The Effects of Dexmedetomidine on Children Undergoing Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.2.1. Selection Criteria
2.2.2. Exclusion Criteria Were the Following
2.2.3. Extraction of Data
2.3. Quality and Risk of Bias Assessment
2.4. Statistical Methods
3. Results
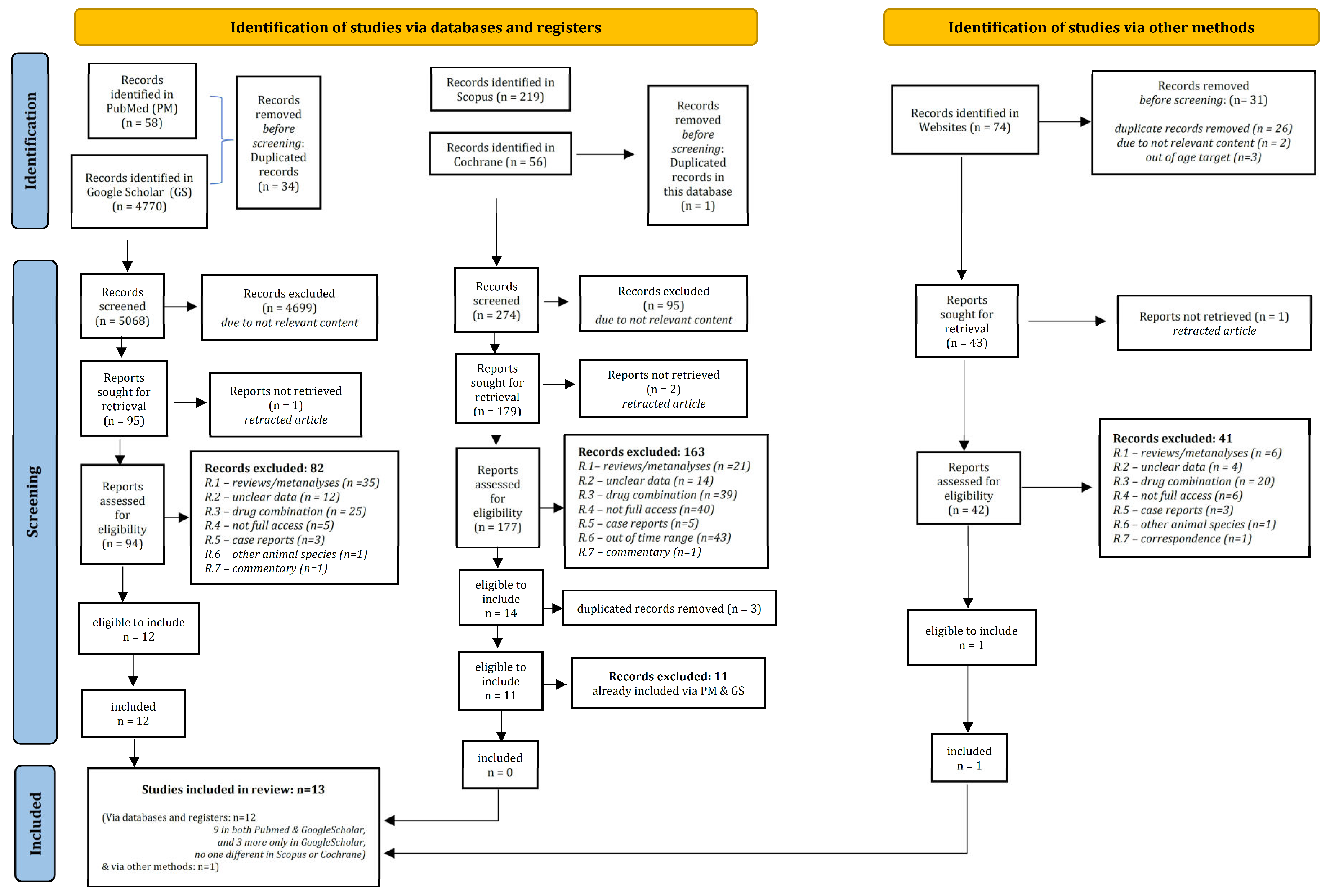
3.1. Study Selection—Characteristics of Included Records
3.2. Quality Assessment—Risk of Bias
