Clinical Diagnosis and Management of Fetal Alcohol Spectrum Disorder and Sensory Processing Disorder in Children
Abstract
1. Introduction
2. Biochemical Mechanisms That Influence FASD
3. Diagnostic Criteria of Fetal Alcohol Spectrum Disorders
3.1. Fetal Alcohol Spectrum Disorders Diagnostic Criteria
3.2. Prenatal Alcohol Exposure
3.3. Paternal Influences on FASD
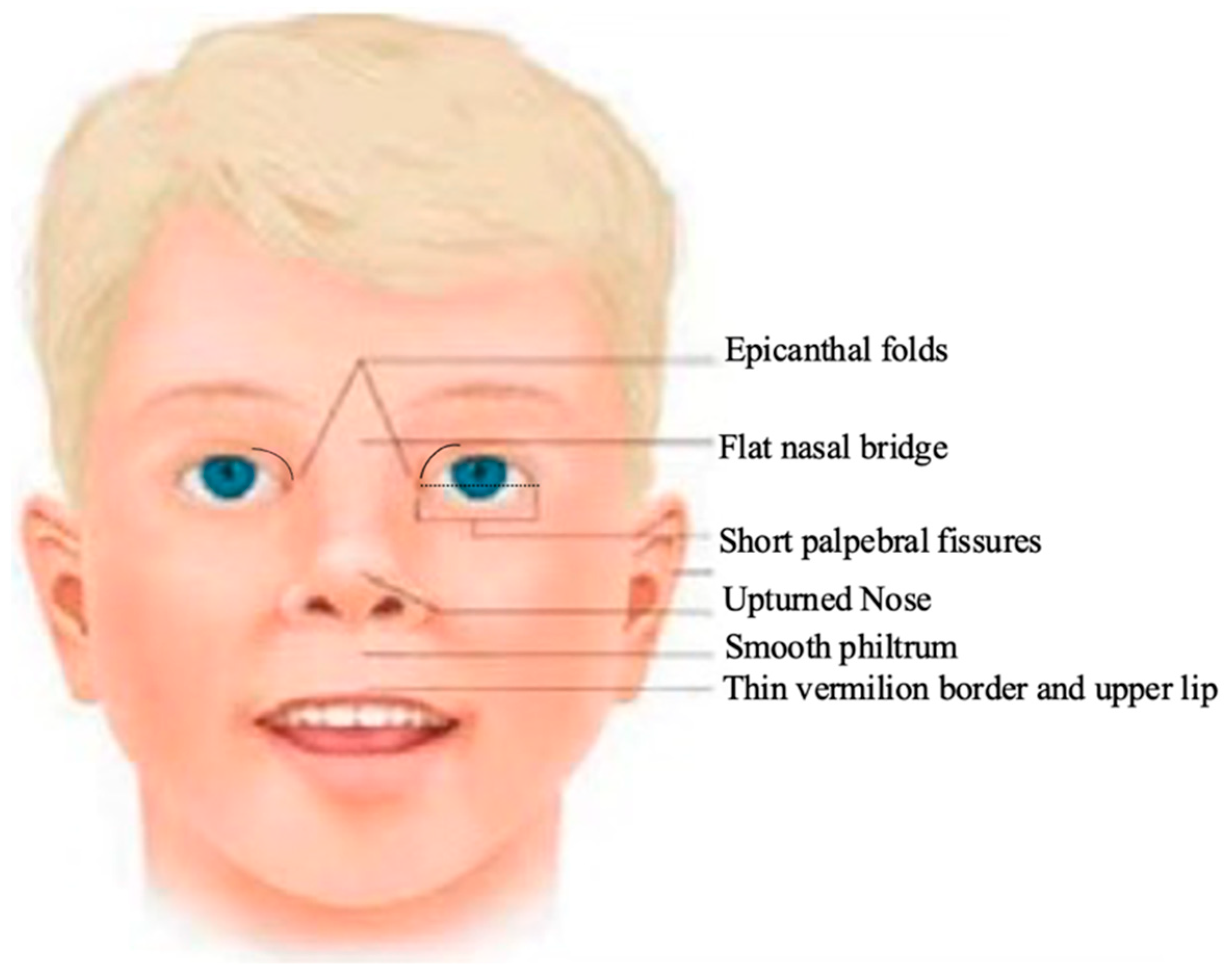
3.4. Facial Dysmorphology
3.5. Growth Deficiency
3.6. Central Nervous System Dysfunctions in Fetal Alcohol Spectrum Disorders
4. Fetal Alcohol Disorders
4.1. Fetal Alcohol Syndrome
4.2. Partial Fetal Alcohol Syndrome
4.3. Alcohol Related Neurodevelopmental Disorder
4.4. Alcohol Related Birth Defects
5. Sensory Processing Disorders
5.1. Sensory Processing Disorder
5.2. Sensory Process Disorder Subtypes
6. Correlative Relationship between Fetal Alcohol Spectrum Disorder and Sensory Processing Disorders
7. Therapeutic Management Considerations
7.1. Therapy
7.2. Therapeutic Interventions
7.2.1. Sleep-Based Therapies
7.2.2. Sensory Integration
7.2.3. Cognitive Therapies
7.3. Therapy Intervention Benefits
7.4. Intervention Effectiveness
7.5. Complexity of Interventional Choices for the Practitioner
8. Limitations
9. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Cook, J.L.; Green, C.R.; Lilley, C.M.; Anderson, S.M.; Baldwin, M.E.; Chudley, A.E.; Conry, J.L.; LeBlanc, N.; Loock, C.A.; Lutke, J.; et al. Fetal alcohol spectrum disorder: A guideline for diagnosis across the lifespan. CMAJ 2016, 188, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Dörrie, N.; Föcker, M.; Freunscht, I.; Hebebrand, J. Fetal alcohol spectrum disorders. Eur. Child. Adolesc. Psychiatry 2014, 23, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers 2023, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 2018, 319, 474–483. [Google Scholar] [CrossRef]
- Wang, R.; Martin, C.; Lei, A.; Hausknecht, K.; Turk, M.; Micov, V.; Kwarteng, F.; Ishiwari, K.; Oubraim, S.; Wang, A.; et al. Prenatal ethanol exposure impairs sensory processing and habituation to visual stimuli, effects normalized by enrichment of postnatal environment. Alcohol. Clin. Exp. Res. 2022, 45, 891–906. [Google Scholar] [CrossRef]
- Franklin, L.; Deitz, J.; Jirikowic, T.; Astley, S. Children with fetal alcohol spectrum disorders: Problem behaviors and sensory processing. Am. J. Occup. Ther. 2008, 62, 265–273. [Google Scholar] [CrossRef]
- Miller, L.J.; Nielsen, D.M.; Schoen, S.A.; Brett-Green, B.A. Perspectives on sensory processing disorder: A call for translational research. Front. Integr. Neurosci. 2009, 3, 597. [Google Scholar] [CrossRef] [PubMed]
- Kalberg, W.O.; Buckley, D. FASD: What types of intervention and rehabilitation are useful? Neurosci. Biobehav. Rev. 2007, 31, 278–285. [Google Scholar] [CrossRef]
