The Current Role of the sFlt-1/PlGF Ratio and the Uterine–Umbilical–Cerebral Doppler Ultrasound in Predicting and Monitoring Hypertensive Disorders of Pregnancy: An Update with a Review of the Literature
Abstract
:1. Introduction
2. Pre-Eclampsia Screening
3. Prediction and Clinical Management of Pre-Eclampsia and Other Adverse Pregnancy Outcomes Using the sFlt-1/PlGF Ratio
4. Prediction and Clinical Management of Pre-Eclampsia and Other Adverse Pregnancy Outcomes Using Doppler Velocimetry Indices
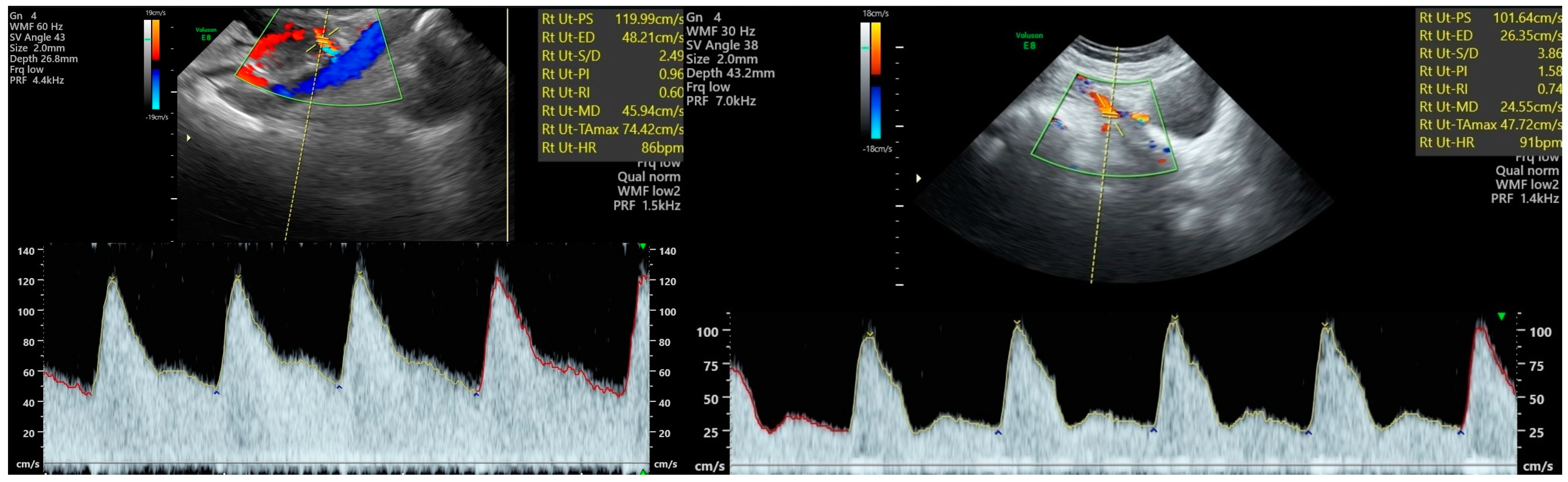
5. Uterine Artery Doppler—Technique and Reference Parameters
6. Other Vascular Structures Evaluated in the Pregnancy Follow-Up—Doppler Technique and Reference Parameters
7. Relevant Data from the Recent Literature Regarding the Value of the sFlt-1/PlGF Ratio
8. Relevant Data from the Recent Literature Regarding the Value of Doppler Velocimetry
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Spong, C.Y.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M.; Sheffield, J.S. Williams Obstetrics 24th Edition; Medical: New York, NY, USA, 2014; pp. 728–732. [Google Scholar]
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Khan, B.; Yar, R.A.; Khakwani, A.K.; Karim, S.; Ali, H.A. Preeclampsia Incidence and Its Maternal and Neonatal Outcomes with Associated Risk Factors. Cureus 2022, 14, e31143. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Magley, M.; Hinson, M.R. Eclampsia. [Updated 30 January 2023]; In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Filipek, A.; Jurewicz, E. Preeclampsia—A disease of pregnant women. Postepy Biochem. 2018, 64, 229–232. [Google Scholar] [CrossRef]
- Alese, M.O.; Moodley, J.; Naicker, T. Preeclampsia and HELLP syndrome, the role of the liver. J. Matern.-Fetal Neonatal Med. 2021, 34, 117–123. [Google Scholar] [CrossRef]
- Chaemsaithong, P.; Sahota, D.S.; Poon, L.C. First trimester preeclampsia screening and prediction. Am. J. Obstet. Gynecol. 2020, 226, S1071–S1097. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Schutte, A.E.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Corominas, A.I.; Medina, Y.; Balconi, S.; Casale, R.; Farina, M.; Martínez, N.; Damiano, A.E. Assessing the Role of Uric Acid as a Pre-dictor of Preeclampsia. Front. Physiol. 2022, 12, 785219. [Google Scholar] [CrossRef]
- Piani, F.; Agnoletti, D.; Baracchi, A.; Scarduelli, S.; Verde, C.; Tossetta, G.; Montaguti, E.; Simonazzi, G.; Degli Esposti, D.; Borghi, C. Serum uric acid to creatinine ratio and risk of preeclampsia and adverse pregnancy outcomes. J. Hypertens. 2023, 41, 1333–1338. [Google Scholar] [CrossRef]
- Sotiriadis, A.; Hernandez-Andrade, E.; Costa, F.d.S.; Ghi, T.; Glanc, P.; Khalil, A.; Martins, W.; Odibo, A.; Papageorghiou, A.; Salomon, L.; et al. ISUOG Practice Guidelines: Role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet. Gynecol. 2018, 53, 7–22. [Google Scholar] [CrossRef]
- Allen, R.E.; Rogozinska, E.; Cleverly, K.; Aquilina, J.; Thangaratinam, S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 194–201. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, X.; Zhou, M.; Deng, Y.; Li, Y.; Peng, C. sFlt-1: A double regulator in angiogenesis-related diseases. Curr. Pharm. Des. 2021, 27, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.; Hennessy, A.; Makris, A. Placental growth factor and pre-eclampsia. J. Hum. Hypertens. 2017, 31, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy clas-sification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [PubMed]
- Stepan, H.; Galindo, A.; Hund, M.; Schlembach, D.; Sillman, J.; Surbek, D.; Vatish, M. Clinical Utility of sFlt-1 and PlGF in Screening, Prediction, Diagnosis and Monitoring of Pre-eclampsia and Fetal Growth Restriction. Obstet. Gynecol. Surv. 2023, 78, 451–453. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Raheja, S.; Tuli, A.; Raghunandan, C.; Agarwal, S. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch. Gynecol. Obstet. 2011, 285, 417–422. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Salmeri, N.; Candiani, M.; Farina, A. Reference ranges of uterine artery pulsatility index from first to third trimester based on serial Doppler measurements: Longitudinal cohort study. Ultrasound Obstet. Gynecol. 2023, 61, 474–480. [Google Scholar] [CrossRef]
