatric surgery effectively reduced pain scores after 24 h and effectively reduced the use of opioids [151]. However, as the authors point out, “While the present study has found that IV acetaminophen leads to a statistically significant decrease in pain score of 0.66 (95% CI 0.28 to 1.03) calculated using a 10-point VAS (Visual Analogue Scale), the results are not definitively higher than the minimally clinically important difference (MCID) associated with pain which ranges from 0.8 to 4 on a 10-point scale. Furthermore, while this review found an opioid-sparing effect of 6.44 mg in MED (morphine equivalent doses), this translates to less than a 10% reduction in morphine used” [151]. Thus, the presumed benefits of analgesia using acetaminophen should be critically evaluated rather than taken for granted by caregivers, keeping in mind the risks to neurodevelopment during early brain development.
It is within clinical contexts involving Category 5 use of acetaminophen that regulatory agencies and physician associations need to carefully evaluate guidelines, considering the benefits and risks of acetaminophen and of alternative pharmacological and non-pharmacological-based management strategies for pain and discomfort. In addition, more research is urgently needed to find safe alternatives. We had previously hypothesized that intravenous acetaminophen, a preparation of acetaminophen containing antioxidants known to counteract the toxic effects of acetaminophen poisoning, would be safe for neurodevelopment. However, even at doses lower than the acceptable dose currently used in humans, repeated administration of the drug mixture to laboratory rats during the neonatal period resulted in dramatically increased anxiety later in life compared to controls [6]. It could be argued that lowering the dose of acetaminophen might eliminate adverse effects on neurodevelopment. However, lowering the dose of the drug by 50% dramatically reduces the effectiveness of the drug [152]. With this in mind, based on laboratory animal studies, there is no known safe and effective dose of acetaminophen during early periods of brain development at the present time. This does not, however, rule out the potential for future development of acetaminophen-based therapeutics that effectively avoid any adverse neurodevelopmental consequences.
Change can and does happen. An advisory panel for the US Food and Drug Administration (FDA) recently determined that oral phenylephrine at the currently recommended dose is ineffective at relieving nasal and sinus congestion [153]. This determination is quite remarkable because oral phenylephrine has been considered safe and effective since the 1970s and is an active ingredient in a wide range of popular medications, including Sudafed PE®, Tylenol Cold®, Alka-Seltzer Plus Cold®, and numerous Mucinex® formulations. As a result, the world’s largest healthcare company, CVS Health Corporation, will no longer sell products with phenylephrine as the sole active ingredient. It is time for another major and far more important change, as regulatory agencies, academies of obstetricians, and academies of pediatricians are encouraged to acknowledge the profound dangers of acetaminophen during neurodevelopment and the lack of convincing data demonstrating long-term benefits from pediatric use of the drug during sensitive periods of brain development.
Author Contributions
Conceptualization, W.P. and R.A.; investigation, W.P. and D.B.-L.; resources, W.P., L.G.A., J.P.J., L.W., D.B.-L. and Z.K.; writing—original draft preparation, W.P. and L.W.; writing—review and editing, W.P., L.G.A., J.P.J., R.A., L.W., D.B.-L. and Z.K.; funding acquisition, W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by generous donations to WPLab, Inc., a non-profit corporation based in Durham, North Carolina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to John Poulton, Susan Poulton, and Tabitha J. Parker for their support. The authors also thank Edel Pons Suárez and Mark Fowler for helpful discussions. In addition, the authors thank R. Randal Bollinger, Jasmine Cendejas-Hernandez, and Susanne Meza-Keuthen for carefully reading the manuscript and engaging in thoughtful discussion.
Conflicts of Interest
Authors William Parker, Lauren G. Anderson, John P. Jones, Rachel Anderson and Zacharoula Konsoula were employed by the company “WPLab, Inc.”. The authors all work for non-profit (non-commercial) institutions. The authors declare no conflicts of interest.
