Neurodevelopmental Outcome and Neuroimaging of Very Low Birth Weight Infants from an Italian NICU Adopting the Family-Centered Care Model
Abstract
:1. Introduction
2. Aims of the Study
- (1)
- Describe neurodevelopmental outcomes at 24 months of corrected age (CA) in a cohort of VLBW infants admitted to a single Italian NICU adopting FCC.
- (2)
- Identify perinatal risk factors for severe neurodevelopmental outcomes.
- (3)
- Evaluate the correlation between brain MRI findings at TEA and neurodevelopmental outcomes.
3. Materials and Methods
3.1. Study Design
3.2. Family-Centered Care
3.3. Brain Magnetic Resonance Imaging (MRI) Protocol and Classification
- -
- Germinal Matrix Hemorrhage–Intraventricular Hemorrhage (GMH-IVH): The germinal matrix is a structure that is normally visible on imaging and undergoes involution as the fetus ages, leaving behind only remnants in the caudothalamic notch and roof of the temporal horns after 32 weeks of gestation. In our study, an irregular contour in a subependymal area with a low T2 signal or accompanying IVH was categorized as GMH-IVH. It is important to note that hemorrhage may not be immediately evident at TEA.
- -
- Hemorrhagic Parenchymal Infarction (HPI): When fully developed, HPI is characterized by a focal bulging or outpouching of the ventricular contour, usually unilateral. This is often accompanied by a low T2 signal component, indicating previous hemorrhage.
- -
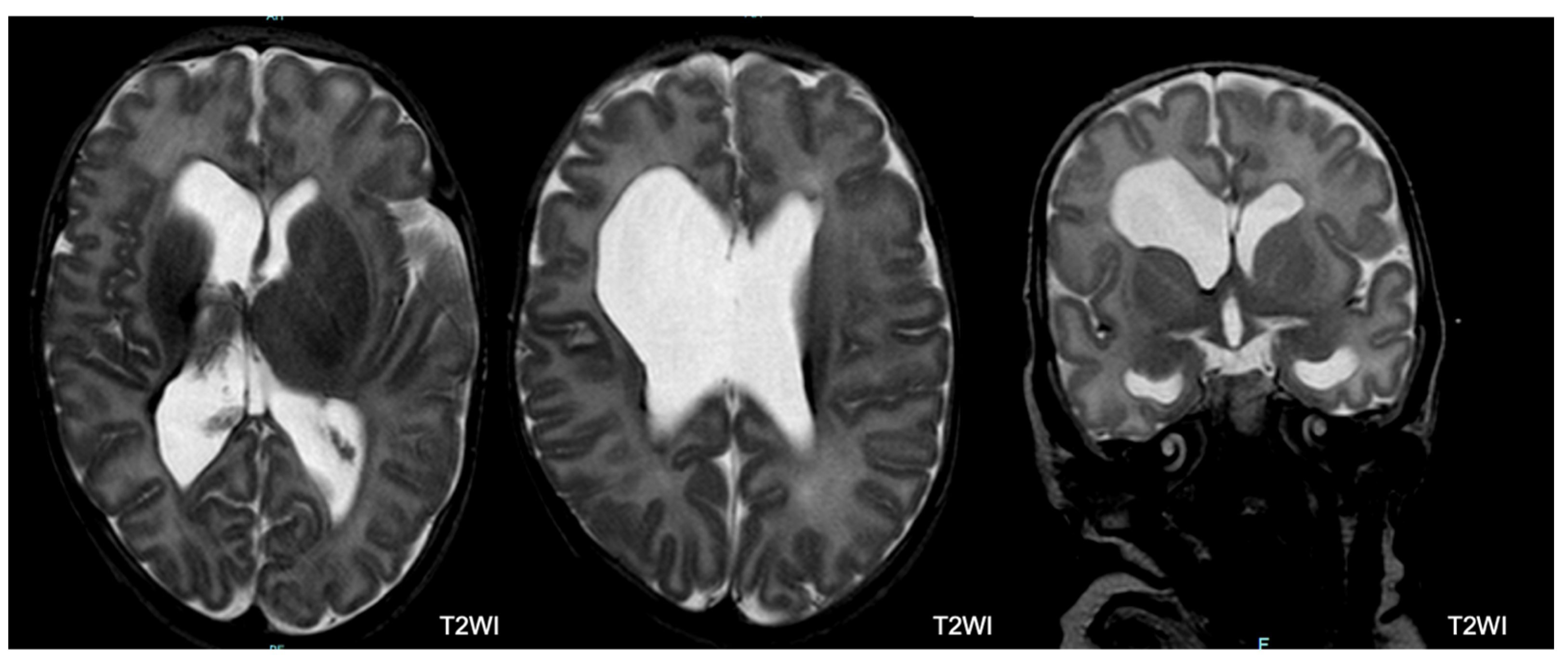
- Periventricular Leukomalacia (PVL): PVL typically manifests as multiple bilateral periventricular cysts in a symmetric distribution, initially appearing separate from the ventricle. Solitary or unilateral cysts are more likely to be venous infarcts or connatal cysts. At TEA, the cysts may no longer be visible. Our study used the following criteria to diagnose PVL: residual bilateral periventricular cysts, dilated/angulated posterior aspects of the lateral ventricles, and associated white matter volume loss. This is often accompanied by thalamic atrophy and abnormal myelination.
- -
- Punctate White Matter Lesions (PWML): PWML are small areas of injury in the white matter with no consistent sonographic appearance. In our study, PWML are defined as small foci of high T1 signal in the white matter, less commonly seen on T2 sequences. The conspicuity of these lesions decreases over time, suggesting a likely higher original lesion load.
- -
- Subependymal Cysts: Subependymal cysts, characterized by thin walls, are typically located at the caudothalamic notch. These cysts may or may not be the sequelae of a germinal matrix hemorrhage.
- -