- Oloyede, O.A.; Iketubosin, F. Uterine artery Doppler study in second trimester of pregnancy. Pan Afr. Med. J. 2013, 15, 87. [Google Scholar] [CrossRef]
- Zalud, I.; Broady, A.J. Guidelines for the Doppler Assessment of the Umbilical and Middle Cerebral Arteries in Obstetrics. Donald Sch. J. Ultrasound Obstet. Gynecol. 2015, 10, 418–421. [Google Scholar] [CrossRef]
- Radswiki, T.; Murphy, A.; Weerakkody, Y. Umbilical arterial Doppler assessment. Radiopaedia 2011. [Google Scholar] [CrossRef]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Biswas, A.; Huang, X.; Lee, K.J.; Li, T.K.; Masuyama, H.; Ohkuchi, A.; Park, J.S.; Saito, S.; Tan, K.H.; et al. Short-term prediction of adverse outcomes using the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor) ratio in Asian women with suspected preeclampsia. Hypertension 2019, 74, 164–172. [Google Scholar] [CrossRef]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction or diagnosis of preeclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.J.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: Ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet. Gynecol. 2019, 53, 367–375. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; O’Sullivan, J.; Ohuma, E.O.; Harrington, D.; Szafranski, P.; Black, R.; Mackillop, L.; Impey, L.; Greenwood, C.; James, T.; et al. Faculty Opinions recommendation of Randomized interventional study on prediction of preeclampsia/eclampsia in women with suspected preeclampsia: INSPIRE. Hypertension 2020, 74, 983–990. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; O’Sullivan, J.; Ohuma, E.O.; James, T.; Papageorghiou, A.T.; Knight, M.; Vatish, M. Performance of soluble fms-like tyro-sine kinase-1-to-placental growth factor ratio of ≥85 for ruling in preeclampsia within 4 weeks. Am. J. Obstet. Gynecol. 2021, 224, 322–323. [Google Scholar] [CrossRef]
- Caillon, H.; Tardif, C.; Dumontet, E.; Winer, N.; Masson, D. Evaluation of sFlt-1/PlGF Ratio for Predicting and Improving Clinical Management of Pre-eclampsia: Experience in a Specialized Perinatal Care Center. Ann. Lab. Med. 2018, 38, 95–101. [Google Scholar] [CrossRef]
- Perales, A.; Delgado, J.L.; de la Calle, M.; García-Hernández, J.A.; Escudero, A.I.; Campillos, J.M.; Sarabia, M.D.; Laíz, B.; Duque, M.; Navarro, M.; et al. sFlt-1/PlGF for prediction of early-onset pre-eclampsia: STEPS (Study of Early Pre-eclampsia in Spain). Ultrasound Obstet. Gynecol. 2017, 50, 373–382. [Google Scholar] [CrossRef]
- Diguisto, C.; Piver, E.; Le Gouge, A.; Eboue, F.; Le Vaillant, C.; Maréchaud, M.; Goua, V.; Giraudeau, B.; Perrotin, F. First trimester uterine artery Doppler, sFlt-1 and PlGF to predict preeclampsia in a high-risk population. J. Matern. Neonatal Med. 2017, 30, 1514–1519. [Google Scholar] [CrossRef]
- Soundararajan, R.; Suresh, S.C.; Mueller, A.; Heimberger, S.; Avula, S.; Sathyanarayana, C.; Mahesh, S.; Madhuprakash, S.; Rana, S. Real life outpatient biomarker use in management of hypertensive pregnancies in third trimester in a low resource Setting: RO-BUST study. Pregnancy Hypertens. 2021, 23, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Y.; Wright, D.; Koutoulas, L.; Akolekar, R.; Nicolaides, K.H. Comparison of screening for pre-eclampsia at 31–34 weeks’ ges-tation by sFlt-1/PlGF ratio and a method combining maternal factors with sFlt-1 and PlGF. Ultrasound Obstet. Gynecol. 2017, 49, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, I.; Simón, E.; Gómez-Arriaga, P.I.; Quezada, M.S.; García-Burguillo, A.; López-Jiménez, E.A.; Galindo, A. Clinical implementa-tion of the sFlt-1/PlGF ratio to identify preeclampsia and fetal growth restriction: A prospective cohort study. Pregnancy Hypertens. 2018, 13, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ciciu, E.; Paṣatu-Cornea, A.-M.; Dumitru, S.; Petcu, L.C.; Salim, C.; Tuta, L.-A. Utility of sFtl-1 and Placental Growth Factor Ratio for Adequate Preeclampsia Management. Healthcare 2023, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, F.; Guo, Q.; Wang, Y.; He, P.; Zhang, H.; Wang, S. The clinical value of PlGF and the sFlt1/PlGF ratio in the management of hypertensive pregnancy disorders: A retrospective real-world study in China. Clin. Chim. Acta 2022, 528, 90–97. [Google Scholar] [CrossRef]