References
- Patel, E.; Jones Iii, J.P., 3rd; Bono-Lunn, D.; Kuchibhatla, M.; Palkar, A.; Cendejas Hernandez, J.; Sarafian, J.T.; Lawton, V.G.; Anderson, L.G.; Konsoula, Z.; et al. The safety of pediatric use of paracetamol (acetaminophen): A narrative review of direct and indirect evidence. Minerva Pediatr. 2022, 74, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jones, J.; Anderson, L.; Konsoula, Z.; Nevison, C.; Reissner, K.; Parker, W. Acetaminophen causes neurodevelopmental injury in susceptible babies and children: No valid rationale for controversy. Clin. Exp. Pediatr. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Herrington, J.A.; Guss Darwich, J.; Harshaw, C.; Brigande, A.M.; Leif, E.B.; Currie, P.J. Elevated ghrelin alters the behavioral effects of perinatal acetaminophen exposure in rats. Dev. Psychobiol. 2022, 64, e22252. [Google Scholar] [CrossRef] [PubMed]
- Harshaw, C.; Warner, A.G. Interleukin-1β-induced inflammation and acetaminophen during infancy: Distinct and interactive effects on social-emotional and repetitive behavior in C57BL/6J mice. Pharmacol. Biochem. Behav. 2022, 220, 173463. [Google Scholar] [CrossRef]
- Viberg, H.; Eriksson, P.; Gordh, T.; Fredriksson, A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicol. Sci. 2014, 138, 139–147. [Google Scholar] [CrossRef]
- Suda, N.; Hernandez, J.C.; Poulton, J.; Jones, J.P.; Konsoula, Z.; Smith, C.; Parker, W. Therapeutic doses of paracetamol with co-administration of cysteine and mannitol during early development result in long term behavioral changes in laboratory rats. PLoS ONE 2021, 16, e0253543. [Google Scholar] [CrossRef]
- Philippot, G.; Gordh, T.; Fredriksson, A.; Viberg, H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): Characterization of a critical period. J. Appl. Toxicol. 2017, 37, 1174–1181. [Google Scholar] [CrossRef]
- Dean, S.L.; Knutson, J.F.; Krebs-Kraft, D.L.; McCarthy, M.M. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur. J. Neurosci. 2012, 35, 1218–1229. [Google Scholar] [CrossRef]
- Parker, W.; Hornik, C.D.; Bilbo, S.; Holzknecht, Z.E.; Gentry, L.; Rao, R.; Lin, S.S.; Herbert, M.R.; Nevison, C.D. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J. Int. Med. Res. 2017, 45, 407–438. [Google Scholar] [CrossRef]
- Schultz, S.T.; Klonoff-Cohen, H.S.; Wingard, D.L.; Akshoomoff, N.A.; Macera, C.A.; Ji, M. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder. The results of a parent survey. Autism 2008, 12, 293–307. [Google Scholar] [CrossRef]
- Frisch, M.; Simonsen, J. Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year-old boys: National cohort study in Denmark. J. R. Soc. Med. 2015, 108, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Virk, J.; Olsen, J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism Res. Off. J. Int. Soc. Autism Res. 2016, 9, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Alemany, S.; Avella-García, C.; Liew, Z.; García-Esteban, R.; Inoue, K.; Cadman, T.; López-Vicente, M.; González, L.; Riaño Galán, I.; Andiarena, A.; et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur. J. Epidemiol. 2021, 36, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Rimland, B. The autism increase: Research needed on the vaccine connection. Autism Res. Rev. Int. 2000, 14, 3–6. [Google Scholar]
- Wastesson, J.W.; Martikainen, J.E.; Zoëga, H.; Schmidt, M.; Karlstad, Ø.; Pottegård, A. Trends in Use of Paracetamol in the Nordic Countries. Basic Clin. Pharmacol. Toxicol. 2018, 123, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Delobel-Ayoub, M.; Saemundsen, E.; Gissler, M.; Ego, A.; Moilanen, I.; Ebeling, H.; Rafnsson, V.; Klapouszczak, D.; Thorsteinsson, E.; Arnaldsdóttir, K.M.; et al. Prevalence of Autism Spectrum Disorder in 7–9-Year-Old Children in Denmark, Finland, France and Iceland: A Population-Based Registries Approach within the ASDEU Project. J. Autism Dev. Disord. 2020, 50, 949–959. [Google Scholar] [CrossRef]
- Philippot, G.; Hosseini, K.; Yakub, A.; Mhajar, Y.; Hamid, M.; Buratovic, S.; Fredriksson, R. Paracetamol (Acetaminophen) and its Effect on the Developing Mouse Brain. Front. Toxicol. 2022, 4, 867748. [Google Scholar] [CrossRef]
- Kanno, S.I.; Tomizawa, A.; Yomogida, S.; Hara, A. Glutathione peroxidase 3 is a protective factor against acetaminophen-induced hepatotoxicity in vivo and in vitro. Int. J. Mol. Med. 2017, 40, 748–754. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Wright, C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef]
- Posadas, I.; Santos, P.; Blanco, A.; Muñoz-Fernández, M.; Ceña, V. Acetaminophen induces apoptosis in rat cortical neurons. PLoS ONE 2010, 5, e15360. [Google Scholar] [CrossRef]
