Ultrasonographic Evaluation of Gastric Content and Volume in Pediatric Patients Undergoing Elective Surgery: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Framework
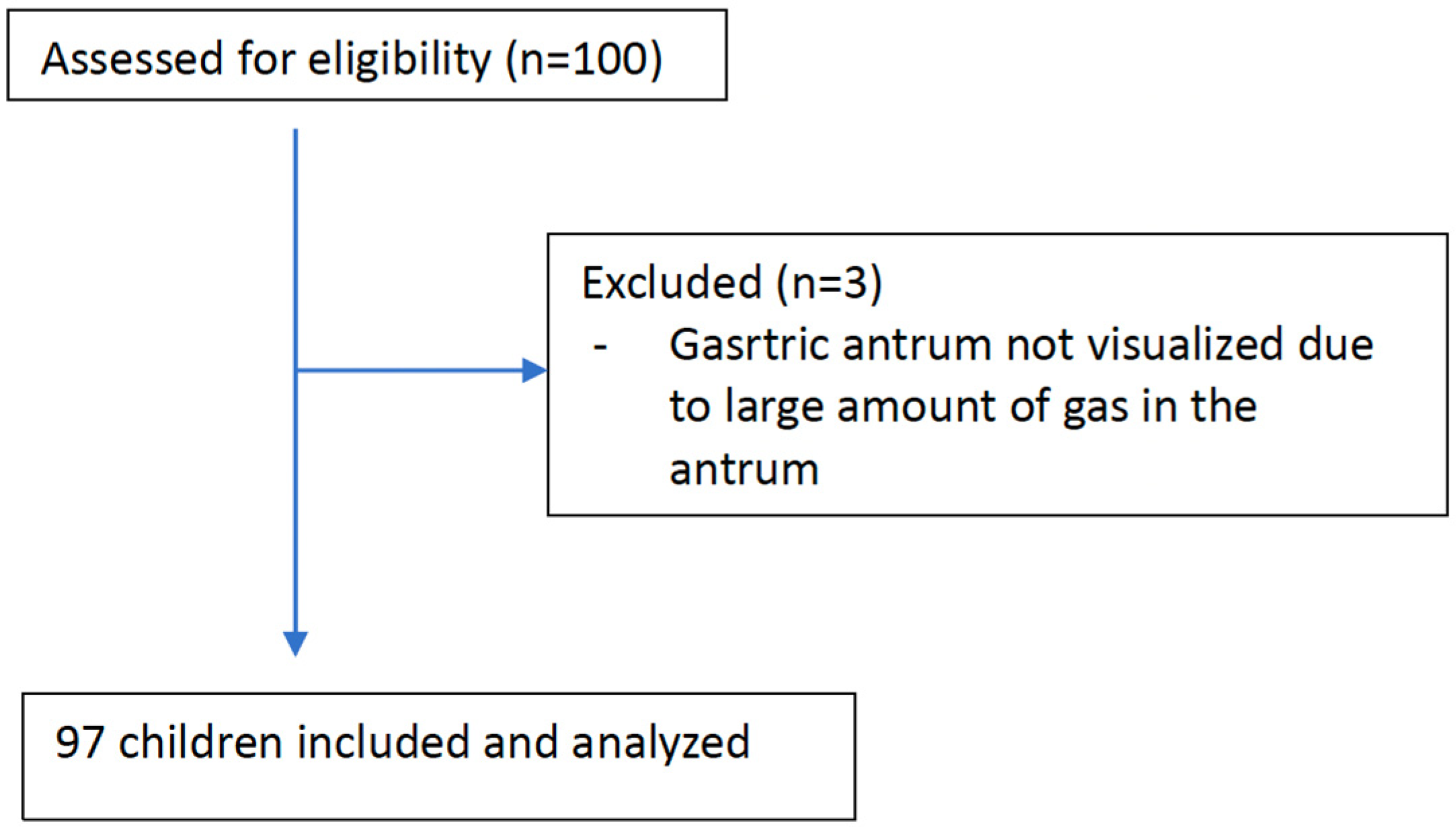
2.2. Participant Selection
2.3. Gastric Antrum Ultrasound Examination
2.4. Sample Size and Statistical Assessment
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouvet, L.; Bellier, N.; Gagey-Riegel, A.-C.; Desgranges, F.-P.; Chassard, D.; Siqueira, M.D.Q. Ultrasound assessment of the prevalence of increased gastric contents and volume in elective pediatric patients: A prospective cohort study. Pediatr. Anesth. 2018, 28, 906–913. [Google Scholar] [CrossRef]
- Borland, L.M.; Sereika, S.M.; Woelfel, S.K.; Saitz, E.W.; Carrillo, P.A.; Lupin, J.L.; Motoyama, E.K. Pulmonary aspiration in pediatric patients during general anesthesia: Incidence and outcome. J. Clin. Anesth. 1998, 10, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Lee, S.Y. Pulmonary aspiration under GA: A 13-year audit in a tertiary pediatric unit. Pediatr. Anesth. 2016, 26, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.W. Pulmonary aspiration in pediatric anesthetic practice in the UK: A prospective survey of specialist pediatric centers over a one-year period. Pediatr. Anesth. 2013, 23, 702–711. [Google Scholar] [CrossRef]
- Cook, T.M.; Woodall, N.; Frerk, C.; Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1 Anaesth. 2015, 106, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M.; Frerk, C. Chapter 19: Aspiration of gastric contents and of blood. In Fourth National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society; Cook, T.M., Woodall, N., Frerk, C., Eds.; Major Complications of Airway Management in the United Kingdom; Report and Findings; Royal College of Anaesthetists: London, UK, 2011; pp. 155–164. [Google Scholar]
- Warner, M.A.; Warner, M.E.; Warner, D.O.; Warner, L.O.; Warner, E.J. Perioperative Pulmonary Aspiration in Infants and Children. Surv. Anesthesiol. 1999, 43, 334. [Google Scholar] [CrossRef]
- Tiret, L.; Nivoche, Y.; Hatton, F.; Desmonts, J.M.; Vourc’H, G. Complications related to anaesthesia in infants and children. Br. J. Anaesth. 1988, 61, 263–269. [Google Scholar] [CrossRef]
- Habre, W.; Disma, N.; Virág, K.; Becke, K.; Hansen, T.G.; Jöhr, M.; Leva, B.; Morton, N.S.; Vermeulen, P.M.; Zielinska, M.; et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): A prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir. Med. 2017, 5, 412–425. [Google Scholar] [CrossRef]
- Engelhardt, T.; Webster, N.R. Pulmonary aspiration of gastric contents in anaesthesia. Br. J. Anaesth. 1999, 83, 453–460. [Google Scholar] [CrossRef]