- Cerebellar Hemorrhage: Cerebellar hemorrhage is defined by size, with small hemorrhages smaller than 5 mm and large hemorrhages larger than 5 mm. At TEA, cerebellar atrophy may be present with minimal evidence of hemorrhage. It is noteworthy to mention that the germinal matrix is located on the outer surface of the cerebellum, so hemorrhages may involve the cerebellar cortex.
- -
- Abnormal Myelin: Abnormal myelin is defined as a diffuse excessively high T2 signal intensity in the subcortical white matter.
- -
- Unclassified Lesions: This category included findings that did not conform to the lesion definitions above. Lesions were considered major if associated with lobar tissue or multiple areas of tissue loss.
3.4. Neurodevelopmental Follow-Up
4. Statistical Analysis
5. Results
5.1. Neurodevelopmental Outcome
5.2. Brain MRI Abnormalities
5.3. MRI and Outcome
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Cavallo, M.C.; Gugiatti, A.; Fattore, G.; Gerzeli, S.; Barbieri, D.; Zanini, R.; Neonatal Adequate Care for Quality of Life (NEO-ACQUA) Study Group. Cost of care and social consequences of very low birth weight infants without premature- related morbidities in Italy. Ital. J. Pediatr. 2015, 41, 59. [Google Scholar] [CrossRef]
- Inder, T.E.; Volpe, J.J.; Anderson, P.J. Defining the Neurologic Consequences of Preterm Birth. N. Engl. J. Med. 2023, 389, 441–453. [Google Scholar] [CrossRef]
- Rysavy, M.A.; Li, L.; Bell, E.F.; Das, A.; Hintz, S.R.; Stoll, B.J.; Vohr, B.R.; Carlo, W.A.; Shankaran, S.; Walsh, M.C.; et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N. Engl. J. Med. 2015, 372, 1801–1811. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Lugli, L.; Pugliese, M.; Plessi, C.; Berardi, A.; Guidotti, I.; Ancora, G.; Grandi, S.; Gargano, G.; Braibanti, S.; Sandri, F.; et al. Neuroprem: The Neuro-developmental outcome of very low birth weight infants in an Italian region. Ital. J. Pediatr. 2020, 46, 26. [Google Scholar] [CrossRef]
- Lui, K.; Lee, S.K.; Kusuda, S.; Adams, M.; Vento, M.; Reichman, B.; Darlow, B.A.; Lehtonen, L.; Modi, N.; Norman, M.; et al. International Network for Evaluation of Outcomes (iNeo) of neonates Investigators. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J. Pediatr. 2019, 215, 32–40.e14. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Lugli, L.; Bedetti, L.; Guidotti, I.; Pugliese, M.; Picciolini, O.; Roversi, M.F.; DellaCasa Muttini, E.; Lucaccioni, L.; Bertoncelli, N.; Ancora, G.; et al. Neuroprem 2: An Italian Study of Neurodevelopmental Outcomes of Very Low Birth Weight Infants. Front. Pediatr. 2021, 9, 697100. [Google Scholar] [CrossRef]
- Altimier, L.; Phillips, R. Neonatal Integrative Developmental Care Model: Seven Neuroprotective Core Measures for Family-Centered Developmental Care. Neonatal Infant. Rev. 2016, 4, 230–244. [Google Scholar] [CrossRef]