- Donovan, A.P.; Basson, M.A. The neuroanatomy of autism—A developmental perspective. J. Anat. 2017, 230, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Sokhadze, E.M.; Casanova, E.L.; Opris, I.; Abujadi, C.; Marcolin, M.A.; Li, X. Translational Neuroscience in Autism: From Neuropathology to Transcranial Magnetic Stimulation Therapies. Psychiatr. Clin. N. Am. 2020, 43, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zielke, H.R.; Yeh, D.; Yang, P. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism Res. Off. J. Int. Soc. Autism Res. 2018, 11, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.N.; Zhang, H.; Shu, Y.; Chen, S.; Hu, Y.Y.; Zhou, M. The neonatal levels of TSB, NSE and CK-BB in autism spectrum disorder from Southern China. Transl. Neurosci. 2016, 7, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Stancioiu, F.; Bogdan, R.; Dumitrescu, R. Neuron-Specific Enolase (NSE) as a Biomarker for Autistic Spectrum Disease (ASD). Life 2023, 13, 1736. [Google Scholar] [CrossRef]
- Anvik, J.O. Acetaminophen toxicosis in a cat. Can. Vet. J. = La Rev. Vet. Can. 1984, 25, 445–447. [Google Scholar]
- Savides, M.C.; Oehme, F.W.; Nash, S.L.; Leipold, H.W. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicol. Appl. Pharmacol. 1984, 74, 26–34. [Google Scholar] [CrossRef]
- Court, M.H. Feline drug metabolism and disposition: Pharmacokinetic evidence for species differences and molecular mechanisms. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1039–1054. [Google Scholar] [CrossRef]
- Lautz, L.S.; Jeddi, M.Z.; Girolami, F.; Nebbia, C.; Dorne, J. Metabolism and pharmacokinetics of pharmaceuticals in cats (Felix sylvestris catus) and implications for the risk assessment of feed additives and contaminants. Toxicol. Lett. 2021, 338, 114–127. [Google Scholar] [CrossRef]
- Miller, R.P.; Roberts, R.J.; Fischer, L.J. Acetaminophen elimination kinetics in neonates, children, and adults. Clin. Pharmacol. Ther. 1976, 19, 284–294. [Google Scholar] [CrossRef]
- Cook, S.F.; Stockmann, C.; Samiee-Zafarghandy, S.; King, A.D.; Deutsch, N.; Williams, E.F.; Wilkins, D.G.; Sherwin, C.M.; van den Anker, J.N. Neonatal Maturation of Paracetamol (Acetaminophen) Glucuronidation, Sulfation, and Oxidation Based on a Parent-Metabolite Population Pharmacokinetic Model. Clin. Pharmacokinet 2016, 55, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Cendejas-Hernandez, J.; Sarafian, J.; Lawton, V.; Palkar, A.; Anderson, L.; Lariviere, V.; Parker, W. Paracetamol (Acetaminophen) Use in Infants and Children was Never Shown to be Safe for Neurodevelopment: A Systematic Review with Citation Tracking. Eur. J. Pediatr. 2022, 181, 1835–1857. [Google Scholar] [CrossRef]
- Hall, C.; Smith, M. Increased cGMP Enforcement Has Gone International: South Korean Action against Johnson & Johnson Serves as Warning. White Collar Watch 2013; June. Available online: https://www.jdsupra.com/legalnews/increased-cgmp-enforcement-has-gone-inte-53643/ (accessed on 13 December 2023).
- Kim, Y.S.; Leventhal, B.L.; Koh, Y.J.; Fombonne, E.; Laska, E.; Lim, E.C.; Cheon, K.A.; Kim, S.J.; Kim, Y.K.; Lee, H.; et al. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry 2011, 168, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Baird, G. 2.64% of South Korean children aged 7 to 12 have autism spectrum disorders. Evid. Based Ment. Health 2012, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Raz, R.; Weisskopf, M.G.; Davidovitch, M.; Pinto, O.; Levine, H. Differences in autism spectrum disorders incidence by sub-populations in Israel 1992–2009: A total population study. J. Autism Dev. Disord. 2015, 45, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Levaot, Y.; Meiri, G.; Dinstein, I.; Menashe, I.; Shoham-Vardi, I. Autism Prevalence and Severity in Bedouin-Arab and Jewish Communities in Southern Israel. Community Ment. Health J. 2019, 55, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J. A history of drug advertising: The evolving roles of consumers and consumer protection. Milbank Q. 2006, 84, 659–699. [Google Scholar] [CrossRef]
- Tovo-Rodrigues, L.; Schneider, B.C.; Martins-Silva, T.; Del-Ponte, B.; Loret de Mola, C.; Schuler-Faccini, L.; Vianna, F.S.L.; Munhoz, T.N.; Entiauspe, L.; Silveira, M.F.; et al. Is intrauterine exposure to acetaminophen associated with emotional and hyperactivity problems during childhood? Findings from the 2004 Pelotas birth cohort. BMC Psychiatry 2018, 18, 368. [Google Scholar] [CrossRef]