- Nikuei, P.; Rajaei, M.; Roozbeh, N.; Mohseni, F.; Poordarvishi, F.; Azad, M.; Haidari, S. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth 2020, 20, 80. [Google Scholar] [CrossRef]
- Leaños-Miranda, A.; Graciela Nolasco-Leaños, A.; Ismael Carrillo-Juárez, R.; José Molina-Pérez, C.; Janet Sillas-Pardo, L.; Manuel Jiménez-Trejo, L.; Isordia-Salas, I.; Leticia Ramírez-Valenzuela, K. Usefulness of the sFlt-1/PlGF (Soluble fms-Like Tyrosine Ki-nase-1/Placental Growth Factor) Ratio in Diagnosis or Misdiagnosis in Women with Clinical Diagnosis of Preeclampsia. Hypertension 2020, 76, 892–900. [Google Scholar] [CrossRef]
- Baltajian, K.; Bajracharya, S.; Salahuddin, S.; Berg, A.H.; Geahchan, C.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A.; Rana, S. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. Am. J. Obstet. Gynecol. 2016, 215, 89.e1–89.e10. [Google Scholar] [CrossRef]
- Peguero, A.; Fernandez-Blanco, L.; Mazarico, E.; Benitez, L.; Gonzalez, A.; Youssef, L.; Crispi, F.; Hernandez, S.; Figueras, F. Added prognostic value of longitudinal changes of angiogenic factors in early-onset severe pre-eclampsia: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2020, 128, 158–165. [Google Scholar] [CrossRef]
- Garcia-Manau, P.; Mendoza, M.; Bonacina, E.; Garrido-Gimenez, C.; Fernandez-Oliva, A.; Zanini, J.; Catalan, M.; Tur, H.; Serrano, B.; Carreras, E. Soluble fms-like tyrosine kinase to placental growth factor ratio in different stages of early-onset fetal growth re-striction and small for gestational age. Acta Obstet. Gynecol. Scand. 2021, 100, 119–128. [Google Scholar] [CrossRef]
- Dröge, L.A.; Perschel, F.H.; Stütz, N.; Gafron, A.; Frank, L.; Busjahn, A.; Henrich, W.; Verlohren, S. Prediction of Preeclampsia-Related Adverse Outcomes with the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor)-Ratio in the Clinical Routine: A Real-World Study. Hypertension 2021, 77, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.R.; Jeong, D.H.; Lee, J.Y.; Woo, E.Y.; Shin, G.T.; Kim, S.Y. sFlt-1/PlGF ratio as a predictive and prognostic marker for preeclampsia. J. Obstet. Gynaecol. Res. 2021, 47, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.; Rouvali, A.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of small for gestational age neonates: Screening by maternal factors, fetal biometry, and biomarkers at 35–37 weeks’ gestation. Am. J. Obstet. Gynecol. 2019, 220, 486.e1–486.e11. [Google Scholar] [CrossRef] [PubMed]
- Gaccioli, F.; Sovio, U.; Cook, E.; Hund, M.; Charnock-Jones, D.S.; Smith, G.C.S. Screening for fetal growth restriction using ultrasound and the sFLT1/PlGF ratio in nulliparous women: A prospective cohort study. Lancet Child Adolesc. Health 2018, 2, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, S.; Mueller, A.; Ratnaparkhi, R.; Perdigao, J.L.; Rana, S. Angiogenic factor abnormalities and risk of peripartum com-plications and prematurity among urban predominantly obese parturients with chronic hypertension. Pregnancy Hypertens. 2020, 20, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gaccioli, F.; Sovio, U.; Gong, S.; Cook, E.; Charnock-Jones, D.S.; Smith, G.C.S. Increased Placental sFLT1 (Soluble fms-Like Tyrosine Kinase Receptor-1) Drives the Antiangiogenic Profile of Maternal Serum Preceding Preeclampsia but Not Fetal Growth Re-striction. Hypertension 2023, 80, 325–334. [Google Scholar] [CrossRef]
- Palma Dos Reis, C.R.; Brás, S.; Meneses, T.; Cerdeira, A.S.; Vatish, M.; Martins, A.T. The sFlt1/PlGF ratio predicts faster fetal deteriora-tion in early fetal growth restriction: A historical cohort study. Acta Obstet. Gynecol. Scand. 2023, 102, 635–643. [Google Scholar] [CrossRef]
