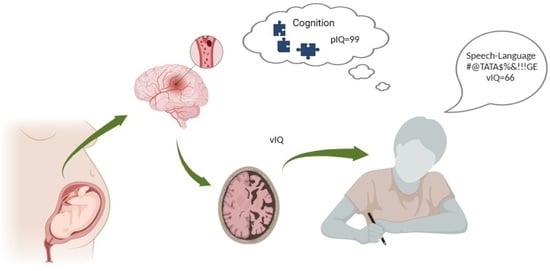
Arterial Presumed Perinatal Ischemic Stroke: A Mini Review and Case Report of Cognitive and Speech-Language Profiles in a 5-Year-Old Girl
Abstract
1. Introduction
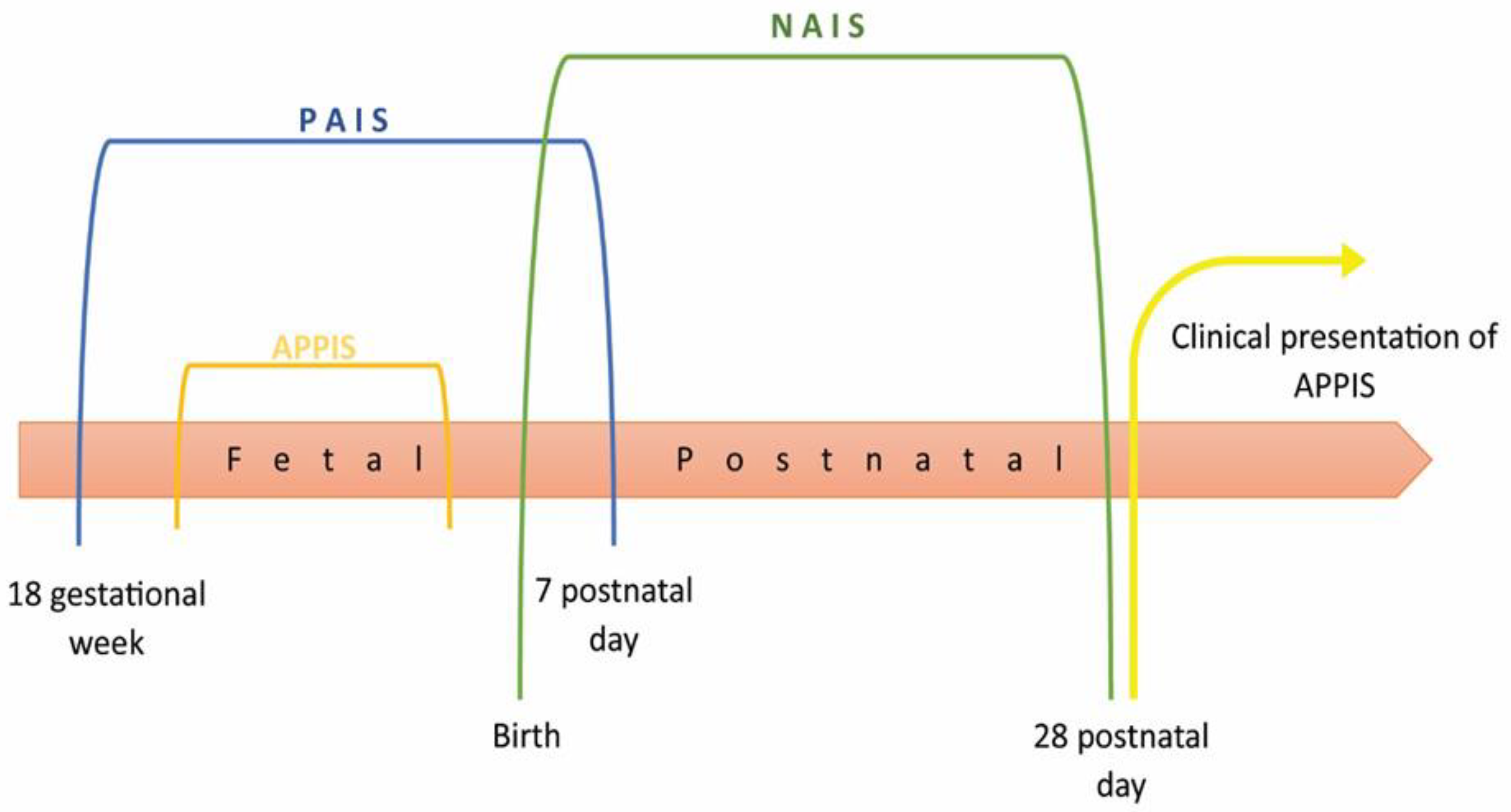
1.1. Epidemiology and Pathophysiology
1.2. Etiology and Risk Factors of Perinatal Stroke
1.3. Clinical Manifestations and Diagnostics of Perinatal Stroke
1.4. Outcomes
1.5. Follow-Up and Therapy
2. Case Presentation
2.1. Case Report
2.2. Data Collection
3. Results
3.1. Cognitive Profile
3.2. Speech-Language Profile
4. Discussion
4.1. Cognitive Profile
4.2. Speech-Language Profile
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSVT | Cerebral sinovenous thrombosis |
| PVHI | Periventricular haemorrhagic infractions |
| PAIS | Perinatal arterial ischemic stroke |
| APPIS | Arterial presumed perinatal ischemic stroke |
| NAIS | Neonatal arterial ischemic stroke |
| PAS | Perinatal arterial stroke |
| PIS | Perinatal ischemic stroke |
| USCP | Unilateral spastic cerebral palsy |
| MRI | Magnetic resonance imaging |
| DWI | Diffusion-weighted imaging |
| T1W | T1-weighted imaging |
| T2W | T2-weighted imaging |
| MCA | Middle cerebral artery |
| PPIS | Presumed perinatal ischemic strokes |
| IQ | Intelligence quotient |
| vIQ | Verbal intelligence quotient |
| pIQ | Performance intelligence quotient |
| fMRI | Functional magnetic resonance imaging |
| FCT | Front-central-temporal |
| EEG | Electroencephalography |
| NREM | Non-rapid eye movement |
| IEPSP | Institute for Experimental Phonetics and Speech Pathology |
| KSAFA | Kostic’s selective auditory filter amplifier |
| REVISK | Serbian adaptation of the Wechsler Intelligence Scale for Children-Revised |
| SLP | Speech and language pathologist |
| SEPAC | The scale for evaluation of psychophysiological abilities of children |
| PPVT | Peabody Picture Vocabulary Test |
References
- Kirton, A.; Deveber, G. Life after perinatal stroke. Stroke 2013, 44, 3265–3271. [Google Scholar] [CrossRef]
- Chabrier, S.; Husson, B.; Dinomais, M.; Landrieu, P.; Nguyen The Tich, S. New insights (and new interrogations) in perinatal arterial ischemic stroke. Thromb. Res. 2011, 127, 13–22. [Google Scholar] [CrossRef]
- Dehkharghani, S. Stroke; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- van der Aa, N.E.; Benders, M.J.; Groenendaal, F.; de Vries, L.S. Neonatal stroke: A review of the current evidence on epidemiology, pathogenesis, diagnostics and therapeutic options. Acta Paediatr. 2014, 103, 356–364. [Google Scholar] [CrossRef]
- Nelson, K.B.; Lynch, J.K. Stroke in newborn infants. Lancet Neurol. 2004, 3, 150–158. [Google Scholar] [CrossRef]
- Dunbar, M.; Kirton, A. Perinatal stroke: Mechanisms, management, and outcomes of early cerebrovascular brain injury. Lancet Child Adolesc. Health 2018, 2, 666–676. [Google Scholar] [CrossRef]
- Mastrangelo, M.; Giordo, L.; Ricciardi, G.; De Michele, M.; Toni, D.; Leuzzi, V. Acute ischemic stroke in childhood: A comprehensive review. Eur. J. Pediatr. 2022, 181, 45–58. [Google Scholar] [CrossRef]
- Golomb, M.R.; Fullerton, H.J.; Nowak-Gottl, U.; Deveber, G.; International Pediatric Stroke Study Group. Male predominance in childhood ischemic stroke: Findings from the international pediatric stroke study. Stroke 2009, 40, 52–57. [Google Scholar] [CrossRef]