- Perlas, A.; Chan, V.W.S.; Lupu, C.M.; Mitsakakis, N.; Hanbidge, A. Ultrasound Assessment of Gastric Content and Volume. Anesthesiology 2009, 111, 82–89. [Google Scholar] [CrossRef]
- Frykholm, P.; Disma, N.; Andersson, H.; Beck, C.; Bouvet, L.; Cercueil, E.; Elliott, E.; Hofmann, J.; Isserman, R.; Klaucane, A.; et al. Pre-operative fasting in children. Eur. J. Anaesthesiol. 2022, 39, 4–25. [Google Scholar] [CrossRef]
- Bouvet, L.; Desgranges, F.-P.; Aubergy, C.; Boselli, E.; Dupont, G.; Allaouchiche, B.; Chassard, D. Prevalence and factors predictive of full stomach in elective and emergency surgical patients: A prospective cohort study. Br. J. Anaesth. 2017, 118, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Evain, J.-N.; Durand, Z.; Dilworth, K.; Sintzel, S.; Courvoisier, A.; Mortamet, G.; Desgranges, F.-P.; Bouvet, L.; Payen, J.-F. Assessing gastric contents in children before general anesthesia for acute extremity fracture: An ultrasound observational cohort study. J. Clin. Anesth. 2021, 77, 110598. [Google Scholar] [CrossRef] [PubMed]
- Boretsky, K.R.; Perlas, A. Gastric Ultrasound Imaging to Direct Perioperative Care in Pediatric Patients: A Report of 2 Cases. A A Pract. 2019, 13, 443–445. [Google Scholar] [CrossRef]
- Grover, G.; Berkowitz, C.D.; Lewis, R.J. Parental Recall After a Visit to the Emergency Department. Clin. Pediatr. 1994, 33, 194–201. [Google Scholar] [CrossRef]
- Kelly, C.; Shulman, V.; Khine, H.; Avner, J.R. Parental Perception of the Passage of Time During a Stressful Event. Pediatr. Emerg. Care 2007, 23, 376–379. [Google Scholar] [CrossRef]
- Perlas, A.; Davis, L.; Khan, M.; Mitsakakis, N.; Chan, V.W. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth. Analg. 2011, 113, 93–97. [Google Scholar] [CrossRef]
- Perlas, A.; Mitsakakis, N.; Liu, L.; Cino, M.; Haldipur, N.; Davis, L.; Cubillos, J.; Chan, V. Validation of a Mathematical Model for Ultrasound Assessment of Gastric Volume by Gastroscopic Examination. Obstet. Anesth. Dig. 2013, 116, 357–363. [Google Scholar] [CrossRef]
- Perlas, A.; Van de Putte, P.; Van Houwe, P.; Chan, V.W.S. I-AIM framework for point-of-care gastric ultrasound. Br. J. Anaesth. 2015, 116, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Perlas, A.; Arzola, C.; Van de Putte, P. Point-of-care gastric ultrasound and aspiration risk assessment: A narrative review. Can. J. Anaesth. 2018, 65, 437–448. [Google Scholar] [CrossRef]
- Bouvet, L.; Miquel, A.; Chassard, D.; Boselli, E.; Allaouchiche, B.; Benhamou, D. Could a single standardized ultrasonographic measurement of antral area be of interest for assessing gastric contents? A preliminary report. Eur. J. Anaesthesiol. 2009, 26, 1015–1019. [Google Scholar] [CrossRef]