- Soni, R.; Tscherning Wel-Wel, C.; Robertson, N.J. Neuroscience meets nurture: Challenges of prematurity and the critical role of family-centred and developmental care as a key part of the neuroprotection care bundle. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 242–249. [Google Scholar] [CrossRef]
- Bertoncelli, N.; Lugli, L.; Bedetti, L.; Lucaccioni, L.; Bianchini, A.; Boncompagni, A.; Cipolli, F.; Cosimo, A.C.; Cuomo, G.; Di Giuseppe, M.; et al. Parents’ Experience in an Italian NICU Implementing NIDCAP-Based Care: A Qualitative Study. Children 2022, 9, 1917. [Google Scholar] [CrossRef]
- Molloy, E.J.; El-Dib, M.; Juul, S.E.; Benders, M.; Gonzalez, F.; Bearer, C.; Wu, Y.W.; Robertson, N.J.; Hurley, T.; Branagan, A.; et al. Newborn Brain Society Guidelines and Publications Committee. Neuroprotective therapies in the NICU in term infants: Present and future. Pediatr. Res. 2023, 93, 1819–1827. [Google Scholar] [CrossRef]
- Parodi, A.; Govaert, P.; Horsch, S.; Bravo, M.C.; Ramenghi, L.A.; eurUS.Brain Group. Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr. Res. 2020, 87 (Suppl. S1), 13–24. [Google Scholar] [CrossRef]
- Edwards, A.D.; Redshaw, M.E.; Kennea, N.; Rivero-Arias, O.; Gonzales-Cinca, N.; Nongena, P.; Ederies, M.; Falconer, S.; Chew, A.; Omar, O.; et al. Effect of MRI on preterm infants and their families: A randomized trial with nested diagnostic and economic evaluation. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F15–F21. [Google Scholar] [CrossRef]
- Counsell, S.J.; Edwards, A.D.; Chew, A.T.M.; Anjari, M.; Dyet, L.E.; Srinivasan, L.; Boardman, J.P.; Allsop, J.M.; Hajnal, J.V.; Rutherford, M.A.; et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 2008, 131, 3201–3208. [Google Scholar] [CrossRef]
- Hintz, S.R.; Barnes, P.D.; Bulas, D.; Slovis, T.L.; Finer, N.N.; Wrage, L.A.; Das, A.; Tyson, J.E.; Stevenson, D.K.; Carlo, W.A.; et al. SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics 2015, 135, e32–e42. [Google Scholar] [CrossRef]
- Tam, E.W.; Rosenbluth, G.; Rogers, E.E.; Ferriero, D.M.; Glidden, D.; Goldstein, R.B.; Glass, H.C.; Piecuch, R.E.; Barkovich, A.J. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J. Pediatr. 2011, 158, 245–250. [Google Scholar] [CrossRef]
- Arulkumaran, S.; Tusor, N.; Chew, A.; Falconer, S.; Kennea, N.; Nongena, P.; Hajnal, J.V.; Counsell, S.J.; Rutherford, M.A.; Edwards, A.D. MRI Findings at Term-Corrected Age and Neurodevelopmental Outcomes in a Large Cohort of Very Preterm Infants. AJNR Am. J. Neuroradiol. 2020, 41, 1509–1516. [Google Scholar] [CrossRef]
- Bashinsky, A.L. Retinopathy of Prematurity. N. Carol. Med. J. 2017, 78, 124–128. [Google Scholar] [CrossRef]