- Binder, J.; Palmrich, P.; Pateisky, P.; Kalafat, E.; Kuessel, L.; Zeisler, H.; Munkhbaatar, M.; Windsperger, K.; Thilaganathan, B.; Khalil, A. The Prognostic Value of Angiogenic Markers in Twin Pregnancies to Predict Delivery Due to Maternal Complications of Preeclampsia. Hypertension 2020, 76, 176–183. [Google Scholar] [CrossRef]
- Binder, J.; Kalafat, E.; Palmrich, P.; Pateisky, P.; Khalil, A. Should angiogenic markers be included in diagnostic criteria of superim-posed pre-eclampsia in women with chronic hypertension? Ultrasound Obstet. Gynecol. 2022, 59, 192–201. [Google Scholar] [CrossRef]
- Duhig, K.E.; Seed, P.T.; Placzek, A.; Sparkes, J.; Hendy, E.; Gill, C.; Brockbank, A.; Shennan, A.H.; Thangaratinam, S.; Chappell, L.C. Prog-nostic indicators of severe disease in late preterm pre-eclampsia to guide decision making on timing of delivery: The PEA-COCK study. Pregnancy Hypertens. 2021, 24, 90–95. [Google Scholar] [CrossRef]
- Roberts, A.; Adekanmi, A.J.; Akinmoladun, J.A.; Adeyinka, A.O. Uterine and umbilical artery doppler in women with pre-eclampsia and their pregnancy outcomes. Niger. Postgrad. Med. J. 2019, 26, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Trongpisutsak, A.; Phupong, V. Prediction of preeclampsia using a combination of serum micro RNA-210 and uterine artery Doppler ultrasound. Sci. Prog. 2021, 104, 00368504211036856. [Google Scholar] [CrossRef] [PubMed]
- Soongsatitanon, A.; Phupong, V. Prediction of preeclampsia using first trimester placental protein 13 and uterine artery Dop-pler. J. Matern.-Fetal Neonatal Med. 2022, 35, 4412–4417. [Google Scholar] [CrossRef]
- Oancea, M.; Grigore, M.; Ciortea, R.; Diculescu, D.; Bodean, D.; Bucuri, C.; Strilciuc, S.; Rada, M.; Mihu, D. Uterine Artery Doppler Ul-trasonography for First Trimester Prediction of Preeclampsia in Individuals at Risk from Low-Resource Settings. Medicina 2020, 56, 428. [Google Scholar] [CrossRef] [PubMed]
- Prakansamut, N.; Phupong, V. Serum SHARP1 and uterine artery Doppler for the prediction of preeclampsia. Sci. Rep. 2019, 9, 12266. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razik, M.; Mostafa, A.; Taha, S.; Salah, A. Combined Doppler ultrasound and platelet indices for prediction of preeclampsia in high-risk pregnancies. J. Matern.-Fetal Neonatal Med. 2019, 32, 4128–4132. [Google Scholar] [CrossRef]
- Ratiu, D.; Hide-Moser, K.; Morgenstern, B.; Gottschalk, I.; Eichler, C.; Ludwig, S.; Grüttner, B.; Mallmann, P.; Thangarajah, F. Doppler Indices and Notching Assessment of Uterine Artery Between the 19th and 22nd Week of Pregnancy in the Prediction of Pregnancy Outcome. In Vivo 2019, 33, 2199–2204. [Google Scholar] [CrossRef]
- Barati, M.; Shahbazian, N.; Ahmadi, L.; Masihi, S. Diagnostic evaluation of uterine artery Doppler sonography for the prediction of adverse pregnancy outcomes. J. Res. Med. Sci. 2014, 19, 515–519. [Google Scholar]
- Llurba, E.; Carreras, E.; Gratacós, E.; Juan, M.; Astor, J.; Vives, A.; Hermosilla, E.; Calero, I.; Millán, P.; García-Valdecasas, B.; et al. Maternal History and Uterine Artery Doppler in the Assessment of Risk for Development of Early- and Late-Onset Preeclampsia and Intrauterine Growth Restriction. Obstet. Gynecol. Int. 2009, 2009, 275613. [Google Scholar] [CrossRef]
- Maged, A.M.; Saad, H.; Meshaal, H.; Salah, E.; Abdelaziz, S.; Omran, E.; Deeb, W.S.; Katta, M. Maternal serum homocysteine and uterine artery Doppler as predictors of preeclampsia and poor placentation. Arch. Gynecol. Obstet. 2017, 296, 475–482. [Google Scholar] [CrossRef]
- Medjedovic, E.; Kurjak, A. The Importance of Doppler Analysis of Uterine Circulation in Pregnancy for a Better Understanding of Preeclampsia. Med. Arch. 2021, 75, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yue, C. Abnormal uterine artery Doppler ultrasound during gestational 21–23 weeks associated with pre-eclampsia. Int. J. Gynecol. Obstet. 2022, 161, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.; Karouni, F.; Katsanevakis, E.; Kapaya, H. Implementation of Uterine Artery Doppler Scanning: Improving the Care of Women and Babies High Risk for Fetal Growth Restriction. J. Pregnancy 2023, 2023, 1506447. [Google Scholar] [CrossRef] [PubMed]
