Clinical and Microbiological Characteristics of Deep Neck Abscesses in Pediatrics: Analysis of a Case Series from a 3rd Level Pediatric Hospital
Abstract
:1. Introduction
2. Materials and Methods
3. Results
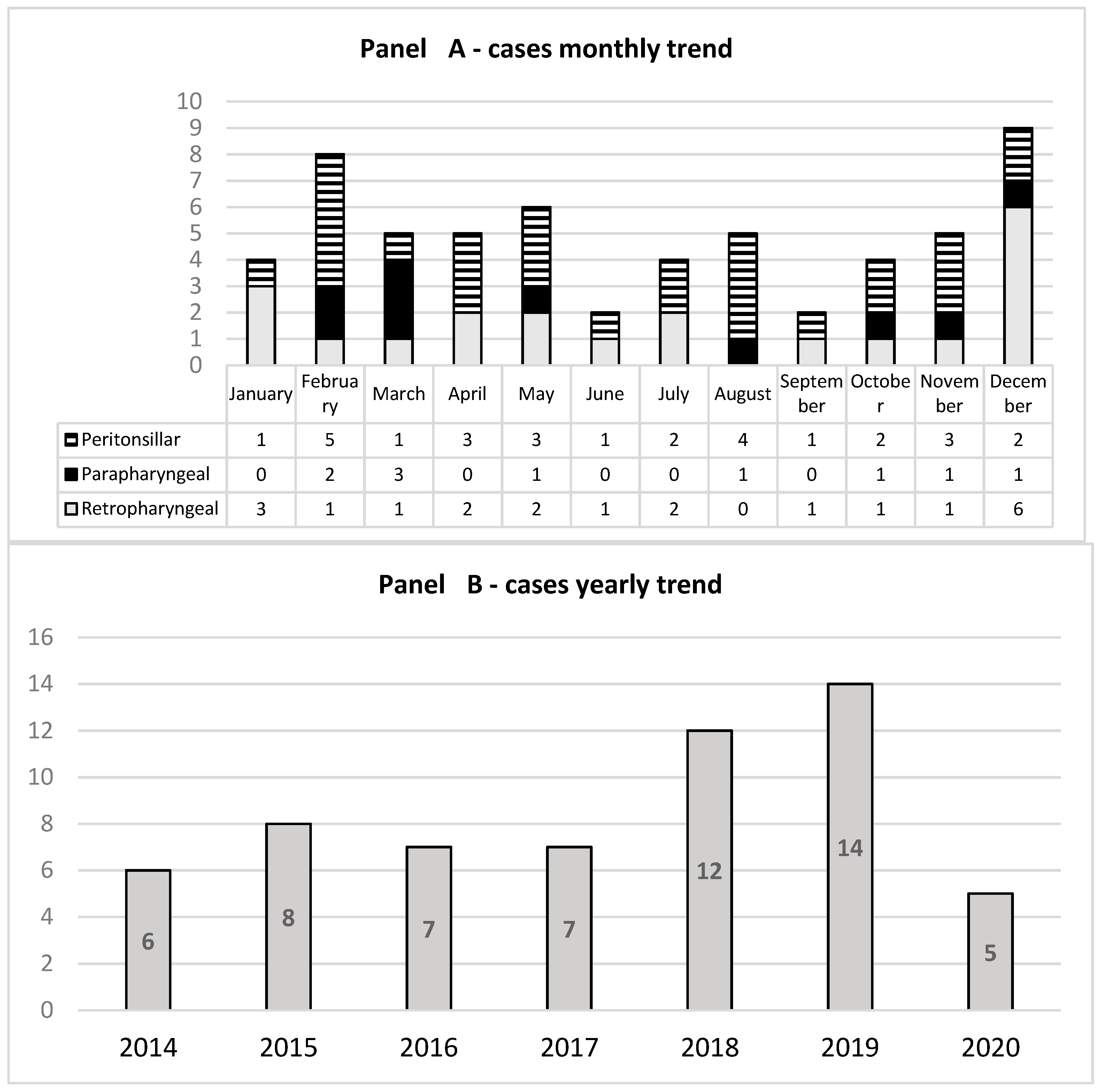
3.1. Clinical and Laboratory Features
3.2. Microbiological Features
3.3. Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wald, R.; Wald, E.R.; Guerra, N.; Byers, C. Upper Respiratory Tract Infections in Young Children: Duration of and Frequency of Complications. Pediatrics 1991, 87, 129–133. [Google Scholar] [PubMed]
- Jain, A.; Singh, I.; Meher, R.; Raj, A.; Rajpurohit, P.; Prasad, P. Deep Neck Space Abscesses in Children below 5 Years of Age and Their Complications. Int. J. Pediatr. Otorhinolaryngol. 2018, 109, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.; Allen, S.M.; Stocks, R.M.S.; Thompson, J.W. Deep Neck Infection. Otolaryngol. Clin. N. Am. 2008, 41, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, C.; Shah, R.K. Pediatric Peritonsillar Abscess: An Overview. Infect. Disord. Drug Targets 2012, 12, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, C.M.; Howell, R.; Amorn, M.; Budacki, R.; Choi, S.; Pena, M. Complications in Pediatric Deep Neck Space Abscesses. Otolaryngol.—Head Neck Surg. 2011, 144, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Donà, D.; Gastaldi, A.; Campagna, M.; Montagnani, C.; Galli, L.; Trapani, S.; Pierossi, N.; De Luca, M.; D’Argenio, P.; Tucci, F.M.; et al. Deep Neck Abscesses in Children: An Italian Retrospective Study. Pediatr. Emerg. Care 2021, 37, e1358. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; De Guido, C.; Pappalardo, M.; Laudisio, S.; Meccariello, G.; Capoferri, G.; Rahman, S.; Vicini, C.; Principi, N. Retropharyngeal, Parapharyngeal and Peritonsillar Abscesses. Children 2022, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Mayor, G.P.; Millán, J.M.S.; Martínez-Vidal, A. Is Conservative Treatment of Deep Neck Space Infections Appropriate? Head Neck 2001, 23, 126–133. [Google Scholar] [CrossRef]
- Tansey, J.B.; Hamblin, J.; Mamidala, M.; Thompson, J.; Mclevy, J.; Wood, J.; Sheyn, A. Dexamethasone Use in the Treatment of Pediatric Deep Neck Space Infections. Ann. Otol. Rhinol. Laryngol. 2020, 129, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Kirse, D.J.; Roberson, D.W. Surgical Management of Retropharyngeal Space Infections in Children. Laryngoscope 2001, 111, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Pong, A.L.; Bradley, J.S. Guidelines for the Selection of Antibacterial Therapy in Children. Pediatr. Clin. N. Am. 2005, 52, 869–894. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Pierrot, S.; Contencin, P.; Morisseau-Durand, M.P.; Manach, Y.; Couloigner, V. Retropharyngeal Infections in Children. Treatment Strategies and Outcomes. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Betsch, A.; Wiskirchen, J.; Trübenbach, J.; Manncke, K.H.; Belka, C.; Claussen, C.D.; Duda, S.H. CT-Guided Percutaneous Drainage of Intra-Abdominal Abscesses: APACHE III Score Stratification of 1-Year Results. Eur. Radiol. 2002, 12, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper Respiratory Tract Infections. Indian J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Lamagni, T.L.; Darenberg, J.; Luca-Harari, B.; Siljander, T.; Efstratiou, A.; Henriques-Normark, B.; Vuopio-Varkila, J.; Bouvet, A.; Creti, R.; Ekelund, K.; et al. Epidemiology of Severe Streptococcus Pyogenes Disease in Europe. J. Clin. Microbiol. 2008, 46, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Page, C.; Biet, A.; Zaatar, R.; Strunski, V. Parapharyngeal Abscess: Diagnosis and Treatment. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.H.; Davis, B.; Clemans-Taylor, B.L.; Littenberg, B.; Estrada, C.A.; Centor, R.M. Rapid Antigen Group A Streptococcus Test to Diagnose Pharyngitis: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e111727. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, N.; Quezada, M.; Asmar, B.I. Retropharyngeal Abscess in Children: The Rising Incidence of Methicillin-Resistant Staphylococcus Aureus. Pediatr. Infect. Dis. J. 2012, 31, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.K.C.; Brown, C.; Mills, N.; Spielmann, P.; Neeff, M. To Drain or Not to Drain—Management of Pediatric Deep Neck Abscesses: A Case-Control Study. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, S.C.; Porter, J.J.; Monuteaux, M.C.; Watters, K.; Hudgins, J.D. Variation in the Management of Children With Deep Neck Infections. Hosp. Pediatr. 2021, 11, 277–283. [Google Scholar] [CrossRef] [PubMed]
| Parapharyngeal | Peritonsillar | Retropharyngeal | Total | p | ||
|---|---|---|---|---|---|---|
| Age (years) | Median | 6.38 | 10.7 | 3.05 | 0.01 | |
| 1° Q | 5.06 | 6.53 | 2 | |||
| 3° Q | 8.92 | 13.7 | 9.69 | |||
| Sex | M | 7 | 19 | 10 | 36 | 0.29 |
| F | 3 | 9 | 11 | 23 | ||
| Total | 10 | 28 | 21 | 59 | ||
| Clinical presentation | Trismus | 8 | 21 | 15 | 44 | 0.897 |