- Grunt, S.; Mazenauer, L.; Buerki, S.E.; Boltshauser, E.; Mori, A.C.; Datta, A.N.; Fluss, J.; Mercati, D.; Keller, E.; Maier, O.; et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics 2015, 135, 1220–1228. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Cheong, J.L.; Diez-Sebastian, J.; Mercuri, E.; Dubowitz, L.M.; Cowan, F.M. Risk Factors for Neonatal Arterial Ischemic Stroke: The Importance of the Intrapartum Period. J. Pediatr. 2016, 173, 62–68.e1. [Google Scholar] [CrossRef] [PubMed]
- Fluss, J.; Garcia-Tarodo, S.; Granier, M.; Villega, F.; Ferey, S.; Husson, B.; Kossorotoff, M.; Muehlethaler, V.; Lebon, S.; Chabrier, S. Perinatal arterial ischemic stroke related to carotid artery occlusion. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2016, 20, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Croen, L.A.; Lindan, C.; Nash, K.B.; Yoshida, C.K.; Ferriero, D.M.; Barkovich, A.J.; Wu, Y.W. Predictors of outcome in perinatal arterial stroke: A population-based study. Ann. Neurol. 2005, 58, 303–308. [Google Scholar] [CrossRef]
- Felling, R.J.; Sun, L.R.; Maxwell, E.C.; Goldenberg, N.; Bernard, T. Pediatric arterial ischemic stroke: Epidemiology, risk factors, and management. Blood Cells Mol. Dis. 2017, 67, 23–33. [Google Scholar] [CrossRef]
- Lehman, L.L.; Rivkin, M.J. Perinatal arterial ischemic stroke: Presentation, risk factors, evaluation, and outcome. Pediatr. Neurol. 2014, 51, 760–768. [Google Scholar] [CrossRef]
- Munoz, D.; Hidalgo, M.J.; Balut, F.; Troncoso, M.; Lara, S.; Barrios, A.; Parra, P. Risk Factors for Perinatal Arterial Ischemic Stroke: A Case-Control Study. Cell Med. 2018, 10, 2155179018785341. [Google Scholar] [CrossRef]
- Curry, C.J.; Bhullar, S.; Holmes, J.; Delozier, C.D.; Roeder, E.R.; Hutchison, H.T. Risk factors for perinatal arterial stroke: A study of 60 mother-child pairs. Pediatr. Neurol. 2007, 37, 99–107. [Google Scholar] [CrossRef]
- Ilves, P.; Laugesaar, R.; Loorits, D.; Kolk, A.; Tomberg, T.; Lõo, S.; Talvik, I.; Kahre, T.; Talvik, T. Presumed Perinatal Stroke: Risk Factors, Clinical and Radiological Findings. J. Child Neurol. 2016, 31, 621–628. [Google Scholar] [CrossRef]
- Harteman, J.C.; Groenendaal, F.; Kwee, A.; Welsing, P.M.; Benders, M.J.; de Vries, L.S. Risk factors for perinatal arterial ischaemic stroke in full-term infants: A case-control study. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F411–F416. [Google Scholar] [CrossRef]
- Cluver, L.; Lachman, J.M.; Sherr, L.; Wessels, I.; Krug, E.; Rakotomalala, S.; Blight, S.; Hillis, S.; Bachman, G.; Green, O.; et al. Parenting in a time of COVID-19. Lancet 2020, 395, e64. [Google Scholar] [CrossRef] [PubMed]
- Gacio, S.; Muñoz Giacomelli, F.; Klein, F. Presumed perinatal ischemic stroke: A review. Arch. Argent. Pediatr. 2015, 113, 449–455. [Google Scholar] [PubMed]
- Kocaman, C.; Yilmaz, Y. Etiological analysis of presumed perinatal stroke. Brain Dev. 2012, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Mirsky, D.M.; Beslow, L.A.; Amlie-Lefond, C.; Krishnan, P.; Laughlin, S.; Lee, S.; Lehman, L.; Rafay, M.; Shaw, D.; Rivkin, M.J.; et al. Pathways for Neuroimaging of Childhood Stroke. Pediatr. Neurol. 2017, 69, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Dudink, J.; Mercuri, E.; Al-Nakib, L.; Govaert, P.; Counsell, S.J.; Rutherford, M.A.; Cowan, F.M. Evolution of Unilateral Perinatal Arterial Ischemic Stroke on Conventional and Diffusion-Weighted MR Imaging. Am. J. Neuroradiol. 2009, 30, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Kirton, A.; Shroff, M.; Pontigon, A.-M.; deVeber, G. Risk factors and presentations of periventricular venous infarction vs arterial presumed perinatal ischemic stroke. Arch. Neurol. 2010, 67, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Biarge, M.; Ferriero, D.M.; Cowan, F.M. Chapter 11—Perinatal arterial ischemic stroke. In Handbook of Clinical Neurology; de Vries, L.S., Glass, H.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 239–266. [Google Scholar]