- Bouvet, L.; Mazoit, J.-X.; Chassard, D.; Allaouchiche, B.; Boselli, E.; Benhamou, D. Clinical Assessment of the Ultrasonographic Measurement of Antral Area for Estimating Preoperative Gastric Content and Volume. Anesthesiology 2011, 114, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.O.; Walker, A.M.; Yeung, A.K.; Lardner, D.R.; Yee, K.; Mulvey, J.M.; Perlas, A. Ultrasound assessment of gastric volume in the fasted pediatric patient undergoing upper gastrointestinal endoscopy: Development of a predictive model using endoscopically suctioned volumes. Pediatr. Anesth. 2014, 25, 301–308. [Google Scholar] [CrossRef]
- Moser, J.J.; Walker, A.M.; Spencer, A.O. Point-of-care paediatric gastric sonography: Can antral cut-off values be used to diagnose an empty stomach? Br. J. Anaesth. 2017, 119, 943–947. [Google Scholar] [CrossRef]
- Schmitz, A.; Thomas, S.; Melanie, F.; Rabia, L.; Klaghofer, R.; Weiss, M.; Kellenberger, C. Ultrasonographic gastric antral area and gastric contents volume in children. Pediatr. Anesth. 2011, 22, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Schmidt, A.R.; Buehler, P.K.; Schraner, T.; Frühauf, M.; Weiss, M.; Klaghofer, R.; Kellenberger, C.J. Gastric ultrasound as a preoperative bedside test for residual gastric contents volume in children. Pediatr. Anesth. 2016, 26, 1157–1164. [Google Scholar] [CrossRef]
- Van de Putte, P.; Perlas, A. Gastric sonography in the severely obese surgical patient: A feasibility study. Anesth. Analg. 2014, 119, 1105–1110. [Google Scholar] [CrossRef]
- Kruisselbrink, R.; Arzola, C.; Jackson, T.; Okrainec, A.; Chan, V.; Perlas, A. Ultrasound assessment of gastric volume in severely obese individuals: A validation study. Br. J. Anaesth. 2016, 118, 77–82. [Google Scholar] [CrossRef]
- Van de Putte, P.; Perlas, A. The link between gastric volume and aspiration risk. In search of the Holy Grail? Anaesthesia 2018, 73, 274–279. [Google Scholar] [CrossRef]
- Desgranges, F.-P.; Riegel, A.-C.G.; Aubergy, C.; Siqueira, M.D.Q.; Chassard, D.; Bouvet, L. Ultrasound assessment of gastric contents in children undergoing elective ear, nose and throat surgery: A prospective cohort study. Anaesthesia 2017, 72, 1351–1356. [Google Scholar] [CrossRef]
- Leviter, J.; Steele, D.W.; Constantine, E.; Linakis, J.G.; Amanullah, S. “Full stomach” despite the wait: Point-of-care gastric ultrasound at the time of procedural sedation in the pediatric emergency department. Acad. Emerg. Med. 2019, 26, 752–760. [Google Scholar] [CrossRef]
- Cook-Sather, S.D.; Liacouras, C.A.; Previte, J.P.; Markakis, D.A.; Schreiner, M.S. Gastric fluid measurement by blind aspiration in paediatric patients: A gastroscopic evaluation. Can. J. Anaesth. 1997, 44, 168–172. [Google Scholar] [CrossRef]
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology 2017, 126, 376–393.