- Običan, S.G.; Odibo, L.; Tuuli, M.G.; Rodriguez, A.; Odibo, A.O. Third trimester uterine artery Doppler indices as predictors of preeclampsia and neonatal small for gestational age. J. Matern. Neonatal Med. 2019, 33, 3484–3489. [Google Scholar] [CrossRef]
- Chilumula, K.; Saha, P.K.; Muthyala, T.; Saha, S.C.; Sundaram, V.; Suri, V. Prognostic role of uterine artery Doppler in early- and late-onset preeclampsia with severe features. J. Ultrasound 2020, 24, 303–310. [Google Scholar] [CrossRef]
| Nr. | Authors | Study Type | N | Objective | Conclusion |
|---|---|---|---|---|---|
| 1 | Verlohren, 2014 [23] | Prospective, multicentric | 877 | To establish gestational age-dependent cut-offs for the use of sFlt-1/PlGF ratio as a prognostic tool for PE | For early-onset PE, the sFlt-1/PlGF ratio ≤33 had the highest likelihood of a negative test, whereas values ≥ 85 had the highest likelihood of a positive test. For late-onset PE, the cut-offs were ≤ 33 and the rule-out and rule-in were ≥110 PE. |
| 2 | Zeisler, 2016—PROGNOSIS study [24] | Prospective, multicentric | 1050 | To assess if a low sFlt-1/PlGF ratio (at or below a cut-off) predicts the absence of PE within 1 week and whether a high ratio (above the cut-off) rules in PE within 4 weeks | sFlt-1/PlGF ≤ 38 ruled out PE within one week, regardless of gestational age, in women with clinically suspected PE (NPV 99.3%). sFlt-1/PlGF > 38 predicts PE occurrence within 4 weeks and a shorter time until delivery. |
| 3 | Bian, 2019—PROGNOSIS Asia study [25] | Prospective, multicentric | 764 | To assess the value of a sFlt-1/PlGF ratio for ruling out PE within 1 week To evaluate the predictive value of the ratio for fetal adverse outcomes | sFlt-1/PlGF ≤ 38 ruled out PE within one week with a similar performance as that of compared to PROGNOSIS study (NPV 98.6%). sFlt-1/PlGF was also a predicitve marker of PE at any time during pregnancy, as well as fetal adverse outcomes, shorter pregnancy duration, and pre-term delivery. |
| 4 | Zeisler, 2019 [26] | Prospective, multicentric | 550 |
To assess the predictive value of the sFlt-1/PlGF ratio to rule out the onset of PE within 4 weeks in patients with suspected PE To assess the value of repeated measurements |
In women with a sFlt-1/PlGF ratio of ≤38, PE can be ruled out for up to 4 weeks with a NPV of ≥94%. Retesting 2 or 3 weeks after the initial test improves risk stratification and decision-making. |
| 5 | Cerdeira, 2019—INSPIRE study [28] | Prospective, monocentric | 370 | To assess the sFlt-1/PlGF ratio’s ability to detect PE in daily clinical practice in cases of high-risk women | sFlt-1/PlGF ≤ 38 (low risk)/ > 38 (high risk) rules out/in PE within one week with better sensitivity and specificity than clinical practice alone, significantly improving clinical precision. |
| 6 | Caillon, 2018 [30] | Prospective, monocentric | 67 | To evaluate the routine use of the sFlt-1/PlGF ratio in a population of high-risk patients to predict PE | A cut-off < 38 for the sFlt-1/PlGF ratio is reliable for ruling out PE within 4 weeks (NPV 100%) |
| 7 | Perales, 2017—STEPS study [31] | Prospective, multicentric | 819 | To evaluate the sFlt-1/PlGF ratio as a predictive marker of early-onset PE in women at risk of PE | Early-onset PE: the sFlt-1/PlGF ratio measured between 20 and 28 weeks can improve prediction for women at risk. Late-onset PE: Only sFlt-1/PlGF measured at 28 weeks improves prediction. |
| 8 | Jeon, 2021 [44] | Prospective, monocentric | 73 | To assess sFlt-1/PlGF’s usefulness in terms of predicting adverse pregnancy outcomes in PE | sFlt-1/PlGF ≥ 85 (high-risk) predicted pre-term birth, reduced neonatal weight, and the need for neonatal intensive care. |