- Fitzgerald, K.C.; Williams, L.S.; Garg, B.P.; Golomb, M.R. Epilepsy in children with delayed presentation of perinatal stroke. J. Child Neurol. 2007, 22, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Murias, K.; Brooks, B.; Kirton, A.; Iaria, G. A review of cognitive outcomes in children following perinatal stroke. Dev. Neuropsychol. 2014, 39, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Monge, S.; Boudia, B.; Ritz, A.; Abbas-Chorfa, F.; Rabilloud, M.; Iwaz, J.; BÉRard, C. A 7-year longitudinal follow-up of intellectual development in children with congenital hemiplegia. Dev. Med. Child Neurol. 2009, 51, 959–967. [Google Scholar] [CrossRef]
- McLinden, A.; Baird, A.D.; Westmacott, R.; Anderson, P.E.; deVeber, G. Early Cognitive Outcome After Neonatal Stroke. J. Child Neurol. 2007, 22, 1111–1116. [Google Scholar] [CrossRef]
- Ricci, D.; Mercuri, E.; Barnett, A.; Rathbone, R.; Cota, F.; Haataja, L.; Rutherford, M.; Dubowitz, L.; Cowan, F. Cognitive Outcome at Early School Age in Term-Born Children with Perinatally Acquired Middle Cerebral Artery Territory Infarction. Stroke 2008, 39, 403–410. [Google Scholar] [CrossRef]
- Chilosi, A.M.; Cipriani, P.; Bertuccelli, B.; Pfanner, L.; Cioni, G. Early cognitive and communication development in children with focal brain lesions. J. Child Neurol. 2001, 16, 309–316. [Google Scholar] [CrossRef]
- Levine, S.C.; Kraus, R.; Alexander, E.; Suriyakham, L.W.; Huttenlocher, P.R. IQ decline following early unilateral brain injury: A longitudinal study. Brain Cogn. 2005, 59, 114–123. [Google Scholar] [CrossRef]
- van Buuren, L.M.; van der Aa, N.E.; Dekker, H.C.; Vermeulen, R.J.; van Nieuwenhuizen, O.; van Schooneveld, M.M.; de Vries, L.S. Cognitive outcome in childhood after unilateral perinatal brain injury. Dev. Med. Child Neurol. 2013, 55, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Westmacott, R.; MacGregor, D.; Askalan, R.; deVeber, G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke 2009, 40, 2012–2019. [Google Scholar] [CrossRef]
- Ballantyne, A.O.; Spilkin, A.M.; Hesselink, J.; Trauner, D.A. Plasticity in the developing brain: Intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain 2008, 131, 2975–2985. [Google Scholar] [CrossRef]
- Wagenaar, N.; Martinez-Biarge, M.; van der Aa, N.E.; van Haastert, I.C.; Groenendaal, F.; Benders, M.; Cowan, F.M.; de Vries, L.S. Neurodevelopment After Perinatal Arterial Ischemic Stroke. Pediatrics 2018, 142, e20174164. [Google Scholar] [CrossRef]
- Bosenbark, D.D.; Krivitzky, L.; Ichord, R.; Vossough, A.; Bhatia, A.; Jastrzab, L.E.; Billinghurst, L. Clinical predictors of attention and executive functioning outcomes in children after perinatal arterial ischemic stroke. Pediatr. Neurol. 2017, 69, 79–86. [Google Scholar] [CrossRef]
- Newport, E.L.; Seydell-Greenwald, A.; Landau, B.; Turkeltaub, P.E.; Chambers, C.E.; Martin, K.C.; Rennert, R.; Giannetti, M.; Dromerick, A.W.; Ichord, R.N.; et al. Language and developmental plasticity after perinatal stroke. Proc. Natl. Acad. Sci. USA 2022, 119, e2207293119. [Google Scholar] [CrossRef]