- Tréluyer, L.; Chevallier, M.; Jarreau, P.H.; Baud, O.; Benhammou, V.; Gire, C.; Marchand-Martin, L.; Marret, S.; Pierrat, V.; Ancel, P.Y.; et al. Intraventricular Hemorrhage in Very Preterm Children: Mortality and Neurodevelopment at Age 5. Pediatrics 2023, 151, e2022059138. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Bowerman, R.A.; Donn, S.M.; Silver, T.M.; Jaffe, M.H. Natural history of neonatal periventricular/intraventricular hemorrhage and its complications: Sonographic observations. AJR Am. J. Roentgenol. 1984, 143, 1041–1052. [Google Scholar] [CrossRef]
- Agut, T.; Alarcon, A.; Cabañas, F.; Bartocci, M.; Martinez-Biarge, M.; Horsch, S.; eurUS.brain group. Preterm white matter injury: Ultrasound diagnosis and classification. Pediatr. Res. 2020, 87 (Suppl. S1), 37–49. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Ramamoorthy, C. Bronchopulmonary dysplasia. Paediatr. Anaesth. 2022, 32, 174–180. [Google Scholar] [CrossRef]
- Gómez-Cantarino, S.; García-Valdivieso, I.; Moncunill-Martínez, E.; Yáñez-Araque, B.; Ugarte Gurrutxaga, M.I. Developing a Family-Centered Care Model in the Neonatal Intensive Care Unit (NICU): A New Vision to Manage Healthcare. Int. J. Environ. Res. Public. Health 2020, 17, 7197. [Google Scholar] [CrossRef]
- Pennell, C.; Whittingham, K.; Boyd, R.; Sanders, M.; Colditz, P. Prematurity and parental self-efficacy: The Preterm Parenting & Self-Efficacy Checklist. Infant. Behav. Dev. 2012, 35, 678–688. [Google Scholar] [CrossRef]
- Amiel-Tison, C.; Gosselin, J. Neurological Development from Birth to Six Years. Guide for Examination and Evaluation; Johns Hopkins University Press: Baltimore, MD, USA, 2000. [Google Scholar]
- Griffiths, R. Griffith Mental Development Scales—Extended Revised—GMDS-ER, 2006 Giunti; Organizzazioni Speciali: Firenze, Italy, 2006. [Google Scholar]
- Bell, E.F.; Hintz, S.R.; Hansen, N.I.; Bann, C.M.; Wyckoff, M.H.; DeMauro, S.B.; Walsh, M.C.; Vohr, B.R.; Stoll, B.J.; Carlo, W.A.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013–2018. JAMA 2022, 327, 248–263. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child. Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Pierrat, V.; Marchand-Martin, L.; Arnaud, C.; Kaminski, M.; Resche-Rigon, M.; Lebeaux, C.; Bodeau-Livinec, F.; Morgan, A.S.; Goffinet, F.; Marret, S.; et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 2017, 358, j3448. [Google Scholar] [CrossRef]
- Serenius, F.; Källén, K.; Blennow, M.; Ewald, U.; Fellman, V.; Holmström, G.; Lindberg, E.; Lundqvist, P.; Maršál, K.; Norman, M.; et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013, 309, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Roberts, G.; Anderson, P.J. Victorian Infant Collaborative Study Group. Outcomes at age 2 years of infants < 28 weeks' gestational age born in Victoria in 2005. J Pediatr. 2010, 156, 49–53.e1. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y. Neonatal Research Network of Japan. Neurodevelopmental outcomes of very low birth weight infants in the Neonatal Research Network of Japan: Importance of neonatal intensive care unit graduate follow-up. Clin. Exp. Pediatr. 2021, 64, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, J.E.; Hansen, N.I.; Bell, E.F.; Sridhar, A.; Carlo, W.A.; Hintz, S.R.; Vohr, B.R.; Colaizy, T.T.; Duncan, A.F.; Wyckoff, M.H.; et al. National Institute of Child Health and Human Development Neonatal Research Network. Outcomes of Extremely Preterm Infants With Birth Weight Less Than 400 g. JAMA Pediatr. 2019, 173, 434–445. [Google Scholar] [CrossRef]
- Longo, S.; Caporali, C.; Pisoni, C.; Borghesi, A.; Perotti, G.; Tritto, G.; Olivieri, I.; La Piana, R.; Tonduti, D.; Decio, A.; et al. Neurodevelopmental outcome of preterm very low birth weight infants admitted to an Italian tertiary center over an 11-year period. Sci. Rep. 2021, 11, 16316. [Google Scholar] [CrossRef]
- Olivieri, I.; Bova, S.M.; Urgesi, C.; Ariaudo, G.; Perotto, E.; Fazzi, E.; Stronati, M.; Fabbro, F.; Balottin, U.; Orcesi, S. Outcome of extremely low birth weight infants: What’s new in the third millennium? Neuropsychological profiles at four years. Early Hum. Dev. 2012, 88, 241–250. [Google Scholar] [CrossRef]
- Barnes-Davis, M.E.; Williamson, B.J.; Merhar, S.L.; Holland, S.K.; Kadis, D.S. Extremely preterm children exhibit altered cortical thickness in language areas. Sci. Rep. 2020, 10, 10824. [Google Scholar] [CrossRef]
- Barre, N.; Morgan, A.; Doyle, L.W.; Anderson, P.J. Language abilities in children who were very preterm and/or very low birth weight: A meta-analysis. J. Pediatr. 2011, 158, 766–774.e1. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Spencer-Smith, M.; Haebich, K.M.; Burnett, A.; Scratch, S.E.; Cheong, J.L.Y.; Doyle, L.W.; Wiley, J.F.; Anderson, P.J. Language Trajectories of Children Born Very Preterm and Full Term from Early to Late Childhood. J. Pediatr. 2018, 202, 86–91.e1. [Google Scholar] [CrossRef]
- Van Noort-van der Spek, I.L.; Franken, M.C.; Weisglas-Kuperus, N. Language functions in preterm-born children: A systematic review and meta-analysis. Pediatrics 2012, 129, 745–754. [Google Scholar] [CrossRef]
- Filippa, M.; Panza, C.; Ferrari, F.; Frassoldati, R.; Kuhn, P.; Balduzzi, S.; D’Amico, R. Systematic review of maternal voice interventions demonstrates increased stability in preterm infants. Acta Paediatr. 2017, 106, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Diggikar, S.; Gurumoorthy, P.; Trif, P.; Mudura, D.; Nagesh, N.K.; Galis, R.; Vinekar, A.; Kramer, B.W. Retinopathy of prematurity and neurodevelopmental outcomes in preterm infants: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1055813. [Google Scholar] [CrossRef] [PubMed]