| 9 | Diguisto, 2017 [32] | Prospective, multicentric | 226 | To evaluate the accuracy of angiogenic biomarkers in the first trimester for PE screening in a high-risk population | PlGF and the sFlt-1/PlGF ratio are useful first-trimester markers involved in the prediction of PE. |
| 10 | Soundararajan, 2021—ROBUST study [33] | Prospective, monocentric | 50 | To evaluate if sFlt-1/PlGF may be used to predict the severity of PE among women at high risk | Patients with a sFlt-1/PlGF ratio > 85 carried a significant risk of developing severe PE associated with pre-term birth. |
| 11 | Tan, 2017 [34] | Prospective, monocentric | 8063 | To estimate at 31–34 weeks of gestation the patient-specific risk of PE via a combination of maternal characteristics and the sFlt-1/PlGF ratio To compare the performance of screening to that achieved only using the sFlt-1/PlGF ratio | Similar performance at 31–34 weeks in predicting delivery within the next four weeks due to PE between the combination and sFlt-1/PLGF alone. The combination displayed superior performance in the prediction of delivery due to PE four weeks after the assessment. |
| 12 | Ciciu, 2023 [36] | Prospective, monocentric | 127 | To assess the clinical utility of the sFtl-1/PIGF ratio in determining the diagnosis and severity of PE | sFlt-1/PlGF values may help differentiate between mild forms and severe forms of PE. sFlt-1/PlGF values can discriminate between PE and gestational hypertension. |
| 13 | Nikuei, 2020 [38] | Prospective, monocentric | 58 | To evaluate diagnostic accuracy of the sFlt-1/PlGF ratio for different forms of PE | The sFlt-1/PlGF ratio showed higher accuracy in terms of differentiating between PE and non-PE patients than for differentiating between severe or early-onset forms of PE. |
| 14 | Leanos-Miranda, 2020 [39] | Prospective, monocentric | 810 | To compare outcomes according to the degree of angiogenic imbalance, as assessed based on the levels of sFlt-1/PlGF in patients already diagnosed with PE (severe imbalance: ratio ≥ 85, mild imbalance: ratio 39–84, no imbalance: ratio ≤ 38) | Rates of pre-term delivery, delivery within 14 days, and delivery of a SGA infant were significantly higher among patients with severe angiogenic imbalance than patients with mild and no imbalance and among patients with mild imbalance vs. no imbalance. HELLP syndrome occurred only in the severe imbalance group. |
| 15 | Baltajian, 2016 [40] | Prospective, monocentric | 100 | To analyze sequential levels of plasma angiogenic factors among patients admitted due to PE | sFlt-1/PlGF ≥ 85 predicted a shorter time to delivery than sFlt-1/PlGF < 85 (6 days vs. 14 days). Significantly higher median sFlt-1/PlGF on admission for patients who presented adverse outcomes (205.9 vs. 47.5). |
| 16 | Peguero, 2021 [41] | Prospective, multicentric | 63 | To assess the potential influence of longitudinal changes in sFlt1/PlGF levels on the prediction of adverse outcomes among women with early-onset severe PE | Longitudinal daily changes in the sFlt-1/PlGF ratio correlated with a shorter time until delivery. Significantly increased daily changes in sFlt-1 values were recorded for those women who developed adverse outcomes: 1047 vs. 342 pg/mL/day. |
| 17 | Garcia-Manau, 2020 [42] | Prospective, monocentric | 207 | To compare sFlt-1/PlGF values and pregnancy outcomes among early-onset SGA/FGR stages | sFlt-1/PlGF values at diagnosis allowed the stratification of FGR severity. |
| 18 | Herraiz, 2018 [35] | Prospective, monocentric | 5601 | To analyze the usefulness of a clinical protocol for early detection of PE and FGR based on the measurement of the sFlt-1/PlGF ratio at 24–28 weeks of gestation | The sFlt-1/PlGF ratio and >95th centile, when measured at 24–28 weeks, were effective at predicting early-onset PE with FGR requiring delivery before 32 weeks. |
| 19 | Gaccioli, 2018 [46] | Prospective, monocentric | 4098 | To determine the effectiveness of sFlt-1/PlGF in predicting adverse pregnancy outcomes associated with the delivery of a SGA | A sFlt-1/PlGF ratio > 5.78 at 28 weeks was highly predictive of PE with pre-term delivery when the infant was SGA. sFlt-1/PlGF values > 38 at 36 weeks were predictive of the term delivery of a SGA infant with maternal PE. |