- Staudt, M.; Lidzba, K.; Grodd, W.; Wildgruber, D.; Erb, M.; Krägeloh-Mann, I. Right-Hemispheric Organization of Language Following Early Left-Sided Brain Lesions: Functional MRI Topography. NeuroImage 2002, 16, 954–967. [Google Scholar] [CrossRef]
- Feldman, H.M.; MacWhinney, B.; Sacco, K. Sentence processing in children with early unilateral brain injury. Brain Lang. 2002, 83, 335–352. [Google Scholar] [CrossRef]
- Booth, J.R.; MacWhinney, B.; Thulborn, K.R.; Sacco, K.; Voyvodic, J.T.; Feldman, H.M. Developmental and Lesion Effects in Brain Activation During Sentence Comprehension and Mental Rotation. Dev. Neuropsychol. 2000, 18, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Newport, E.L.; Landau, B.; Seydell-Greenwald, A.; Turkeltaub, P.E.; Chambers, C.E.; Dromerick, A.W.; Carpenter, J.; Berl, M.M.; Gaillard, W.D. Revisiting Lenneberg’s Hypotheses About Early Developmental Plasticity: Language Organization after Left-Hemisphere Perinatal Stroke. Biolinguistics 2017, 11, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Jacola, L.M.; Schapiro, M.B.; Schmithorst, V.J.; Byars, A.W.; Strawsburg, R.H.; Szaflarski, J.P.; Plante, E.; Holland, S.K. Functional Magnetic Resonance Imaging Reveals Atypical Language Organization in Children Following Perinatal Left Middle Cerebral Artery Stroke. Neuropediatrics 2006, 37, 46–52. [Google Scholar] [CrossRef]
- Tillema, J.-M.; Byars, A.W.; Jacola, L.M.; Schapiro, M.B.; Schmithorst, V.J.; Szaflarski, J.P.; Holland, S.K. Reprint of “Cortical reorganization of language functioning following perinatal left MCA stroke” [Brain and Language 105 99–111]. Brain Lang. 2008, 106, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Kirton, A.; Deveber, G.; Pontigon, A.M.; Macgregor, D.; Shroff, M. Presumed perinatal ischemic stroke: Vascular classification predicts outcomes. Ann. Neurol. 2008, 63, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Chabrier, S.; Peyric, E.; Drutel, L.; Deron, J.; Kossorotoff, M.; Dinomais, M.; Lazaro, L.; Lefranc, J.; Thébault, G.; Dray, G.; et al. Multimodal Outcome at 7 Years of Age after Neonatal Arterial Ischemic Stroke. J. Pediatr. 2016, 172, 156–161.e3. [Google Scholar] [CrossRef] [PubMed]
- François, C.; Ripollés, P.; Ferreri, L.; Muchart, J.; Sierpowska, J.; Fons, C.; Solé, J.; Rebollo, M.; Zatorre, R.J.; Garcia-Alix, A.; et al. Right Structural and Functional Reorganization in Four-Year-Old Children with Perinatal Arterial Ischemic Stroke Predict Language Production. eNeuro 2019, 6, 0447-18.2019. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, S.; Jeličić, L.; Marisavljević, M.; Fatić, S.; Gavrilović, A.; Subotić, M. Can EEG Correlates Predict Treatment Efficacy in Children with Overlapping ASD and SLI Symptoms: A Case Report. Diagnostics 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Ćirović, M.; Jeličić, L.; Maksimović, S.; Fatić, S.; Marisavljević, M.; Bošković Matić, T.; Subotić, M. EEG Correlates of Cognitive Functions in a Child with ASD and White Matter Signal Abnormalities: A Case Report with Two-and-a-Half-Year Follow-Up. Diagnostics 2023, 13, 2878. [Google Scholar] [CrossRef]
- Avila, L.; Riesgo, R.; Pedroso, F.; Goldani, M.; Danesi, M.; Ranzan, J.; Sleifer, P. Language and focal brain lesion in childhood. J. Child Neurol. 2010, 25, 829–833. [Google Scholar] [CrossRef]