- Vlenterie, R.; Wood, M.E.; Brandlistuen, R.E.; Roeleveld, N.; van Gelder, M.M.; Nordeng, H. Neurodevelopmental problems at 18 months among children exposed to paracetamol in utero: A propensity score matched cohort study. Int. J. Epidemiol. 2016, 45, 1998–2008. [Google Scholar] [CrossRef]
- Liew, Z.; Ritz, B.; Virk, J.; Arah, O.A.; Olsen, J. Prenatal Use of Acetaminophen and Child IQ: A Danish Cohort Study. Epidemiology 2016, 27, 912–918. [Google Scholar] [CrossRef]
- Liew, Z.; Bach, C.C.; Asarnow, R.F.; Ritz, B.; Olsen, J. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int. J. Epidemiol. 2016, 45, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Azuine, R.E.; Zhang, Y.; Hou, W.; Hong, X.; Wang, G.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure with Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry 2020, 77, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Avella-Garcia, C.B.; Julvez, J.; Fortuny, J.; Rebordosa, C.; Garcia-Esteban, R.; Galan, I.R.; Tardon, A.; Rodriguez-Bernal, C.L.; Iniguez, C.; Andiarena, A.; et al. Acetaminophen use in pregnancy and neurodevelopment: Attention function and autism spectrum symptoms. Int. J. Epidemiol. 2016, 45, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, E.; Handal, M.; Selmer, R.; Brandlistuen, R.E.; Skurtveit, S. Language competence and communication skills in 3-year-old children after prenatal exposure to analgesic opioids. Pharmacoepidemiol. Drug Saf. 2017, 26, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; Rebordosa, C.; Lee, P.C.; Olsen, J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014, 168, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ystrom, E.; Gustavson, K.; Brandlistuen, R.E.; Knudsen, G.P.; Magnus, P.; Susser, E.; Davey Smith, G.; Stoltenberg, C.; Suren, P.; Haberg, S.E.; et al. Prenatal Exposure to Acetaminophen and Risk of ADHD. Pediatrics 2017, 140, e20163840. [Google Scholar] [CrossRef]
- Thompson, J.M.; Waldie, K.E.; Wall, C.R.; Murphy, R.; Mitchell, E.A. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS ONE 2014, 9, e108210. [Google Scholar] [CrossRef]
- Stergiakouli, E.; Thapar, A.; Davey Smith, G. Association of Acetaminophen Use During Pregnancy with Behavioral Problems in Childhood: Evidence against Confounding. JAMA Pediatr. 2016, 170, 964–970. [Google Scholar] [CrossRef]
- Brandlistuen, R.E.; Ystrom, E.; Nulman, I.; Koren, G.; Nordeng, H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int. J. Epidemiol. 2013, 42, 1702–1713. [Google Scholar] [CrossRef]
- Freed, G.L.; Clark, S.J.; Butchart, A.T.; Singer, D.C.; Davis, M.M. Parental vaccine safety concerns in 2009. Pediatrics 2010, 125, 654–659. [Google Scholar] [CrossRef]
- Bazzano, A.; Zeldin, A.; Schuster, E.; Barrett, C.; Lehrer, D. Vaccine-related beliefs and practices of parents of children with autism spectrum disorders. Am. J. Intellect. Dev. Disabil. 2012, 117, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.D.; Krajbich, I.; Way, B.M. Acetaminophen influences social and economic trust. Sci. Rep. 2019, 9, 4060. [Google Scholar] [CrossRef]
- Dewall, C.N.; Macdonald, G.; Webster, G.D.; Masten, C.L.; Baumeister, R.F.; Powell, C.; Combs, D.; Schurtz, D.R.; Stillman, T.F.; Tice, D.M.; et al. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychol. Sci. 2010, 21, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Durso, G.R.O.; Luttrell, A.; Way, B.M. Over-the-Counter Relief from Pains and Pleasures Alike: Acetaminophen Blunts Evaluation Sensitivity to Both Negative and Positive Stimuli. Psychol. Sci. 2015, 26, 750–758. [Google Scholar] [CrossRef]
- Randles, D.; Kam, J.W.Y.; Heine, S.J.; Inzlicht, M.; Handy, T.C. Acetaminophen attenuates error evaluation in cortex. Soc. Cogn. Affect. Neurosci. 2016, 11, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Hutabarat, R.M.; Unadkat, J.D.; Kushmerick, P.; Aitken, M.L.; Slattery, J.T.; Smith, A.L. Disposition of drugs in cystic fibrosis. III Acetaminophen. Clin. Pharmacol. Ther. 1991, 50, 695–701. [Google Scholar] [CrossRef]
- Kearns, G.L. Hepatic drug metabolism in cystic fibrosis: Recent developments and future directions. Ann. Pharmacother. 1993, 27, 74–79. [Google Scholar] [CrossRef]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation deficit in “low-functioning” autistic children: A pilot study. Biol. Psychiatry 1999, 46, 420–424. [Google Scholar] [CrossRef]
- Guengerich, F.P. A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol. Res. 2021, 37, 1–23. [Google Scholar] [CrossRef]