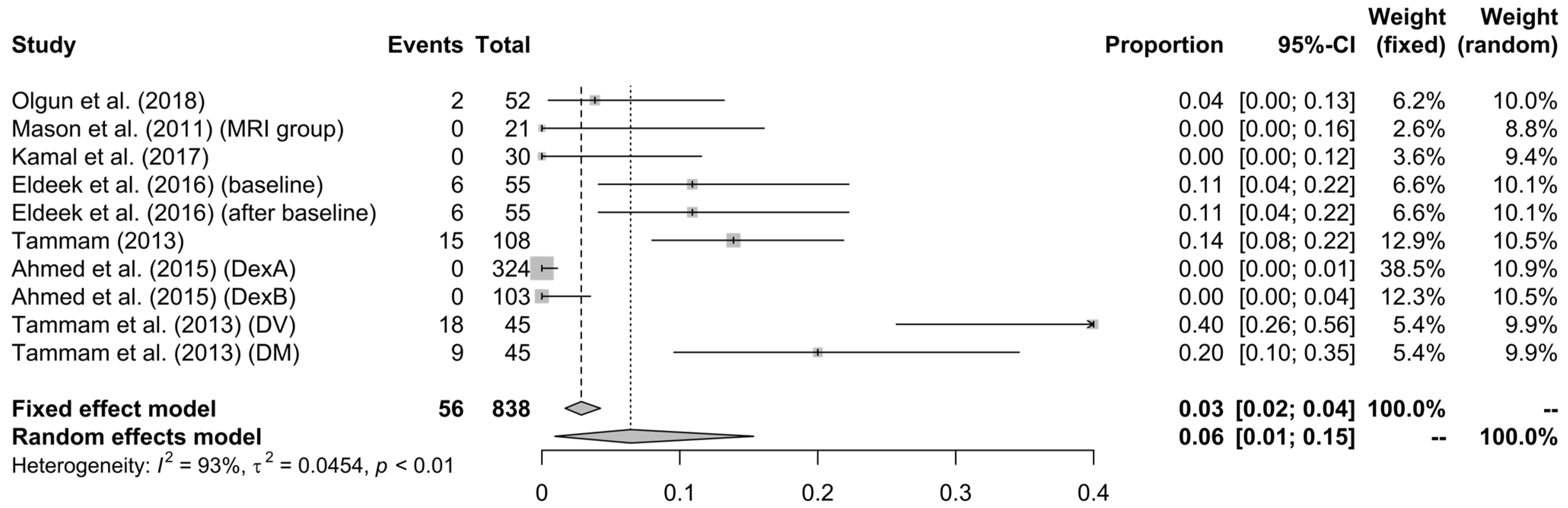
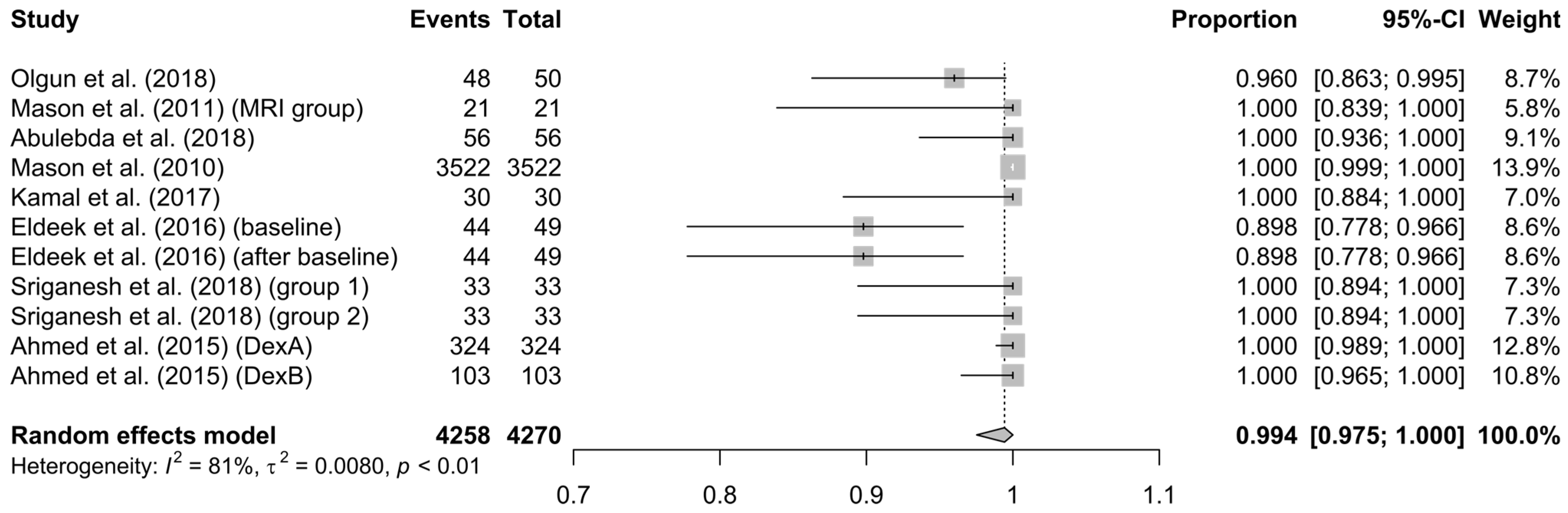
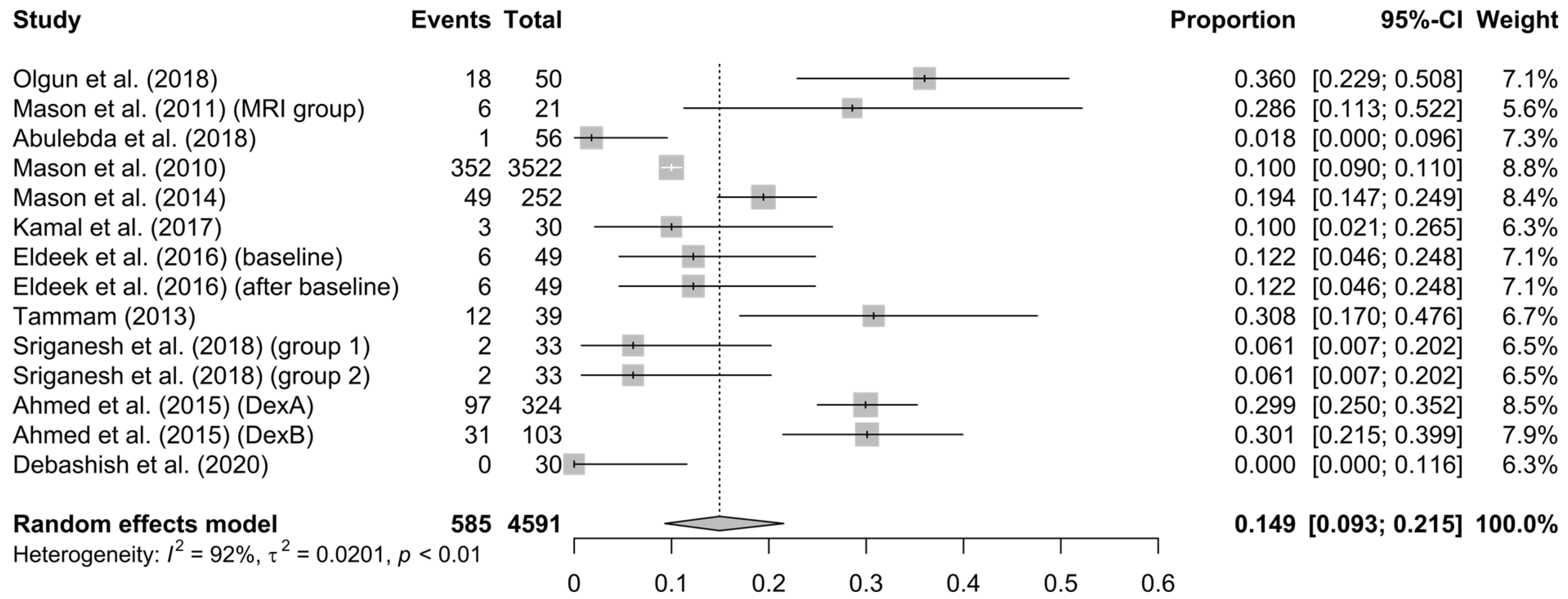
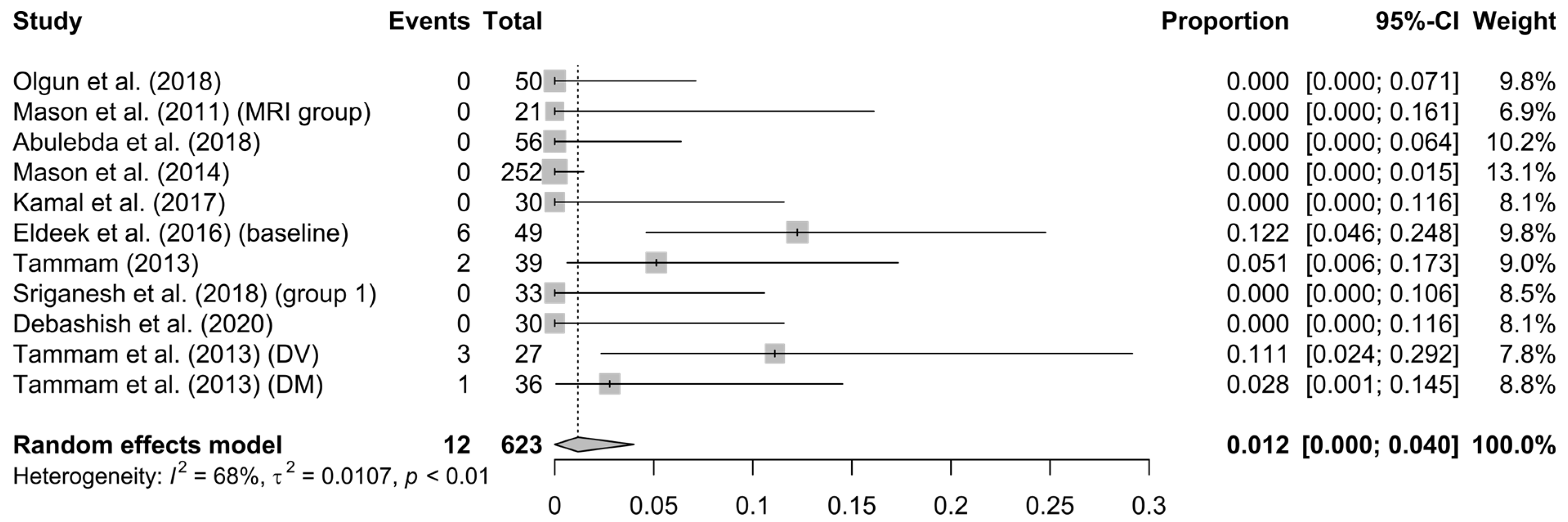
3.3. Analysis’ Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahinovic, M.M.; Struys, M.; Absalom, A.R. Clinical Pharmacokinetics and Pharmacodynamics of Propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Electronic Medicines Compendium (EMC). Propofol 10 mg/mL (1%) Emulsion for Injection/Infusion. Available online: https://www.medicines.org.uk/emc/product/11319/smpc (accessed on 24 April 2023).
- Electronic Medicines Compendium (EMC). Chloral Hydrate 143 mg/5 mL Oral Solution. Available online: https://www.medicines.org.uk/emc/product/8136/smpc (accessed on 24 April 2023).
- Gauillard, J.; Cheref, S.; Vacherontrystram, M.N.; Martin, J.C. Chloral hydrate: A hypnotic best forgotten? L’Encephale 2002, 28, 200–204. [Google Scholar] [PubMed]
- Blumer, J.L. Clinical pharmacology of midazolam in infants and children. Clin. Pharmacokinet. 1998, 35, 37–47. [Google Scholar] [CrossRef]
- Electronic Medicines Compendium (EMC). Midazolam 1 mg/mL Injection. Available online: https://www.medicines.org.uk/emc/product/6419/smpc (accessed on 24 April 2023).