| No ENT symptoms | 1 | 4 | 2 | 7 | ||
| Total | 9 | 25 | 17 | 51 | ||
| Fever | 8 | 25 | 16 | 49 | 0.463 | |
| Apyrexia | 2 | 3 | 5 | 10 | ||
| Total | 10 | 28 | 21 | 59 | ||
| CRP (mg/dL) | Mean | 13.3 | 6.85 | 12.8 | 0.05 | |
| IC 95% | 8–18 | 3–10 | 9–16 | |||
| WBC (/mmc) | Median | 21,000 | 17,000 | 18,000 | 0.160 | |
| 1st Q | 20,000 | 14,000 | 16,000 | |||
| 3rd Q | 26,000 | 21,000 | 26,000 |
| Drainage Culture Pathogen | Parapharyngeal | Peritonsillar | Retropharyngeal | Total |
|---|---|---|---|---|
| Streptococcus mitis | 1 | 4 | 2 | 7 |
| Methicillin-sensitive Staphylococcus aureus (MSSA) | 5 | 5 | ||
| Streptococcus pyogenes | 5 | 5 | ||
| Streptococcus salivarius | 1 | 2 | 1 | 4 |
| Streptococcu sanginosus | 2 | 2 | ||
| Streptococcus parasanguinis | 1 | 1 | 2 | |
| Streptococcus vestibularis | 2 | 2 | ||
| Methicillin-sensitive Staphylococcus epidermidis (MSSE) | 1 | 1 | ||
| Streptococcus oralis | 1 | 1 | ||
| Streptococcus viridans | 1 | 1 | ||
| Negative culture | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, M.; Saffioti, C.; Mesini, A.; Palmero, C.; D’Agostino, R.; Garofolo, S.; Rossi, A.; Damasio, M.B.; Castagnola, E. Clinical and Microbiological Characteristics of Deep Neck Abscesses in Pediatrics: Analysis of a Case Series from a 3rd Level Pediatric Hospital. Children 2023, 10, 1506. https://doi.org/10.3390/children10091506
Mariani M, Saffioti C, Mesini A, Palmero C, D’Agostino R, Garofolo S, Rossi A, Damasio MB, Castagnola E. Clinical and Microbiological Characteristics of Deep Neck Abscesses in Pediatrics: Analysis of a Case Series from a 3rd Level Pediatric Hospital. Children. 2023; 10(9):1506. https://doi.org/10.3390/children10091506
Chicago/Turabian StyleMariani, Marcello, Carolina Saffioti, Alessio Mesini, Candida Palmero, Roberto D’Agostino, Sabrina Garofolo, Andrea Rossi, Maria Beatrice Damasio, and Elio Castagnola. 2023. "Clinical and Microbiological Characteristics of Deep Neck Abscesses in Pediatrics: Analysis of a Case Series from a 3rd Level Pediatric Hospital" Children 10, no. 9: 1506. https://doi.org/10.3390/children10091506
APA StyleMariani, M., Saffioti, C., Mesini, A., Palmero, C., D’Agostino, R., Garofolo, S., Rossi, A., Damasio, M. B., & Castagnola, E. (2023). Clinical and Microbiological Characteristics of Deep Neck Abscesses in Pediatrics: Analysis of a Case Series from a 3rd Level Pediatric Hospital. Children, 10(9), 1506. https://doi.org/10.3390/children10091506