- Albano, E.; Rundgren, M.; Harvison, P.J.; Nelson, S.D.; Moldéus, P. Mechanisms of N-acetyl-p-benzoquinone imine cytotoxicity. Mol. Pharmacol. 1985, 28, 306–311. [Google Scholar] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. I Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. IV Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973, 187, 211–217. [Google Scholar] [PubMed]
- Du, K.; Farhood, A.; Jaeschke, H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch. Toxicol. 2017, 91, 761–773. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Elwell, C.; Johnson, M.H. Mitochondrial Dysfunction in Autism Spectrum Disorders. Autism—Open Access 2016, 6, 1000190. [Google Scholar] [CrossRef]
- Mischkowski, D.; Crocker, J.; Way, B.M. A Social Analgesic? Acetaminophen (Paracetamol) Reduces Positive Empathy. Front. Psychol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Many, B.T.; Rizeq, Y.K.; Vacek, J.; Cheon, E.C.; Johnson, E.; Hu, Y.Y.; Raval, M.V.; Abdullah, F.; Goldstein, S.D. A contemporary snapshot of circumcision in US children’s hospitals. J. Pediatr. Surg. 2020, 55, 1134–1138. [Google Scholar] [CrossRef]
- Campbell, O.M.; Cegolon, L.; Macleod, D.; Benova, L. Length of Stay after Childbirth in 92 Countries and Associated Factors in 30 Low- and Middle-Income Countries: Compilation of Reported Data and a Cross-sectional Analysis from Nationally Representative Surveys. PLoS Med. 2016, 13, e1001972. [Google Scholar] [CrossRef]
- Tan, C.; Frewer, V.; Cox, G.; Williams, K.; Ure, A. Prevalence and Age of Onset of Regression in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analytical Update. Autism Res. Off. J. Int. Soc. Autism Res. 2021, 14, 582–598. [Google Scholar] [CrossRef]
- Owings, M.; Uddin, S.; Williams, S. Trends in Circumcision for Male Newborns in U.S. Hospitals: 1979–2010. In Centers for Disease Control and Prevention; Statistics NCfH: Hyattsville, MD, USA, 2013; pp. 1–5. [Google Scholar]
- Clissold, S.P. Paracetamol and phenacetin. Drugs 1986, 32 (Suppl. S4), 46–59. [Google Scholar] [CrossRef]
- Mahmud, S.; Rosen, N. History of NSAID Use in the Treatment of Headaches Pre and Post-industrial Revolution in the United States: The Rise and Fall of Antipyrine, Salicylic Acid, and Acetanilide. Curr. Pain Headache Rep. 2019, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Morris, D., Haddy, A., Eds.; Analgesic contents of patent medicines of the early 20th century: Bromo-Seltzer and Antikamnia. In Proceedings of the 264th ACS National Meeting, Chicago, IL, USA, 21–25 August 2022. [Google Scholar]
- Ninan, B.; Wertheimer, A. Withdrawing Drugs in the U.S. Versus Other Countries. Inov. Pharm. 2012, 3, 87. [Google Scholar] [CrossRef][Green Version]
- Asperger, H. Die “Autistischen Psychopathen” im Kindesalter. Arch. Für Psychiatr. Und Nervenkrankh. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020; Morbidity and Mortality Weekly Report Surveillance Summaries; CDC: Washington, DC, USA, 2002. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-the Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Chen, D.; Xu, Y.; Lan, L.; Zhao, S.; Liu, Q.; Snijders, A.M.; Xia, Y. Maternal exposure to pesticides and autism or attention-deficit/hyperactivity disorders in offspring: A meta-analysis. Chemosphere 2023, 313, 137459. [Google Scholar] [CrossRef] [PubMed]
- Modick, H.; Schütze, A.; Pälmke, C.; Weiss, T.; Brüning, T.; Koch, H.M. Rapid determination of N-acetyl-4-aminophenol (paracetamol) in urine by tandem mass spectrometry coupled with on-line clean-up by two dimensional turbulent flow/reversed phase liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 925, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, G.; Weiss, T.; Modick, H.; Käfferlein, H.U.; Brüning, T.; Koch, H.M. N-Acetyl-4-aminophenol (paracetamol), N-acetyl-2-aminophenol and acetanilide in urine samples from the general population, individuals exposed to aniline and paracetamol users. Int. J. Hyg. Environ. Health 2014, 217, 592–599. [Google Scholar] [CrossRef]
- Eiche, A.; Bexell, G.; Sandelin, K. Genotoxicity of p-aminophenol in somatic and germ line cells of Drosophila melanogaster. Mutat. Res. 1990, 240, 87–92. [Google Scholar] [CrossRef]
- Hogestatt, E.D.; Jonsson, B.A.; Ermund, A.; Andersson, D.A.; Bjork, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef]
- Agency UEP. Title 40—Protection of Environment. Chapter I—Environmental Protection Agency (continued). Subchapter E—Pesticide Programs. Part 180—Tolerances and Exemptions for Pesticide Chemical Residues in Food; 2014. Available online: https://www.govinfo.gov/app/details/CFR-2016-title40-vol26/CFR-2016-title40-vol26-part180 (accessed on 13 December 2023).