- Gagey, A.-C.; Siqueira, M.d.Q.; Desgranges, F.-P.; Combet, S.; Naulin, C.; Chassard, D.; Bouvet, L. Ultrasound assessment of the gastric contents for the guidance of the anaesthetic strategy in infants with hypertrophic pyloric stenosis: A prospective cohort study. Br. J. Anaesth. 2016, 116, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Kellenberger, C.J.; Liamlahi, R.; Fruehauf, M.; Klaghofer, R.; Weiss, M. Residual gastric contents volume does not differ following 4 or 6 h fasting after a light breakfast—A magnetic resonance imaging investigation in healthy non-anaesthetised school-age children. Acta Anaesthesiol. Scand. 2011, 56, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Kellenberger, C.; Lochbuehler, N.; Fruehauf, M.; Klaghofer, R.; Weiss, M. Effect of different quantities of a sugared clear fluid on gastric emptying and residual volume in children: A crossover study using magnetic resonance imaging. Br. J. Anaesth. 2012, 108, 644–647. [Google Scholar] [CrossRef]
- Gagey, A.C.; de Queiroz Siqueira, M.; Monard, C.; Combet, S.; Cogniat, B.; Desgranges, F.-P.; Robinson, P.; Chassard, D.; Bouvet, L. The effect of pre-operative gastric ultrasound examination on the choice of general anaesthetic induction technique for non-elective pediatric surgery. A prospective cohort study. Anesthesia 2018, 73, 304–312. [Google Scholar] [CrossRef]
- Falconer, R.; Skouras, C.; Carter, T.; Greenway, L.; Paisley, A.M. Preoperative fasting: Current practice and areas for improvement. Updat. Surg. 2013, 66, 31–39. [Google Scholar] [CrossRef]
- Engelhardt, T.; Wilson, G.; Horne, L.; Weiss, M.; Schmitz, A. Are you hungry? Are you thirsty?—Fasting times in elective outpatient pediatric patients. Pediatr. Anesth. 2011, 21, 964–968. [Google Scholar] [CrossRef]
- Andersson, H.; Hellström, P.M.; Frykholm, P. Introducing the 6-4-0 fasting regimen and the incidence of prolonged preoperative fasting in children. Pediatr. Anesth. 2017, 28, 46–52. [Google Scholar] [CrossRef]
- Newton, R.J.G.; Stuart, G.M.; Willdridge, D.J.; Thomas, M. Using quality improvement methods to reduce clear fluid fasting times in children on a preoperative ward. Pediatr. Anesth. 2017, 27, 793–800. [Google Scholar] [CrossRef]
- Thomas, M.; Morrison, C.; Newton, R.; Schindler, E. Consensus statement on clear fluids fasting for elective pediatric general anesthesia. Pediatr. Anesth. 2018, 28, 411–414. [Google Scholar] [CrossRef]
- Williams, C.; Johnson, P.A.; Guzzetta, C.E.; Guzzetta, P.C.; Cohen, I.T.; Sill, A.M.; Vezina, G.; Cain, S.; Harris, C.; Murray, J. Pediatric Fasting Times Before Surgical and Radiologic Procedures: Benchmarking Institutional Practices Against National Standards. J. Pediatr. Nurs. 2014, 29, 258–267. [Google Scholar] [CrossRef]
- Al-Robeye, A.M.; Barnard, A.N.; Bew, S. Thirsty work: Exploring children’s experiences of preoperative fasting. Pediatr. Anesth. 2019, 30, 43–49. [Google Scholar] [CrossRef]