- Wolke, D.; Söhne, B.; Ohrt, B.; Riegel, K. Follow-up of preterm children: Important to document dropouts. Lancet 1995, 345, 447. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Rossi, C.; Guidotti, I.; Gallo, C.; Della Casa, E.; Bertoncelli, N.; Coccolini, E.; Ferrari, F. Preterm birth and developmental problems in infancy and preschool age Part II: Cognitive, neuropsychological and behavioural outcomes. J. Matern. Fetal Neonatal Med. 2013, 26, 1653–1657. [Google Scholar] [CrossRef]








| Neurodevelopmental Outcome | n (%) |
|---|---|
| Normal | 173 (77.9) |
| Minor sequelae | 34 (15.3) |
| DQ between 76 and 87 | 10 (4.5) a |
| Clumsiness | 19 (8.6) |
| Major sequelae | 15 (6.8) |
| Disabling CP | 3 (1.4) |
| Non-disabling CP | 5 (2.3) |
| Severe visual impairment (peripheral or central b) | 5 (2.3) c |
| DQ < 70 without other impairments | 6 (2.7) |
| GA 22–25 Weeks | GA 26–29 Weeks | GA 30–33 Weeks | GA > 33 Weeks | p Value | |
|---|---|---|---|---|---|
| n = 19 | n = 114 | n = 80 | n = 9 | ||
| Normal, n (%) | 11 (57.9) | 84 (73.7) | 69 (86.3) | 9 (100.0) | 0.067 |
| Minor sequelae, n (%) | 5 (26.3) | 21 (18.4) | 8 (10.0) | 0 (0.0) | |
| Major sequelae, n (%) | 3 (15.8) | 9 (7.9) | 3 (3.8) | 0 (0.0) | |
| CP, n (%) | 1 (5.3) | 6 (5.3) | 0 (0.0) | 0 (0.0) | 0.188 |
| No CP, n (%) | 18 (94.7) | 108 (94.7) | 80 (100.0) | 9 (100.0) |
| All Infants | Normal Outcome | Minor Sequelae | Major Sequelae | p Value | |
|---|---|---|---|---|---|
| n = 222 | n = 173 | n = 34 | n = 15 | ||
| Maternal age, mean (SD), years | 34.3 (5.4) | 34.6 (5.4) | 33.7 (6.1) | 30.7 (3.2) | 0.082 |
| Multiple gestations, n (%) | 69.0 (31.1) | 56.0 (32.4) | 10.0 (29.4) | 3.0 (20.0) | 0.595 |
| Cesarian section, n (%) | 90 (40.6) | 71 (41.0) | 10 (29.4) | 9 (60.0) | 0.126 |
| Antenatal steroid therapy, n (%) | 202 (91.0) | 158 (91.3) | 31 (91.2) | 13 (86.7) | 0.788 |
| Magnesium sulphate, n (%) | 166 (74.8) | 123 (71.1) | 29 (85.3) | 14 (93.3) | 0.051 |
| Gestational age, mean (SD), weeks | 29.0 (2.6) | 29.3 (2.6) | 27.8 (2.3) | 27.6 (2.7) | <0.001 |
| Male, n (%) | 115 (51.8) | 87 (50.3) | 20 (58.8) | 8 (53.3) | 0.656 |
| Ethnicity, n (%) | 71 (32.0) | 44 (25.4) | 21 (61.8) | 6 (40.0) | 0.0001 |
| Birth weight, mean (SD), g | 1118.3 (264.5) | 1154.8 (248.4) | 984.8 (265.6) | 1000.1 (324.2) | 0.001 |
| AP1, mean (SD) | 5.8 (2.11) | 6.0 (2.1) | 5.7 (2.0) | 4.3 (2.1) | 0.009 |
| AP5, mean (SD) | 7.8 (1.6) | 8.0 (1.6) | 7.8 (1.5) | 6.9 (2.3) | 0.086 |
| Cardiac compressions, n (%) | 14 (6.3) | 10 (0.6) | 2 (5.9) | 2 (13.3) | 0.515 |
| Resuscitation, n (%) | 74 (33.3) | 50 (28.9) | 16 (47.1) | 8 (53.3) | 0.054 |
| Epinephrin, n (%) | 9 (4.1) | 6 (3.5) | 1 (2.9) | 2 (13.3) | 0.169 |
| Intubation at birth, n (%) | 76 (34.2) | 51 (29.5) | 17 (50.0) | 8 (53.3) | 0.021 |