- El-Sheikh, E.A.; Ramadan, M.M.; El-Sobki, A.E.; Shalaby, A.A.; McCoy, M.R.; Hamed, I.A.; Ashour, M.B.; Hammock, B.D. Pesticide Residues in Vegetables and Fruits from Farmer Markets and Associated Dietary Risks. Molecules 2022, 27, 8072. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.M.; Le, T.V.; Chu, T.T.Q.; Le, B.N.; Duong, M.D.; Thanh, N.M.; Tac Pham, V.; Minas, H.; Bui, T.T.H. Prevalence of autism spectrum disorders and their relation to selected socio-demographic factors among children aged 18–30 months in northern Vietnam, 2017. Int. J. Ment. Health Syst. 2019, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Nevison, C.; Blaxill, M.; Zahorodny, W. California Autism Prevalence Trends from 1931 to 2014 and Comparison to National ASD Data from IDEA and ADDM. J. Autism Dev. Disord. 2018, 48, 4103–4117. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, L.D.; Rice, C.E.; Barger, B.; Soke, G.N.; Lee, L.C.; Moody, E.; Edmondson-Pretzel, R.; Levy, S.E. DSM-5 criteria for autism spectrum disorder maximizes diagnostic sensitivity and specificity in preschool children. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Feldon, J.; Dammann, O. Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res. 2011, 69 Pt 2, 26R–33R. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Jones, J.P.; Parker, W. Is autism a member of a family of diseases resulting from genetic/cultural mismatches? Implications for treatment and prevention. Autism Res. Treat. 2012, 2012, 910946. [Google Scholar] [CrossRef]
- Becker, K.G. Autism, asthma, inflammation, and the hygiene hypothesis. Med. Hypotheses 2007, 69, 731–740. [Google Scholar] [CrossRef]
- Bickler, S.W.; DeMaio, A. Western diseases: Current concepts and implications for pediatric surgery research and practice. Pediatr. Surg. Int. 2008, 24, 251–255. [Google Scholar] [CrossRef]
- Parker, W.; Patel, E.; Jirků-Pomajbíková, K.; Laman, J.D. COVID-19 morbidity in lower versus higher income populations underscores the need to restore lost biodiversity of eukaryotic symbionts. iScience 2023, 26, 106167. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, F.; Willighagen, E.L.; Kutmon, M.; van Hoften, M.; Curfs, L.M.G.; Evelo, C.T. A resource to explore the discovery of rare diseases and their causative genes. Sci. Data 2021, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.O.; Robson, R.A.; Birkett, D.J. Paracetamol metabolism in pregnancy. Br. J. Clin. Pharmacol. 1986, 22, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cresteil, T. Onset of xenobiotic metabolism in children: Toxicological implications. Food Addit. Contam. 1998, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, K.; Wright, I.M.; Schneider, J.J.; Jones, A.L.; Martin, J.H. Pharmacokinetics in neonatal prescribing: Evidence base, paradigms and the future. Br. J. Clin. Pharmacol. 2015, 80, 1281–1288. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.D.; Charvet, C.J.; Clancy, B.; Darlington, R.B.; Finlay, B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 2013, 33, 7368–7383. [Google Scholar] [CrossRef]
- Lubrano, R.; Paoli, S.; Bonci, M.; Di Ruzza, L.; Cecchetti, C.; Falsaperla, R.; Pavone, P.; Matin, N.; Vitaliti, G.; Gentile, I. Acetaminophen administration in pediatric age: An observational prospective cross-sectional study. Ital. J. Pediatr. 2016, 42, 20. [Google Scholar] [CrossRef][Green Version]
- May, A.; Bauchner, H. Fever Phobia: The Pediatrician’s Contribution. Pediatrics 1992, 90, 851–854. [Google Scholar] [CrossRef]
- Bilenko, N.; Tessler, H.; Okbe, R.; Gorodischer, R. Determinants of antipyretic misuse in children up to 5 years of age: A cross-sectional study. Clin. Ther. 2006, 28, 783–793. [Google Scholar] [CrossRef]
- Poirier, M.P.; Collins, E.P.; McGuire, E. Fever phobia: A survey of caregivers of children seen in a pediatric emergency department. Clin. Pediatr. 2010, 49, 530–534. [Google Scholar] [CrossRef] [PubMed]
- ProPublica. Use Only as Directed2013 June 28, 2018. Available online: https://www.propublica.org/article/tylenol-mcneil-fda-use-only-as-directed (accessed on 20 September 2013).