- Brady, M.C.; Kinn, S.; Ness, V.; O’Rourke, K.; Randhawa, N.; Stuart, P. Preoperative fasting for pre-venting perioperative complications in children. Cochrane Database Syst. Rev. 2009, 7, CD005285. [Google Scholar]
- Dennhardt, N.; Beck, C.; Huber, D.; Sander, B.; Boehne, M.; Boethig, D.; Leffler, A.; Sümpelmann, R. Optimized preoperative fasting times decrease ketone body concentration and stabilize mean arterial blood pressure during induction of anesthesia in children younger than 36 months: A prospective observational cohort study. Pediatr. Anesth. 2016, 26, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Simpao, A.F.; Wu, L.; Nelson, O.; Gálvez, J.A.; Tan, J.M.; Wasey, J.O.; Muhly, W.T.; Tsui, F.-C.; Masino, A.J.; Stricker, P.A. Faculty Opinions recommendation of Preoperative fluid fasting times and postinduction low blood pressure in children: A retrospective analysis. Anesthesiology 2020, 133, 523–533. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.; Yang, H.; Pan, C.; Luo, N.; Li, J.; Yang, F.; Bei, Y.; Cahilog, Z.; Chen, Q.; et al. Ultrasonographic assessment of preoperative gastric volume in patients with dyspepsia: A prospective observational study. BMC Anesthesiol. 2022, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.M.; Cleary, P.W.; Sinnott, M.D. Investigating mixing and emptying for aqueous liquid content from the stomach using a coupled biomechanical-SPH model. Food Funct. 2018, 9, 3202–3219. [Google Scholar] [CrossRef]
- Kozu, H.; Kobayashi, I.; Nakajima, M.; Uemura, K.; Sato, S.; Ichikawa, S. Analysis of Flow Phenomena in Gastric Contents Induced by Human Gastric Peristalsis Using CFD. Food Biophys. 2010, 5, 330–336. [Google Scholar] [CrossRef]
- Pal, A.; Brasseur, J.G.; Abrahamsson, B. A stomach road or ‘Magenstrasse’ for gastric emptying. J. Biomech. 2007, 40, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Brandstaeter, S.; Fuchs, S.L.; Aydin, R.C.; Cyron, C.J. Mechanics of the stomach: A review of an emerging field of biomechanics. Gamm-Mitteilungen 2019, 42, e201900001. [Google Scholar] [CrossRef]
- Kruisselbrink, R.; Arzola, C.; Endersby, R.; Tse, C.; Chan, V.; Perlas, A. Intra- and Interrater Reliability of Ultrasound Assessment of Gastric Volume. Anesthesiology 2014, 121, 46–51. [Google Scholar] [CrossRef] [PubMed]
| n | (%) | ||
|---|---|---|---|
| Gender | Female | 30 | (30.9) |
| Male | 67 | (69.1) | |
| ASA | I | 88 | (90.7) |
| II | 9 | (9.3) | |
| Surgery type | Orthopedic surgery | 2 | (2.1) |
| Pediatric surgery | 83 | (85.6) | |
| ENT surgery | 4 | (4.1) | |
| Urologic surgery | 8 | (8.2) | |
| Gastric content | Grade 0 | 55 | (56.7) |
| Grade 1 | 37 | (38.1) | |
| Grade 2 | 5 | (5.2) | |
| Median (Interquartile Range) | Range (Min–Max) | |
|---|---|---|
| Age (month) | 72.00 (47.00–135.00) | 24–212 |
| Height (cm) | 116.00 (103.00–147.00) | 1.32–180 |
| Weight (kg) | 22.00 (16.50–41.00) | 12–76 |