- Senturias, Y.; Burns, B. Managing children and adolescents with fetal alcohol spectrum disorders in the medical home. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 96–101. [Google Scholar] [CrossRef]
- Fjeldsted, B.; Xue, L. Sensory Processing in Young Children with Fetal Alcohol Spectrum Disorder. Phys. Occup. Ther. Pediatr. 2019, 39, 553–565. [Google Scholar] [CrossRef]
- Ahmed-Landeryou, M.J. Fetal central nervous system development and alcohol—The evidence so far. Fetal Pediatr. Pathol. 2012, 31, 349–359. [Google Scholar] [CrossRef]
- Dou, X.; Menkari, C.E.; Shanmugasundararaj, S.; Miller, K.W.; Charness, M.E. Two alcohol binding residues interact across a domain interface of the L1 neural cell adhesion molecule and regulate cell adhesion. J. Biol. Chem. 2011, 286, 16131–16139. [Google Scholar] [CrossRef]
- Dou, X.; Wilkemeyer, M.F.; Menkari, C.E.; Parnell, S.E.; Sulik, K.K.; Charness, M.E. Mitogen-activated protein kinase modulates ethanol inhibition of cell adhesion mediated by the L1 neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA 2013, 110, 5683–5688. [Google Scholar] [CrossRef] [PubMed]
- Licheri, V.; Brigman, J.L. Altering Cell-Cell Interaction in Prenatal Alcohol Exposure Models: Insight on Cell-Adhesion Molecules During Brain Development. Front. Mol. Neurosci. 2021, 14, 753537. [Google Scholar] [CrossRef] [PubMed]
- Laufer, B.I.; Kapalanga, J.; Castellani, C.A.; Diehl, E.J.; Yan, L.; Singh, S.M. Associative DNA methylation changes in children with prenatal alcohol exposure. Epigenomics 2015, 7, 1259–1274. [Google Scholar] [CrossRef]
- Mishra, N.K.; Shrinath, P.; Rao, R.; Shukla, P.K. Sex-Specific Whole-Transcriptome Analysis in the Cerebral Cortex of FAE Offspring. Cells 2023, 12, 328. [Google Scholar] [CrossRef]
- Fiore, M.; Petrella, C.; Coriale, G.; Rosso, P.; Fico, E.; Ralli, M.; Greco, A.; De Vincentiis, M.; Minni, A.; Polimeni, A.; et al. Markers of Neuroinflammation in the Serum of Prepubertal Children with Fetal Alcohol Spectrum Disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Riley, E.P.; Charness, M.E. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 2019, 18, 760–770. [Google Scholar] [CrossRef]
- Wilhoit, L.F.; Scott, D.A.; Simecka, B.A. Fetal Alcohol Spectrum Disorders: Characteristics, Complications, and Treatment. Community Ment. Health J. 2017, 53, 711–718. [Google Scholar] [CrossRef]
- Brown, J.M.; Bland, R.; Jonsson, E.; Greenshaw, A.J. The Standardization of Diagnostic Criteria for Fetal Alcohol Spectrum Disorder [FASD]: Implications for Research, Clinical Practice and Population Health. Can. J. Psychiatry 2019, 64, 169–176. [Google Scholar] [CrossRef]
- Greenmyer, J.R.; Klug, M.G.; Nkodia, G.; Popova, S.; Hart, B.; Burd, L. High prevalence of prenatal alcohol exposure detected by breathalyzer in the Republic of the Congo, Africa. Neurotoxicol. Teratol. 2020, 80, 106892. [Google Scholar] [CrossRef]