- Walsh, A.; Edwards, H.; Fraser, J. Over-the-counter medication use for childhood fever: A cross-sectional study of Australian parents. J. Paediatr. Child Health 2007, 43, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Lacher, B.; Crain, E.F. Acetaminophen and ibuprofen dosing by parents. Pediatr. Emerg. Care 2000, 16, 394–397. [Google Scholar] [CrossRef]
- Alomar, M.; Alenazi, F.; Alruwaili, N. Accuracy of acetaminophen dosing in children by caregivers in Saudi Arabia. Ann. Saudi Med. 2011, 31, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.G.; Grunfeld, A.F. ‘Fever phobia’ in the emergency department: A survey of children’s caregivers. Eur. J. Emerg. Med. Off. J. Eur. Soc. Emerg. Med. 2006, 13, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Heubi, J.E.; Barbacci, M.B.; Zimmerman, H.J. Therapeutic misadventures with acetaminophen: Hepatoxicity after multiple doses in children. J. Pediatr. 1998, 132, 22–27. [Google Scholar] [CrossRef]
- Arikan, Z.; Teksam, O.; Kara, A.; Kale, G. Determining causes and frequency of misdosing of antipyretics in patients presenting with fever to pediatric emergency. Turk. Arch. Pediatr. 2012, 47, 114–118. [Google Scholar]
- Yavuz, E.; Yayla, E.; Cebeci, S.E.; Kirimli, E.; Gumustakim, R.S.; Cakir, L.; Dogan, S. Parental beliefs and practices regarding childhood fever in Turkish primary care. Niger. J. Clin. Pract. 2017, 20, 93–98. [Google Scholar] [CrossRef]
- Mackowiak, P.A.; Worden, G. Carl Reinhold August Wunderlich and the evolution of clinical thermometry. Clin. Infect. Dis. 1994, 18, 458–467. [Google Scholar] [CrossRef]
- Schmitt, B.D. Fever phobia: Misconceptions of parents about fevers. Am. J. Dis. Child 1980, 134, 176–181. [Google Scholar] [CrossRef]
- Kothari, V.M.; Karnad, D.R. New onset fever in the intensive care unit. J. Assoc. Phys. India 2005, 53, 949–953. [Google Scholar]
- Herzog, L.W.; Coyne, L.J. What is fever? Normal temperature in infants less than 3 months old. Clin. Pediatr. 1993, 32, 142–146. [Google Scholar] [CrossRef]
- Crocetti, M.; Moghbeli, N.; Serwint, J. Fever phobia revisited: Have parental misconceptions about fever changed in 20 years? Pediatrics 2001, 107, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.P.; Davis, P.H.; Gonzalez-del Rey, J.A.; Monroe, K.W. Pediatric emergency department nurses’ perspectives on fever in children. Pediatr. Emerg. Care 2000, 16, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.J.; Hanna-Jumma, S.; Carraretto, M.; Forni, L. The pathophysiological basis and consequences of fever. Crit. Care 2016, 20, 200. [Google Scholar] [CrossRef]
- Sullivan, J.E.; Farrar, H.C. Fever and Antipyretic Use in Children. Pediatrics 2011, 127, 580–587. [Google Scholar] [CrossRef]
- El-Radhi, A.S.M. Fever management: Evidence vs current practice. Clin. Pediatr. 2012, 1, 29–33. [Google Scholar]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Al-Jabi, S.W.; Sweileh, W.M.; Nabulsi, M.M.; Tubaila, M.F.; Awang, R.; Sawalha, A.F. Beliefs and practices regarding childhood fever among parents: A cross-sectional study from Palestine. BMC Pediatr. 2013, 13, 66. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; American Academy of Pediatrics. Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures. Febrile Seizures: Clinical Practice Guideline for the Long-term Management of the Child with Simple Febrile Seizures. Pediatrics 2008, 121, 1281–1286. [Google Scholar] [CrossRef]
- James, L.; Sullivan, J.E.; Roberts, D. The proper use of acetaminophen. Paediatr. Child Health 2011, 16, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Administration USFaD. Consumer Updates—Don’t Double Up. on Acetaminophen; FDA: Silver Spring, MD, USA, 2013. [Google Scholar]
- Greene, J.W.; Craft, L.; Ghishan, F. Acetaminophen poisoning in infancy. Am. J. Dis. Child 1983, 137, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Okasora, K.; Tanabe, T.; Ogino, M.; Yamazaki, S.; Oba, C.; Syabana, K.; Nomura, S.; Shirasu, A.; Inoue, K.; et al. Acetaminophen and Febrile Seizure Recurrences during the Same Fever Episode. Pediatrics 2018, 142, e20181009. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Suto, M.; Tsuji, M.; Sasaki, H.; Takehara, K.; Ishiguro, A.; Kubota, M. Use of antipyretics for preventing febrile seizure recurrence in children: A systematic review and meta-analysis. Eur. J. Pediatr. 2021, 180, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Guo, N.W.; Huang, C.C.; Wang, S.T.; Tsai, J.J. Neurocognitive attention and behavior outcome of school-age children with a history of febrile convulsions: A population study. Epilepsia 2000, 41, 412–420. [Google Scholar] [CrossRef]