| 20 | Heimberger, 2020 [47] | Retrospective, monocentric | 115 | To compare characteristics and outcomes of women with chronic hypertension | sFlt-1/PlGF ≥ 85 increased the risk of pre-term delivery at <34 and <37 weeks, lower gestational age at delivery, superimposed PE, and severe PE. |
| 21 | Binder, 2020 [50] | Retrospective, monocentric | 164 | To evaluate the predictive value of the sFlt-1/PlGF ratio for delivery because of PE in twin pregnancies | sFlt-1/PlGF < 38 was useful in ruling out pre-term delivery due to PE. |
| 22 | Gaccioli, 2023 [48] | Prospective, monocentric | 4212 | To determine the relationship between maternal serum and placental levels of sFlt-1 and PlGF in women with a diagnosis of PE or IUGR | The contribution of sFlt-1 and PlGF to the increased sFlt-1/PlGF ratio are different in PE and IUGR: increased placental sFlt-1 imbalances the ratio in PE, whereas decreased PlGF imbalances the ratio in IUGR. |
| 23 | Palma Dos Reis, 2023 [49] | Prospective, monocentric | 125 | To evaluate if sFlt-1/PlGF ratio predicts faster fetal deterioration in early FGR | sFlt-1/PlGF > 85 predicted faster fetal deterioration, independently of PE, etc. |
| 24 | Binder, 2021 [51] | Retrospective, monocentric | 142 | To investigate the ability of sFlt-1/PlGF to predict superimposed PE or adverse pregnancy outcomes among patients with chronic hypertension | sFlt-1/PlGF significanlty improved the prediction of superimposed PE and adverse outcomes, such as stillbirth, pre-term delivery, etc. |
| 25 | Duhig, 2021—The PEACOCK study [52] | Prospective, multicentric | 36 | To assess the performance of sFlt-1/PlGF in predicting the need for delivery within seven days among women with late pre-term PE | In late pre-term PE, sFlt-1/PlGF did not add value to the clinical assessment. |
| Nr. | Authors | Study Type | N | Objective | Conclusion |
|---|---|---|---|---|---|
| 1 | Adekanmi, 2019 [46] | Prospective, monocentric | 93 | To develop accurate prediction models that identify women at high risk of PE and allow appropriate interventions | Cases that develop PE showed significantly lower uterine and umbilical PSV and EDV and higher uterine RI, PI, and S/D levels. Uterine PI is the best predictor for PE, while a combination of uterine and umbilical PSV predicted severity of PE. |
| 2 | Trongpisutsak, 2021 [47] | Prospective, monocentric | 443 | To assess if uterine artery Doppler at 16–24 weeks can predict PE | The optimal cut-off for PI was 1.025. Uterine diastolic notching also predicted PE. |
| 3 | Soongsatitanon, 2020 [48] | Prospective, monocentric | 353 | To determine the predictive value for PE using uterine PI in the first trimester | Uterine artery PI > 95th centile (2.17) in the first trimester was a marker of PE. |
| 4 | Oancea, 2020 [49] | Prospective, monocentric | 120 | To evaluate the potential of first-trimester uterine artery Doppler regarding early detection of PE in high-risk patients | Uterine PI in first trimester showed moderate predictive power. |
| 5 | Diguisto, 2017 [25] | Prospective, multicentric | 226 | To evaluate the accuracy of uterine artery Doppler in the first trimester for PE screening | Mean PI, lowest PI, mean RI, and bilateral notching were reliable first-trimester parameters in PE screening. |
| 6 | Prakansamut, 2019 [50] | Prospective, monocentric | 405 | To assess if uterine artery Doppler at 11–14 weeks can predict PE | No relevant PI values or presence of uterine notching in PE prediction. |
| 7 | Abdel Razik, 2018 [51] | Prospective, monocentric | 270 | To evaluate the role of uterine artery Doppler at 20–24 weeks in the prediction of PE | Cut-off values for mean PI (≥1.14), as well as mean RI (>0.61), were reported. |