| Mechanical Ventilation, n (%) | 87 (39.2) | 57 (32.9) | 19 (55.9) | 11 (73.3) | 0.0009 |
| nCPAP, n (%) | 167 (75.2) | 132 (76.3) | 24 (70.6) | 11 (73.3) | 0.731 |
| Surfactant, n (%) | 179 (80.6) | 84 (48.6) | 22 (64.7) | 11 (73.3) | 0.068 |
| Oxygen 28 days, n (%) | 102 (46.0) | 68.0 (39.3) | 24.0 (70.6) | 10 (66.7) | 0.002 |
| Bronchopulmonary dysplasia, n (%) | 48 (21.6) | 28 (16.2) | 14 (41.2) | 6 (40.0) | 0.001 |
| PDA, n (%) | 79 (35.6) | 54 (31.2) | 16 (47.1) | 9 (60.0) | 0.026 |
| PDA treated pharmacologically, n (%) | 52 (23.4) | 33 (19.1) | 15 (44.1) | 4 (26.7) | 0.007 |
| NEC, n (%) | 6 (2.7) | 4 (2.3) | 1 (2.9) | 1(6.7) | 0.605 |
| Early-onset sepsis, n (%) | 2 (0.9) | 1 (0.6) | 1 (2.9) | 0 (0) | 0.382 |
| Late-onset sepsis, n (%) | 35 (15.8) | 18 (10.4) | 10 (2.9) | 7 (46.7) | 0.0001 |
| ROP > grade 2, n (%) | 10 (4.5) | 6 (3.5) | 1 (2.9) | 3 (20.0) | 0.0004 |
| ROP surgery, n (%) | 13 (5.9) | 8 (4.6) | 2 (5.9) | 3 (20.0) | 0.052 |
| PIH, n (%) | 51 (23.0) | 33 (19.1) | 8 (23.5) | 10 (66.7) | 0.0001 |
| PVL >1, n (%) | 7 (3.2) | 3 (1.7) | 1 (2.9) | 3 (20.0) | 0.0005 |
| Human milk discharge, n (%) | 154 (69.4) | 125 (72.3) | 23 (67.6) | 6 (40.0) | 0.033 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p Value | OR | CI 95% | p Value | |
| Maternal age | 0.9 | 0.8–1.0 | 0.105 | |||
| Maternal education | 3.6 | 0.4–30.4 | 0.235 | |||
| Multiple gestations | 0.5 | 0.1–2.0 | 0.319 | |||
| Cesarean section | 2.3 | 0.8–6.7 | 0.123 | |||
| Antenatal steroid therapy | 0.6 | 0.1–2.8 | 0.525 | |||
| Magnesium sulphate | 5.1 | 0.7–39.4 | 0.053 | |||
| Gestational age | 0.8 | 0.6–1.0 | 0.027 | |||
| Male | 1.1 | 0.4–3.1 | 0.902 | |||
| Ethnicity | 1.5 | 0.5–4.3 | 0.498 | |||
| Birth weight | 1.0 | 1.0–1.0 | 0.075 | |||
| AP1 | 0.7 | 0.6–0.9 | 0.006 | |||
| AP5 | 0.7 | 0.6–1.0 | 0.027 | |||
| Cardiac compressions in delivery room | 2.5 | 0.5–12.3 | 0.305 | |||
| Any resuscitation in delivery room | 5.3 | 0.6–44.1 | 0.062 | |||
| Epinephrin in delivery room | 4.4 | 0.8–23.2 | 0.125 | |||
| Intubation in delivery room | 2.3 | 0.8–6.7 | 0.119 | |||
| Mechanical Ventilation | 4.7 | 1.4–15.3 | 0.006 | |||
| nCPAP | 0.9 | 0.3–2.9 | 0.836 | |||
| Surfactant | 2.6 | 0.8–8.3 | 0.098 | |||
| Oxygen 28 days | 2.7 | 0.8–9.1 | 0.081 | |||
| Bronchopulmonary dysplasia 36 w | 2.7 | 0.9–8.2 | 0.091 | |||
| PDA | 2.9 | 1.0–8.6 | 0.046 | |||
| PDA treated pharmacologically | 1.2 | 0.4–4.0 | 0.762 | |||
| NEC | 2.9 | 0.3–26.4 | 0.400 | |||
| Early-onset sepsis | 0.0 | 0.0–0.0 | 0.596 | |||
| Late-onset sepsis | 5.6 | 1.9–16.6 | 0.003 | |||
| ROP > grade 2 | 2.0 | 1.3–3.0 | 0.004 | 1.8 | 1.1–2.8 | 0.016 |
| ROP surgery | 4.9 | 1.2–20.3 | 0.048 | |||
| PIH | 8.1 | 2.6–25.0 | <0.001 | 5.6 | 1.7–18.4 | 0.004 |
| Human milk discharge | 0.3 | 0.1–0.8 | 0.015 | |||