- Chapman, S.B.; Max, J.E.; Gamino, J.F.; McGlothlin, J.H.; Cliff, S.N. Discourse plasticity in children after stroke: Age at injury and lesion effects. Pediatr. Neurol. 2003, 29, 34–41. [Google Scholar] [CrossRef]
- Gout, A.; Seibel, N.; Rouvière, C.; Husson, B.; Hermans, B.; Laporte, N.; Kadhim, H.; Grandin, C.; Landrieu, P.; Sébire, G. Aphasia owing to subcortical brain infarcts in childhood. J. Child Neurol. 2005, 20, 1003–1008. [Google Scholar] [CrossRef]
- Lansing, A.E.; Max, J.E.; Delis, D.C.; Fox, P.T.; Lancaster, J.; Manes, F.F.; Schatz, A. Verbal learning and memory after childhood stroke. J. Int. Neuropsychol. Soc. 2004, 10, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Max, J.E.; Bruce, M.; Keatley, E.; Delis, D. Pediatric stroke: Plasticity, vulnerability, and age of lesion onset. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Studer, M.; Boltshauser, E.; Mori, A.C.; Datta, A.; Fluss, J.; Mercati, D.; Hackenberg, A.; Keller, E.; Maier, O.; Marcoz, J.-P. Factors affecting cognitive outcome in early pediatric stroke. Neurology 2014, 82, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Allman, C.; Scott, R.B. Neuropsychological sequelae following pediatric stroke: A nonlinear model of age at lesion effects. Child Neuropsychol. 2013, 19, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Everts, R.; Pavlovic, J.; Kaufmann, F.; Uhlenberg, B.; Seidel, U.; Nedeltchev, K.; Perrig, W.; Steinlin, M. Cognitive functioning, behavior, and quality of life after stroke in childhood. Child Neuropsychol. 2008, 14, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Westmacott, R.; Askalan, R.; Macgregor, D.; Anderson, P.; Deveber, G. Cognitive outcome following unilateral arterial ischaemic stroke in childhood: Effects of age at stroke and lesion location. Dev. Med. Child Neurol. 2010, 52, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Biro, M. REVISK Manual, 2nd ed.; DPS: Belgrade, Serbia, 1998. [Google Scholar]
- Bogavac, I.; Jeličić, L.; Nenadovic, V.; Subotić, M.; Janjić, V. The speech and language profile of a child with Turner Syndrome—A case study. Clin. Linguist. Phon. 2021, 36, 565–578. [Google Scholar] [CrossRef]
- Jeličić, L.; Sovilj, M.; Bogavac, I.; Drobnjak, A.E.; Gouni, O.; Kazmierczak, M.; Subotić, M. The Impact of Maternal Anxiety on Early Child Development During the COVID-19 Pandemic. Front. Psychol. 2021, 12, 792053. [Google Scholar] [CrossRef]
- Rakonjac, M.; Cuturilo, G.; Stevanović, M.; Jovanović, I.; Jeličić-Dobrijević, L.; Mijovic, M.; Drakulić, D. Speech and language abilities of children with the familial form of 22q11.2 deletion syndrome. Genetika 2016, 48, 57–72. [Google Scholar] [CrossRef]
- Dunn, L.M.; Dunn, L.M.; Kovačević, M.; Padovan, N.; Hržica, G.; Kuvač Kraljević, J.; Mustapić, M.; Dobravac, G.; Palmović, M. Peabody Slikovni Test Rječnika, PPVT-IIIHR; Naklada Slap: Zagreb, Croatia, 2010. [Google Scholar]
- Fatić, S.; Stanojević, N.; Stokić, M.; Nenadović, V.; Jeličić, L.; Bilibajkić, R.; Gavrilović, A.; Maksimović, S.; Adamović, T.; Subotić, M. Electroen cephalography correlates of word and non-word listening in children with specific language impairment: An observational study20F0. Medicine 2022, 101, e31840. [Google Scholar] [CrossRef]
- Kostić, Đ.; Vladisavljević, S. Tests for Speech and Language Assessment; Zavod za udžbenike i nastavna sredstva: Belgrade, Serbia, 1983. (In Serbian) [Google Scholar]