- Sottas, C.E.; Anderson, B.J. Dexmedetomidine: The new all-in-one drug in paediatric anaesthesia? Curr. Opin. Anaesthesiol. 2017, 30, 441–451. [Google Scholar] [CrossRef]
- Lois, F.; De Kock, M. Something new about ketamine for pediatric anesthesia? Curr. Opin. Anaesthesiol. 2008, 21, 340–344. [Google Scholar] [CrossRef]
- Electronic Medicines Compendium (EMC). Ketamine 50 mg/mL Injection. Available online: https://www.medicines.org.uk/emc/product/6935/smpc (accessed on 24 April 2023).
- Kang, R.; Shin, Y.H.; Gil, N.S.; Oh, Y.N.; Hahm, T.S.; Jeong, J.S. A retrospective comparison of propofol to dexmedetomidine for pediatric magnetic resonance imaging sedation in patients with mucopolysaccharidosis type II. Paediatr. Anaesth. 2018, 28, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Olutoye, O.A.; Baker, B.W.; Belfort, M.A.; Olutoye, O.O. Food and Drug Administration warning on anesthesia and brain development: Implications for obstetric and fetal surgery. Am. J. Obstet. Gynecol. 2018, 218, 98–102. [Google Scholar] [CrossRef]
- Andropoulos, D.B. Effect of Anesthesia on the Developing Brain: Infant and Fetus. Fetal Diagn. Ther. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Olgun, G.; Ali, M.H. Use of Intranasal Dexmedetomidine as a Solo Sedative for MRI of Infants. Hosp. Pediatr. 2018, 8, 68–71. [Google Scholar] [CrossRef]
- Salerno, S.; Granata, C.; Trapenese, M.; Cannata, V.; Curione, D.; Rossi Espagnet, M.C.; Magistrelli, A.; Toma, P. Is MRI imaging in pediatric age totally safe? A critical reprisal. Radiol. Med. 2018, 123, 695–702. [Google Scholar] [CrossRef]
- Walters, J.L.; Paule, M.G. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol. Teratol. 2017, 60, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.; Nickerson, J.P.; Higgins, T.; Williams, R.K. Pediatric anesthesia and neurotoxicity: What the radiologist needs to know. Pediatr. Radiol. 2018, 48, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Barbi, E.; Mason, K.P. Dexmedetomidine: What’s New for Pediatrics? A Narrative Review. J. Clin. Med. 2020, 9, 2724. [Google Scholar] [CrossRef] [PubMed]
- Janke, E.L.; Samra, S. Dexmedetomidine and neuroprotection. Semin. Anesth. Perioper. Med. Pain 2006, 25, 71–76. [Google Scholar] [CrossRef]
- Nikolic, K.; Agbaba, D. Imidazoline antihypertensive drugs: Selective i(1) -imidazoline receptors activation. Cardiovasc. Ther. 2012, 30, 209–216. [Google Scholar] [CrossRef]
- Lepeltier, H.; Lepetit, A.; Gauberti, M.; Escalard, C.; Salaun, J.P.; Benard, C.; Lesage, A.; Brossier, D.; Goyer, I. Dexmedetomidine sedation vs. inhaled general anesthesia for pediatric MRI: A retrospective cohort study: Dexmedetomidine sedation vs. inhaled general anesthesia for MRI. Arch. Pediatr. 2022, 29, 213–218. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- The Authors. Current Version of RoB 2. Available online: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 24 April 2023).
- R Foundation. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 24 April 2023).
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid.-Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Schwarzer, G. meta: An R Package for Meta-Analysis. R News 2007, 7, 40–45. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Bland, M. Estimating Mean and Standard Deviation from the Sample Size, Three Quartiles, Minimum, and Maximum. Int. J. Stat. Med. Res. 2015, 4, 57–64. [Google Scholar] [CrossRef]
- Deepanshu, S.; Surya, P.U.; Vinay, S.; Sakshi, P.; Ravi, R.K.N. Deep Meta Tool: GUI Tool to Obtain Mean and Standard Deviation (SD) from Median and Interquartile Range (IQR). Available online: https://www.researchsquare.com/article/rs-828102 (accessed on 25 November 2021).