| 8 | Ratiu, 2019 [52] | Prospective, monocentric | 1472 | To evaluate if uterine artery Doppler waveform analysis and the presence of a notch in the second trimester in unselected women with singleton pregnancies correlate with significant differences in common pregnancy outcomes | Bilateral high RI and PI and a notch significantly increased the development of SGA and IUGR. The presence of a notch significantly increases the development of severe PE, HELLP syndrome, and oligohydramnios. Bilateral notching was associated with IUGR or SGA at the screening time. |
| 9 | Barati, 2014 [53] | Prospective, monocentric | 379 | To investigate the predictive value of uterine artery Doppler in the identification of adverse pregnancy outcomes | Mean uterine artery PI > 1.45 measured at 16–22 weeks predicted an increased risk of PE, SGA, and pre-term delivery. |
| 10 | Llurba, 2009 [54] | Prospective, multicentric | 6856 | To examine the value of one-time uterine artery Doppler examination, performed at 20 weeks, in the prediction of PE and IUGR in a population of unselected patients | Doppler screening of the uterine arteries at 20 weeks was a feasible tool for the detection of pregnant women with a high risk for early-onset adverse outcomes, such as PE and IUGR. |
| 11 | Maged, 2017 [55] | Prospective, monocentric | 453 | To evaluate the role of uterine artery Doppler at 18–22 weeks as a predictor of PE and IUGR | Elevated uterine RI was a valuable marker that predicted PE (cut-off 0.55) and IUGR (cut-off 0.54). |
| 12 | Medjedovic, 2021 [56] | Prospective, multicentric | 80 | To investigate ultrasound risk factors for PE | Uterine artery notching (especially if present bilaterally) is a strong predictor. |
| 13 | Ekanem, 2023 [58] | Retrospective, multicentric | To evaluate the role of Doppler examination of uterine arteries at 20–24 weeks of gestation in the screening of IUGR in a population with risk factors | The implementation of uterine artery Doppler screening to predict high-risk IUGR pregnancies should include evaluation of notching besides mean PI at 20–24 weeks. | |
| 14 | Obican, 2019 [59] | Prospective, monocentric | 200 | To assess the third-trimester uterine artery Doppler value in predicting adverse pregnancy outcomes in high-risk women | Left uterine artery notching and PI > 95th centile were associated with SGA, early-onset PE, and PE. |
| 15 | Chilumula, 2021 [60] | Prospective, monocentric | 60 | To correlate uterine artery Doppler results with maternal and neonatal outcomes in early- and late-onset severe PE | In early-onset PE, abnormal uterine Doppler results increased the risk of both maternal and neonatal complications. For late-onset PE, abnormal Doppler only predicted perinatal complications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirilă, C.N.; Mărginean, C.; Chirilă, P.M.; Gliga, M.L. The Current Role of the sFlt-1/PlGF Ratio and the Uterine–Umbilical–Cerebral Doppler Ultrasound in Predicting and Monitoring Hypertensive Disorders of Pregnancy: An Update with a Review of the Literature. Children 2023, 10, 1430. https://doi.org/10.3390/children10091430
Chirilă CN, Mărginean C, Chirilă PM, Gliga ML. The Current Role of the sFlt-1/PlGF Ratio and the Uterine–Umbilical–Cerebral Doppler Ultrasound in Predicting and Monitoring Hypertensive Disorders of Pregnancy: An Update with a Review of the Literature. Children. 2023; 10(9):1430. https://doi.org/10.3390/children10091430
Chicago/Turabian StyleChirilă, Cristian Nicolae, Claudiu Mărginean, Paula Maria Chirilă, and Mirela Liana Gliga. 2023. "The Current Role of the sFlt-1/PlGF Ratio and the Uterine–Umbilical–Cerebral Doppler Ultrasound in Predicting and Monitoring Hypertensive Disorders of Pregnancy: An Update with a Review of the Literature" Children 10, no. 9: 1430. https://doi.org/10.3390/children10091430
APA StyleChirilă, C. N., Mărginean, C., Chirilă, P. M., & Gliga, M. L. (2023). The Current Role of the sFlt-1/PlGF Ratio and the Uterine–Umbilical–Cerebral Doppler Ultrasound in Predicting and Monitoring Hypertensive Disorders of Pregnancy: An Update with a Review of the Literature. Children, 10(9), 1430. https://doi.org/10.3390/children10091430