- Jeličić, L.; Bogavac, I.; Perovic, A. Serbian version of the Multilingual Assessment Instrument for Narratives (MAIN). ZAS Pap. Linguist. 2020, 64, 189–197. [Google Scholar] [CrossRef]
- Vladisavljević, S. Pathologically Underdeveloped Language in Children: Instructions for Speech and Language Development; Zavod za udžbenike i nastavna sredstva: Belgrade, Serbia, 1997. [Google Scholar]
- Vukovic, M.; Vukovic, I.; Stojanovik, V. Investigation of language and motor skills in Serbian speaking children with specific language impairment and in typically developing children. Res. Dev. Disabil. 2010, 31, 1633–1644. [Google Scholar] [CrossRef]
- Carlsson, G. Memory for words and drawings in children with hemiplegic cerebral palsy. Scand. J. Psychol. 1997, 38, 265–273. [Google Scholar] [CrossRef]
- Cioni, G.; Brizzolara, D.; Ferretti, G.; Bertuccelli, B.; Fazzi, B. Visual information processing in infants with focal brain lesions. Exp. Brain Res. 1998, 123, 95–101. [Google Scholar] [CrossRef]
- Schatz, A.M.; Ballantyne, A.O.; Trauner, D.A. A hierarchical analysis of block design errors in children with early focal brain damage. Dev. Neuropsychol. 2000, 17, 75–83. [Google Scholar] [CrossRef]
- Trauner, D.A.; Chase, C.; Walker, P.; Wulfeck, B. Neurologic profiles of infants and children after perinatal stroke. Pediatr. Neurol. 1993, 9, 383–386. [Google Scholar] [CrossRef]
- Wulfeck, B.B.; Trauner, D.A.; Tallal, P.A. Neurologic, cognitive, and linguistic features of infants after early stroke. Pediatr. Neurol. 1991, 7, 266–269. [Google Scholar] [CrossRef]
- Hajek, C.A.; Yeates, K.O.; Anderson, V.; Mackay, M.; Greenham, M.; Gomes, A.; Lo, W. Cognitive outcomes following arterial ischemic stroke in infants and children. J. Child Neurol. 2014, 29, 887–894. [Google Scholar] [CrossRef]
- Lo, W.; Gordon, A.; Hajek, C.; Gomes, A.; Greenham, M.; Perkins, E.; Zumberge, N.; Anderson, V.; Yeates, K.O.; Mackay, M.T. Social competence following neonatal and childhood stroke. Int. J. Stroke 2014, 9, 1037–1044. [Google Scholar] [CrossRef]
- Lo, W.D.; Hajek, C.; Pappa, C.; Wang, W.; Zumberge, N. Outcomes in children with hemorrhagic stroke. JAMA Neurol. 2013, 70, 66–71. [Google Scholar] [CrossRef]
- Carlsson, G.; Uvebrant, P.; Hugdahl, K.; Arvidsson, J.; Wiklund, L.M.; von Wendt, L. Verbal and non-verbal function of children with right-versus left-hemiplegic cerebral palsy of pre-and perinatal origin. Dev. Med. Child Neurol. 1994, 36, 503–512. [Google Scholar] [CrossRef]
- Goodman, R.; Yude, C. IQ and its predictors in childhood hemiplegia. Dev. Med. Child Neurol. 1996, 38, 881–890. [Google Scholar] [CrossRef]
- Kolk, A.; Ennok, M.; Laugesaar, R.; Kaldoja, M.-L.; Talvik, T. Long-term cognitive outcomes after pediatric stroke. Pediatr. Neurol. 2011, 44, 101–109. [Google Scholar] [CrossRef]
- Muter, V.; Taylor, S.; Vargha-Khadem, F. A longitudinal study of early intellectual development in hemiplegic children. Neuropsychologia 1997, 35, 289–298. [Google Scholar] [CrossRef]
- Bates, E.; Thal, D.; Trauner, D.; Fenson, J.; Aram, D.; Eisele, J.; Nass, R. From first words to grammar in children with focal brain injury. Dev. Neuropsychol. 1997, 13, 275–343. [Google Scholar] [CrossRef]
- Marchman, V.A.; Miller, R.; Bates, E.A. Babble and first words in children with focal brain injury. Appl. Psycholinguist. 1991, 12, 1–22. [Google Scholar] [CrossRef]
- Stiles, J.; Nass, R.D.; Levine, S.C.; Moses, P.; Reilly, J.S. Perinatal stroke. Pediatr. Neuropsychol. Res. Theory Pract. 2010, 5, 181–210. [Google Scholar]