- Umer, A.; Lilly, C.; Hamilton, C.; Baldwin, A.; Breyel, J.; Tolliver, A.; Mullins, C.; John, C.; Maxwell, S. Prevalence of alcohol use in late pregnancy. Pediatr. Res. 2020, 88, 312–319. [Google Scholar] [CrossRef]
- Bager, H.; Christensen, L.P.; Husby, S.; Bjerregaard, L. Biomarkers for the Detection of Prenatal Alcohol Exposure: A Review. Alcohol. Clin. Exp. Res. 2017, 41, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Song, L.; Chen, J.; Wang, Q.; Shen, H.; Zhang, S.; Li, X. Association of Preconception Paternal Alcohol Consumption with Increased Fetal Birth Defect Risk. JAMA Pediatr. 2021, 175, 742–743. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.N.; Srikanth, N.; Bhadsavle, S.S.; Thomas, K.R.; Zimmel, K.N.; Basel, A.; Roach, A.N.; Mehta, N.A.; Bedi, Y.S.; Golding, M.C. Preconception paternal ethanol exposures induce alcohol-related craniofacial growth deficiencies in fetal offspring. J. Clin. Investig. 2023, 133, e167624. [Google Scholar] [CrossRef]
- Chang, R.C.; Wang, H.; Bedi, Y.; Golding, M.C. Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics Chromatin 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.N.; Zimmel, K.N.; Basel, A.; Roach, A.N.; Mehta, N.A.; Thomas, K.R.; Dotson, L.J.; Bedi, Y.S.; Golding, M.C. Paternal alcohol exposures program intergenerational hormetic effects on offspring fetoplacental growth. Front. Cell Dev. Biol. 2022, 10, 930375. [Google Scholar] [CrossRef]
- Denny, L.; Coles, S.; Blitz, R. Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Am. Fam. Physician 2017, 96, 515–522. [Google Scholar]
- Lovely, C.B. Animal Models of Gene-Alcohol Interactions. Birth. Defects. Res. 2020, 112, 367–379. [Google Scholar] [CrossRef]
- Bradley, R.; Lakpa, K.L.; Burd, M.; Mehta, S.; Katusic, M.Z.; Greenmyer, J.R. Fetal Alcohol Spectrum Disorder and Iron Homeostasis. Nutrients 2022, 14, 4223. [Google Scholar] [CrossRef]
- Huebner, S.M.; Blohowiak, S.E.; Kling, P.J.; Smith, S.M. Prenatal Alcohol Exposure Alters Fetal Iron Distribution and Elevates Hepatic Hepcidin in a Rat Model of Fetal Alcohol Spectrum Disorders. J. Nutr. 2016, 146, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.; Morse, B.A. Intervention and the Child with FAS. Alcohol. Health Res. World 1994, 18, 67–72. [Google Scholar]
- Warren, K.R.; Foudin, L.L. Alcohol-related birth defects—The past, present, and future. Alcohol. Res. Health 2001, 25, 153–158. [Google Scholar] [PubMed]
- Carr, J.L.; Agnihotri, S.; Keightley, M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol. Clin. Exp. Res. 2010, 34, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Jirikowic, T.L.; Thorne, J.C.; McLaughlin, S.A.; Waddington, T.; Lee, A.K.C.; Astley Hemingway, S.J. Prevalence and patterns of sensory processing behaviors in a large clinical sample of children with prenatal alcohol exposure. Res. Dev. Disabil. 2020, 100, 103617. [Google Scholar] [CrossRef]