- Patano, A.; Inchingolo, A.M.; Laudadio, C.; Azzollini, D.; Marinelli, G.; Ceci, S.; Latini, G.; Rapone, B.; Inchingolo, A.D.; Mancini, A.; et al. Therapeutic Strategies of Primary Molar Infraocclusion: A Systematic Review. Children 2023, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, R.; Riggins, J.; Kariyanna, S.; Calkins, P.; Rotta, A.T. High-dose dexmedetomidine sedation for pediatric MRI. Paediatr. Anaesth. 2011, 21, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.P.; Lubisch, N.B.; Robinson, F.; Roskos, R. Intramuscular dexmedetomidine sedation for pediatric MRI and CT. AJR Am. J. Roentgenol. 2011, 197, 720–725. [Google Scholar] [CrossRef]
- Abulebda, K.; Louer, R.; Lutfi, R.; Ahmed, S.S. A Comparison of Safety and Efficacy of Dexmedetomidine and Propofol in Children with Autism and Autism Spectrum Disorders Undergoing Magnetic Resonance Imaging. J. Autism Dev. Disord. 2018, 48, 3127–3132. [Google Scholar] [CrossRef]
- Mason, K.P.; Zurakowski, D.; Zgleszewski, S.; Prescilla, R.; Fontaine, P.J.; Dinardo, J.A. Incidence and predictors of hypertension during high-dose dexmedetomidine sedation for pediatric MRI. Paediatr. Anaesth. 2010, 20, 516–523. [Google Scholar] [CrossRef]
- Kamal, K.; Asthana, U.; Bansal, T.; Dureja, J.; Ahlawat, G.; Kapoor, S. Evaluation of efficacy of dexmedetomidine versus propofol for sedation in children undergoing magnetic resonance imaging. Saudi J. Anaesth. 2017, 11, 163–168. [Google Scholar] [CrossRef]
- Eldeek, A.M.; Elfawal, S.M.; Allam, M.G. Sedation in children undergoing magnetic resonance imaging comparative study between dexmedetomidine and ketamine. Egypt. J. Anaesth. 2016, 32, 263–268. [Google Scholar] [CrossRef]
- Tammam, T.F. Comparison of the efficacy of dexmedetomidine, ketamine, and a mixture of both for pediatric MRI sedation. Egypt. J. Anaesth. 2013, 29, 241–246. [Google Scholar] [CrossRef]
- Sriganesh, K.; Saini, J.; Theerth, K.; Venkataramaiah, S. Airway Dimensions in Children with Neurological Disabilities During Dexmedetomidine and Propofol Sedation for Magnetic Resonance Imaging Study. Turk. J. Anaesthesiol. Reanim. 2018, 46, 214–221. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Unland, T.; Slaven, J.E.; Nitu, M.E. High dose dexmedetomidine: Effective as a sole agent sedation for children undergoing MRI. Int. J. Pediatr. 2015, 2015, 397372. [Google Scholar] [CrossRef] [PubMed]
- Tammam, T.F.; Wahba, S.S. Quality of MRI pediatric sedation: Comparison between intramuscular and intravenous dexmedetomidine. Egypt. J. Anaesth. 2013, 29, 47–52. [Google Scholar] [CrossRef]
- Paul, D.; Kaur, K.B.; Ray, A.; Jaiswal, A.; Kate, S.; Bhengra, A. Effectiveness of Dexmedetomidine vs Ketamine in Paediatric Patients Undergoing MRI. J. Clin. Diagn. Res. 2020, 14, UC01–UC05. [Google Scholar] [CrossRef]
- Mason, K.P.; Turner, D.P.; Houle, T.T.; Fontaine, P.J.; Lerman, J. Hemodynamic response to fluid management in children undergoing dexmedetomidine sedation for MRI. AJR Am. J. Roentgenol. 2014, 202, W574–W579. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Authors | Publication Year | Type of Study | N | Sub-Groups | N1 | D | D1 | Dose D1 (μg/kg) | Route D1 | Maintenance (μg/kg/h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [13] | Olgun et al. | 2018 | retrospective chart review | 52 | _ | 52 | 50 | 49 | 4 | IN | ΝR |