| Outborn | 3.0 | 0.6–15.0 | 0.170 | |||
| All Infants | Normal Outcome | Minor Sequelae | Major Sequelae | p Value | |
|---|---|---|---|---|---|
| n = 221 | n = 173 | n = 34 | n = 15 | ||
| Any lesion | 143 (64.4) | 105 (60.7) | 24 (70.6) | 14 (93.3) | 0.032 |
| Abnormal myelin | 56 (25.2) | 43 (25.9) | 8 (23.5) | 5 (33.3) | 0.750 |
| Cerebellar hemorrhage >5 mm | 4 (1.8) | 1 (0.6) | 0 (0) | 3 (20.0) | <0.0001 |
| Cerebellar hemorrhage < 5 mm | 9 (4.1) | 6 (3.5) | 1 (2.9) | 2 (13.3) | 0.169 |
| GMH-IVH | 54 (24.3) | 34 (19.7) | 10 (29.4) | 10 (66.7) | 0.0002 |
| HPI | 6 (2.7) | 1 (0.6) | 2 (5.9) | 3 (20.0) | <0.0001 |
| White matter punctate lesions | 11 (5.0) | 9 (5.2) | 1 (2.9) | 1 (6.7) | 0.814 |
| Subependymal cyst | 17 (7.7) | 13 (7.5) | 3 (8.8) | 1 (6.7) | 0.957 |
| Thin corpus callosum | 9 (4.1) | 5 (2.9) | 3 (8.8) | 1 (6.7) | 0.244 |
| Ventricular dilatation | 76 (34.2) | 53 (30.6) | 13 (38.2) | 10 (66.7) | 0.017 |
| PVL | 5 (2.3) | 2 (1.2) | 1 (2.9) | 2 (13.3) | 0.009 |
| Major unclassified | 7 (3.2) | 2 (1.2) | 1 (2.9) | 4 (26.7) | <0.0001 |
| Minor unclassified | 4 (1.8) | 3 (1.7) | 0 (0) | 1 (6.7) | 0.248 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugli, L.; Pugliese, M.; Bertoncelli, N.; Bedetti, L.; Agnini, C.; Guidotti, I.; Roversi, M.F.; Della Casa, E.M.; Cavalleri, F.; Todeschini, A.; et al. Neurodevelopmental Outcome and Neuroimaging of Very Low Birth Weight Infants from an Italian NICU Adopting the Family-Centered Care Model. Children 2024, 11, 12. https://doi.org/10.3390/children11010012
Lugli L, Pugliese M, Bertoncelli N, Bedetti L, Agnini C, Guidotti I, Roversi MF, Della Casa EM, Cavalleri F, Todeschini A, et al. Neurodevelopmental Outcome and Neuroimaging of Very Low Birth Weight Infants from an Italian NICU Adopting the Family-Centered Care Model. Children. 2024; 11(1):12. https://doi.org/10.3390/children11010012
Chicago/Turabian StyleLugli, Licia, Marisa Pugliese, Natascia Bertoncelli, Luca Bedetti, Cristina Agnini, Isotta Guidotti, Maria Federica Roversi, Elisa Muttini Della Casa, Francesca Cavalleri, Alessandra Todeschini, and et al. 2024. "Neurodevelopmental Outcome and Neuroimaging of Very Low Birth Weight Infants from an Italian NICU Adopting the Family-Centered Care Model" Children 11, no. 1: 12. https://doi.org/10.3390/children11010012
APA StyleLugli, L., Pugliese, M., Bertoncelli, N., Bedetti, L., Agnini, C., Guidotti, I., Roversi, M. F., Della Casa, E. M., Cavalleri, F., Todeschini, A., Di Caprio, A., Zini, T., Corso, L., Miselli, F., Ferrari, F., & Berardi, A. (2024). Neurodevelopmental Outcome and Neuroimaging of Very Low Birth Weight Infants from an Italian NICU Adopting the Family-Centered Care Model. Children, 11(1), 12. https://doi.org/10.3390/children11010012