| BMI (kg/m2) | 17.46 (15.80–19.09) | 12.75–23.89 |
| Last solid intake time (h) | 9.00 (8.00–9.00) | 6–12 |
| Last thick liquid intake time (h) | 9.00 (8.00–9.00) | 4–12 |
| Last clear liquid intake time (h) | 4.00 (3.00–6.00) | 2–10 |
| Antral RLD CSA (cm2) | 2.36 (1.44–4.20) | 0.48–6.34 |
| Gastric volume (mL/kg) | 0.46 (0.33–0.72) | 0.1–1.29 |
| Gastric volume (mL) | 13.41 (7.68–20.79) | 2.16–38.1 |
| Grade 0 (n = 55) | Grade 1 (n = 37) | Grade 2 (n = 5) | p a | |
|---|---|---|---|---|
| Antral RLD CSA (cm2) median (IQR) | 1.49 (0.94–2.08) | 4.29 (3.66–4.73) | 5.96 (4.59–6.02) | <0.001 |
| Antral RLD CSA (cm2) mean (95% CI) | 1.58 (1.39–1.77) | 4.25 (3.94–4.57) | 5.44 (4.27–6.60) | |
| Calculated gastric volume (mL/kg) median (IQR) | 0.34 (0.20–0.40) | 0.72 (0.56–0.83) | 0.81 (0.81–1.01) | <0.001 |
| Calculated gastric volume (mL/kg) mean (95% CI) | 0.32 (0.29–0.36) | 0.72 (0.64–0.80) | 0.93 (0.71–1.15) |
| Grade 0 (n = 55) | Grade 1 (n = 37) | Grade 2 (n = 5) | p a | ||||
|---|---|---|---|---|---|---|---|
| Gastric volume > 0.8 mL/kg | 1 | (1.8%) | 13 | (35.1%) | 5 | (100.0%) | <0.001 |
| Gastric volume > 1 mL/kg | 0 | (0.0%) | 4 | (10.8%) | 2 | (40.0%) | 0.002 |
| Gastric volume > 1.25 mL/kg | 0 | (0.0%) | 1 | (2.7%) | 0 | (0.0%) | 0.433 |
| Gastric volume > 1.5 mL/kg | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | * |
| Grade 0 | Grade 1 | Grade 2 | |
|---|---|---|---|
| Rho (p) | Rho (p) | Rho (p) | |
| BMI | 0.542 (<0.001) | 0.143 (0.397) | 0.000 (>0.999) |
| Age (month) | 0.796 (<0.001) | 0.622 (<0.001) | 0.667 (0.219) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirel, A.; Özgünay, Ş.E.; Eminoğlu, Ş.; Balkaya, A.N.; Onur, T.; Kılıçarslan, N.; Gamlı, M. Ultrasonographic Evaluation of Gastric Content and Volume in Pediatric Patients Undergoing Elective Surgery: A Prospective Observational Study. Children 2023, 10, 1432. https://doi.org/10.3390/children10091432
Demirel A, Özgünay ŞE, Eminoğlu Ş, Balkaya AN, Onur T, Kılıçarslan N, Gamlı M. Ultrasonographic Evaluation of Gastric Content and Volume in Pediatric Patients Undergoing Elective Surgery: A Prospective Observational Study. Children. 2023; 10(9):1432. https://doi.org/10.3390/children10091432
Chicago/Turabian StyleDemirel, Asiye, Şeyda Efsun Özgünay, Şermin Eminoğlu, Ayşe Neslihan Balkaya, Tuğba Onur, Nermin Kılıçarslan, and Mehmet Gamlı. 2023. "Ultrasonographic Evaluation of Gastric Content and Volume in Pediatric Patients Undergoing Elective Surgery: A Prospective Observational Study" Children 10, no. 9: 1432. https://doi.org/10.3390/children10091432
APA StyleDemirel, A., Özgünay, Ş. E., Eminoğlu, Ş., Balkaya, A. N., Onur, T., Kılıçarslan, N., & Gamlı, M. (2023). Ultrasonographic Evaluation of Gastric Content and Volume in Pediatric Patients Undergoing Elective Surgery: A Prospective Observational Study. Children, 10(9), 1432. https://doi.org/10.3390/children10091432