- Vicari, S.; Albertoni, A.; Chilosi, A.; Cipriani, P.; Cioni, G.; Bates, E. Plasticity and reorganization during language development in children with early brain injury. Cortex 2000, 36, 31–46. [Google Scholar] [CrossRef]
- Kirton, A.; Westmacott, R.; Deveber, G. Pediatric stroke: Rehabilitation of focal injury in the developing brain. NeuroRehabilitation 2007, 22, 371–382. [Google Scholar] [CrossRef]
- Akshoomoff, N.A.; Feroleto, C.C.; Doyle, R.E.; Stiles, J. The impact of early unilateral brain injury on perceptual organization and visual memory. Neuropsychologia 2002, 40, 539–561. [Google Scholar] [CrossRef]
- Stiles, J.; Stern, C.; Trauner, D.; Nass, R. Developmental change in spatial grouping activity among children with early focal brain injury: Evidence from a modeling task. Brain Cogn. 1996, 31, 46–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stiles, J.; Trauner, D.; Engel, M.; Nass, R. The development of drawing in children with congenital focal brain injury: Evidence for limited functional recovery. Neuropsychologia 1997, 35, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Gibb, R. Brain plasticity and behaviour in the developing brain. J. Can. Acad. Child Adolesc. Psychiatry 2011, 20, 265–276. [Google Scholar] [PubMed]
- Anderson, V.; Spencer-Smith, M.; Wood, A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 2011, 134, 2197–2221. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.; Deotto, A.; Desrocher, M.; deVeber, G.; Westmacott, R. Determinants of cognitive outcomes of perinatal and childhood stroke: A review. Child Neuropsychol. 2016, 22, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Ilves, P.; Tomberg, T.; Kepler, J.; Laugesaar, R.; Kaldoja, M.-L.; Kepler, K.; Kolk, A. Different plasticity patterns of language function in children with perinatal and childhood stroke. J. Child Neurol. 2014, 29, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.S.; Wasserman, S.; Appelbaum, M. Later language development in narratives in children with perinatal stroke. Dev. Sci. 2013, 16, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.L.; Levine, S.C.; Fisher, J.A.; Goldin-Meadow, S. Does linguistic input play the same role in language learning for children with and without early brain injury? Dev. Psychol. 2009, 45, 90. [Google Scholar] [CrossRef]
- Thal, D.J.; Marchman, V.; Stiles, J.; Aram, D.; Trauner, D.; Nass, R.; Bates, E. Early lexical development in children with focal brain injury. Brain Lang. 1991, 40, 491–527. [Google Scholar] [CrossRef]
- Demir, Ö.E.; Levine, S.C.; Goldin-Meadow, S. Narrative skill in children with early unilateral brain injury: A possible limit to functional plasticity. Dev. Sci. 2010, 13, 636–647. [Google Scholar] [CrossRef]
- François, C.; Ripollés, P.; Bosch, L.; Garcia-Alix, A.; Muchart, J.; Sierpowska, J.; Fons, C.; Solé, J.; Rebollo, M.; Gaitán, H. Language learning and brain reorganization in a 3.5-year-old child with left perinatal stroke revealed using structural and functional connectivity. Cortex 2016, 77, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J. The effects of injury to dynamic neural networks in the mature and developing brain. Dev. Psychobiol. 2012, 54, 343–349. [Google Scholar] [CrossRef] [PubMed]

| Maternal | Antenatal | Intrapartum | Fetal |
|---|---|---|---|