| [30] | Siddappa et al. | 2011 | retrospective clinical chart review | 76 | dex1 | 36 | 36 | 2 | IV | 1 | |
| dex2 | 18 | 0 | 2 | IV | 1 | ||||||
| [31] | Mason et al. | 2011 | retrospective review of clinical data | 65 | ΜRI | 21 | 21 | 20 | 2.8 ± 0.6 | IM | NR |
| [32] | Abulebda et al. | 2018 | retrospective chart review | 56 | _ | 56 | 56 | 3.41 ± 1.04 | IV | NR | |
| [33] | Mason et al. | 2010 | RCT—retrospective review of institute data-base | 3522 | total | 3522 | 3522 | 2645 | 3 | IV | 2 |
| [34] | Kamal et al. | 2017 | prospective randomized study | 30 | _ | 30 | 30 | 2 | IV | 1–1.5 | |
| [35] | Eldeek et al. | 2016 | randomized prospective comparative study | 55 | _ | 55 | 49 | 49 | 1 | IV | 0.5–0.75 |
| [36] | Tammam | 2013 | prospective double-blind comparative study | 108 | 108 | 39 | 39 | 3 | IM | NR | |
| [37] | Sriganesh et al. | 2018 | prospective randomized | 36 | _ | 36 | 33 | 33 | 2 | IV | 2 |
| [38] | Ahmed et al. | 2015 | retrospective institutional review | 427 | dexA | 324 | 324 | 324 | 2 | IV | 1 |
| dexB | 103 | 103 | 0 | 2 | IV | 1 | |||||
| [39] | Tammam et al. | 2013 | double-blind, comparative, randomized study | 90 | DV | 45 | 27 | 45 | 1 | IV | 1 |
| DM | 45 | 36 | 45 | 3 | IM | _ | |||||
| [40] | Debashish et al. | 2020 | cross-sectional study | 30 | _ | 30 | 30 | 30 | 1 | IV | 0.2–0.7 |
| [41] | Mason et al. | 2014 | retrospective institute chart review | 1657 | 3 (1 into consideration) | 1657 | 252 | 140 | 3 | IV | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulou, V.-A.; Pouliakis, A.; Alexiou, N.; Ioannidi, P.; Vagiona, D.; Ekmektzoglou, K.; Xanthos, T.; Boutsikou, T.; Iliodromiti, Z.; Iacovidou, N. The Effects of Dexmedetomidine on Children Undergoing Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Children 2023, 10, 948. https://doi.org/10.3390/children10060948
Angelopoulou V-A, Pouliakis A, Alexiou N, Ioannidi P, Vagiona D, Ekmektzoglou K, Xanthos T, Boutsikou T, Iliodromiti Z, Iacovidou N. The Effects of Dexmedetomidine on Children Undergoing Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Children. 2023; 10(6):948. https://doi.org/10.3390/children10060948
Chicago/Turabian StyleAngelopoulou, Valentina-Anastasia, Abraham Pouliakis, Nikolaos Alexiou, Parthena Ioannidi, Dimitra Vagiona, Konstantinos Ekmektzoglou, Theodoros Xanthos, Theodora Boutsikou, Zoi Iliodromiti, and Nikoletta Iacovidou. 2023. "The Effects of Dexmedetomidine on Children Undergoing Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis" Children 10, no. 6: 948. https://doi.org/10.3390/children10060948
APA StyleAngelopoulou, V.-A., Pouliakis, A., Alexiou, N., Ioannidi, P., Vagiona, D., Ekmektzoglou, K., Xanthos, T., Boutsikou, T., Iliodromiti, Z., & Iacovidou, N. (2023). The Effects of Dexmedetomidine on Children Undergoing Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Children, 10(6), 948. https://doi.org/10.3390/children10060948