- Ellenberg, J.H.; Nelson, K.B. Febrile Seizures and Later Intellectual Performance. Arch. Neurol. 1978, 35, 17–21. [Google Scholar] [CrossRef]
- Verity, C.M.; Butler, N.R.; Golding, J. Febrile convulsions in a national cohort followed up from birth. II—Medical history and intellectual ability at 5 years of age. Br. Med. J. (Clin. Res. Ed) 1985, 290, 1311–1315. [Google Scholar] [CrossRef]
- Schnaiderman, D.; Lahat, E.; Sheefer, T.; Aladjem, M. Antipyretic effectiveness of acetaminophen in febrile seizures: Ongoing prophylaxis versus sporadic usage. Eur. J. Pediatr. 1993, 152, 747–749. [Google Scholar] [CrossRef]
- Uhari, M.; Rantala, H.; Vainionpää, L.; Kurttila, R. Effect of acetaminophen and of low intermittent doses of diazepam on prevention of recurrences of febrile seizures. J. Pediatr. 1995, 126, 991–995. [Google Scholar] [CrossRef]
- Strengell, T.; Uhari, M.; Tarkka, R.; Uusimaa, J.; Alen, R.; Lautala, P.; Rantala, H. Antipyretic agents for preventing recurrences of febrile seizures: Randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2009, 163, 799–804. [Google Scholar] [CrossRef]
- Duffner, P.K. Anti-pyretic agents are ineffective in the prevention of febrile seizures. J. Pediatr. 2010, 156, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Monfries, N.; Goldman, R.D. Prophylactic antipyretics for prevention of febrile seizures following vaccination. Can. Fam. Physician 2017, 63, 128–130. [Google Scholar] [PubMed]
- El-Radhi, A.S.; Barry, W. Do antipyretics prevent febrile convulsions? Arch. Dis. Child 2003, 88, 641–642. [Google Scholar] [CrossRef][Green Version]
- Howard, C.R.; Howard, F.M.; Weitzman, M.L. Acetaminophen analgesia in neonatal circumcision: The effect on pain. Pediatrics 1994, 93, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.; Moody, M.A.; Walter, E.B. Effect of antipyretic analgesics on immune responses to vaccination. Hum. Vaccines Immunother. 2016, 12, 2391–2402. [Google Scholar] [CrossRef]
- WHO. Reducing pain at the time of vaccination: WHO position paper, September 2015-Recommendations. Vaccine 2016, 34, 3629–3630. [Google Scholar] [CrossRef] [PubMed]
- Homme, J.H.; Fischer, P.R. Randomised controlled trial: Prophylactic paracetamol at the time of infant vaccination reduces the risk of fever but also reduces antibody response. Evid.—Based Med. 2010, 15, 50–51. [Google Scholar] [CrossRef]
- Young, P.; Saxena, M.; Bellomo, R.; Freebairn, R.; Hammond, N.; van Haren, F.; Holliday, M.; Henderson, S.; Mackle, D.; McArthur, C.; et al. Acetaminophen for Fever in Critically Ill Patients with Suspected Infection. N. Engl. J. Med. 2015, 373, 2215–2224. [Google Scholar] [CrossRef]
- Wrotek, S.; LeGrand, E.K.; Dzialuk, A.; Alcock, J. Let fever do its job: The meaning of fever in the pandemic era. Evol. Med. Public Health 2021, 9, 26–35. [Google Scholar] [CrossRef]
- Thibault, C.; Pelletier, É.; Nguyen, C.; Trottier, E.D.; Doré-Bergeron, M.J.; DeKoven, K.; Roy, A.M.; Piché, N.; Delisle, J.F.; Morin, C.; et al. The Three W’s of Acetaminophen In Children: Who, Why, and Which Administration Mode. J. Pediatr. Pharmacol. Ther. 2023, 28, 20–28. [Google Scholar] [CrossRef]
- Simmons, K.; Ortiz, R.; Kossowsky, J.; Krummenacher, P.; Grillon, C.; Pine, D.; Colloca, L. Pain and placebo in pediatrics: A comprehensive review of laboratory and clinical findings. Pain 2014, 155, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Freo, U.; Ruocco, C.; Valerio, A.; Scagnol, I.; Nisoli, E. Paracetamol: A Review of Guideline Recommendations. J. Clin. Med. 2021, 10, 3420. [Google Scholar] [CrossRef] [PubMed]
- Towheed, T.E.; Maxwell, L.; Judd, M.G.; Catton, M.; Hochberg, M.C.; Wells, G. Acetaminophen for osteoarthritis. Cochrane Database Syst. Rev. 2006, 2006, Cd004257. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Ashoorion, V.; Gmora, S.; Anvari, M.; Hong, D. Intravenous Acetaminophen versus Placebo in Post-bariatric Surgery Multimodal Pain Management: A Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2019, 29, 1420–1428. [Google Scholar] [CrossRef]
- Windorfer, A.; Vogel, C. Investigations concerning serum concentration and temperature following oral application of a new paracetamol preparation (author’s transl). Klin. Padiatr. 1976, 188, 430–434. [Google Scholar]
- FDA. FDA Clarifies Results of Recent Advisory Committee Meeting on Oral Phenylephrine; US Government—FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).