| History of seizures in the family | First pregnancy | Prolonged ruptures of membranes | Male sex |
| History of neurological diseases | Thrombophilia | Abnormal cardiotocography pattern | Congenital heart disease |
| Preeclampsia | Meconium stained amniotic fluid | Hypoglycaemia | |
| Maternal smoking | Emergency caesarean section | Early onset sepsis/meningitis | |
| Assisted vacuum delivery | |||
| Need for resuscitation | |||
| Apgar scores < 7 | |||
| Birth asphyxia |
| Subtests | Score | SD | IQ | |
|---|---|---|---|---|
| Verbal scale | Information | 5 | 0 | 66 |
| Comprehension | 3 | −1 | ||
| Arithmetic | 5 | 0 | ||
| Similarities | 4 | 0 | ||
| Digit Span | 6 | +1 | ||
| Performance scale | Picture Completion | 10 | 0 | 93 |
| Picture Arrangement | 11 | +1 | ||
| Block Design | 5 | −2 | ||
| Object Assembly | 7 | −1 | ||
| Coding | 12 | +2 |
| Instrument | Age Equivalent |
|---|---|
| PPVT | Six years and one month |
| SEPAC—speech and language | Four years and two months |
| SEPAC—sensorimotor | Four years and seven months |
| SEPAC—socioemotional | Five years |
| Comprehension of Grammar Forms | Production of Grammar Forms | |||||
|---|---|---|---|---|---|---|
| + | +/− | − | + | +/− | − | |
| Plural of nouns | √ | √ | ||||
| Gender | √ | √ | ||||
| Word cases | √ | √ | ||||
| Pronouns | √ | √ | ||||
| Spatial prepositions | √ | √ (in, on) | √ (under, above, in front of, behind, next to, between) | |||
| Adverbs | √ | √ | ||||
| Comparison of adjectives | √ | √ | ||||
| Present tense | √ | √ | ||||
| Past tense | √ | √ | ||||
| Future tense | √ | √ | ||||
| Types of Articulation Errors | ||||||
|---|---|---|---|---|---|---|
| Target Consonant | Omission | Substitution | Devoiced | Interdental | ||
| Plosives | Voiceless | p | ||||
| k | ||||||
| t | √ | |||||
| Voiced | b | |||||
| g | √ | |||||
| d | √ | √ | ||||
| Affricates | Voiceless | t͡s | k | |||
| t͡ɕ | k | |||||
| d͡ʑ | k | |||||
| Voiced | t͡ʂ | k | ||||
| d͡ʐ | k | |||||
| Fricatives | Voiceless | f | √ | w | ||
| x | ||||||
| s | ||||||
| z | s | |||||
| Voiced | ʂ | s | ||||
| ʐ | s | |||||
| Approximants | ʋ | √ | w | |||
| j | ||||||
| Nasals | m | |||||
| n | ||||||
| ɲ | √ | |||||
| Laterals | l | √ | ||||
| ʎ | √ | |||||
| Trill | r | √ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogavac, I.; Jeličić, L.; Marisavljević, M.; Bošković Matić, T.; Subotić, M. Arterial Presumed Perinatal Ischemic Stroke: A Mini Review and Case Report of Cognitive and Speech-Language Profiles in a 5-Year-Old Girl. Children 2024, 11, 33. https://doi.org/10.3390/children11010033
Bogavac I, Jeličić L, Marisavljević M, Bošković Matić T, Subotić M. Arterial Presumed Perinatal Ischemic Stroke: A Mini Review and Case Report of Cognitive and Speech-Language Profiles in a 5-Year-Old Girl. Children. 2024; 11(1):33. https://doi.org/10.3390/children11010033
Chicago/Turabian StyleBogavac, Ivana, Ljiljana Jeličić, Maša Marisavljević, Tatjana Bošković Matić, and Miško Subotić. 2024. "Arterial Presumed Perinatal Ischemic Stroke: A Mini Review and Case Report of Cognitive and Speech-Language Profiles in a 5-Year-Old Girl" Children 11, no. 1: 33. https://doi.org/10.3390/children11010033
APA StyleBogavac, I., Jeličić, L., Marisavljević, M., Bošković Matić, T., & Subotić, M. (2024). Arterial Presumed Perinatal Ischemic Stroke: A Mini Review and Case Report of Cognitive and Speech-Language Profiles in a 5-Year-Old Girl. Children, 11(1), 33. https://doi.org/10.3390/children11010033