- Wengel, T.; Hanlon-Dearman, A.C.; Fjeldsted, B. Sleep and sensory characteristics in young children with fetal alcohol spectrum disorder. J. Dev. Behav. Pediatr. 2011, 32, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.M.; Carpenter, H.A.; Marsh, A.M.; McClung, K.A.; Doll, J.D. The Knowledge of Rehabilitation Professionals Concerning Fetal Alcohol Spectrum Disorders. Occup. Ther. Health Care 2016, 30, 69–79. [Google Scholar] [CrossRef]
- Polatajko, H.J.; Cantin, N. Exploring the effectiveness of occupational therapy interventions, other than the sensory integration approach, with children and adolescents experiencing difficulty processing and integrating sensory information. Am. J. Occup. Ther. 2010, 64, 415–429. [Google Scholar] [CrossRef]
- Peadon, E.; Rhys-Jones, B.; Bower, C.; Elliott, E.J. Systematic review of interventions for children with Fetal Alcohol Spectrum Disorders. BMC Pediatr. 2009, 9, 35. [Google Scholar] [CrossRef]
- Riley, E.P.; Mattson, S.N.; Li, T.K.; Jacobson, S.W.; Coles, C.D.; Kodituwakku, P.W.; Adnams, C.M.; Korkman, M.I. Neurobehavioral consequences of prenatal alcohol exposure: An international perspective. Alcohol. Clin. Exp. Res. 2003, 27, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Ordenewitz, L.K.; Weinmann, T.; Schlüter, J.A.; Moder, J.E.; Jung, J.; Kerber, K.; Greif-Kohistani, N.; Heinen, F.; Landgraf, M.N. Evidence-based interventions for children and adolescents with fetal alcohol spectrum disorders—A systematic review. Eur. J. Paediatr. Neurol. 2021, 33, 50–60. [Google Scholar] [CrossRef] [PubMed]


| Intervention Method | Outcomes |
|---|---|
| Sleep-based Therapies | Improves sleeping |
| Sensory Integration | Improves cognition and social abilities. Especially useful for sensory oversensitivity |
| Cognitive Therapies | Improves performance, participation, and self-regulation. Especially useful for motor coordination |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breuer, L.; Greenmyer, J.R.; Wilson, T. Clinical Diagnosis and Management of Fetal Alcohol Spectrum Disorder and Sensory Processing Disorder in Children. Children 2024, 11, 108. https://doi.org/10.3390/children11010108
Breuer L, Greenmyer JR, Wilson T. Clinical Diagnosis and Management of Fetal Alcohol Spectrum Disorder and Sensory Processing Disorder in Children. Children. 2024; 11(1):108. https://doi.org/10.3390/children11010108
Chicago/Turabian StyleBreuer, Lorel, Jacob R. Greenmyer, and Ted Wilson. 2024. "Clinical Diagnosis and Management of Fetal Alcohol Spectrum Disorder and Sensory Processing Disorder in Children" Children 11, no. 1: 108. https://doi.org/10.3390/children11010108
APA StyleBreuer, L., Greenmyer, J. R., & Wilson, T. (2024). Clinical Diagnosis and Management of Fetal Alcohol Spectrum Disorder and Sensory Processing Disorder in Children. Children, 11(1), 108. https://doi.org/10.3390/children11010108





