Exercise Capacity in Very Low Birth Weight Adults: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Analysis
2.5. Quality Assessment
3. Results
3.1. Identified Studies and Characteristics
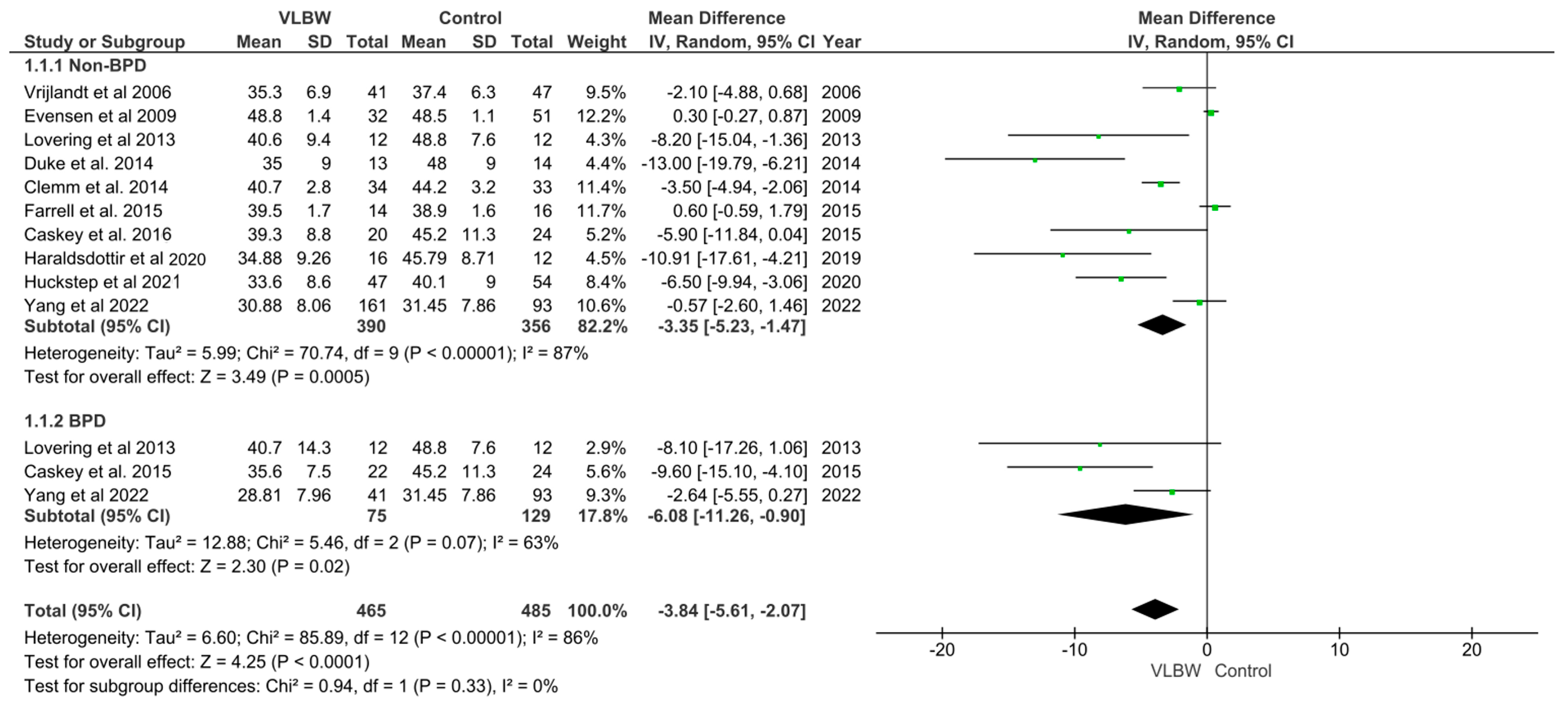
3.2. VO2 Max in VLBW Infants
3.3. Levels of Physical Activity in VLBW Infants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Nutrition Targets 2025. Low Birth Weight Policy Brief. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.5 (accessed on 17 October 2014).
- Jeschke, E.; Biermann, A.; Günster, C.; Böhler, T.; Heller, G.; Hummler, H.D.; Bührer, C. Mortality and major morbidity of very-low-birth-weight infants in Germany 2008-2012: A report based on administrative data. Front. Pediatr. 2016, 4, 23. [Google Scholar] [CrossRef]
- Cartlidge, P.H.; Stewart, J.H. Survival of very low birthweight and very preterm infants in a geographically defined population. Acta Paediatr. 1997, 86, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A.; et al. Preterm heart in adult life cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013, 127, 197. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Bradlow, W.M.; Augustine, D.; Davis, E.F.; Francis, J.; Singhal, A.; Lucas, A.; Neubauer, S.; McCormick, K.; Leeson, P. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013, 128, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; Cooper, J.; Bethell, M.S.; McCracken, C.; Lewandowski, A.J.; Leeson, P.; Neubauer, S.; Harvey, N.C.; Petersen, S.E. Lower birth weight is linked to poorer cardiovascular health in middle-aged population-based adults. Heart 2023, 109, 535–551. [Google Scholar] [CrossRef]
- Greenough, A. Does low birth weight confer a lifelong respiratory disadvantage? Am. J. Respir. Crit. Care Med. 2009, 180, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Huckstep, O.; Williamson, W.; Telles, F.; Burchert, H.; Bertagnolli, M.; Herdman, C.; Arnold, L.; Smillie, R.; Mohamed, A.; Boardman, H.; et al. Physiological stress elicits impaired left ventricular function in preterm-born adults. J. Am. Coll. Cardiol. 2018, 71, 1347–1356. [Google Scholar] [CrossRef]
- Mohamed, A.; Marciniak, M.; Williamson, W.; Huckstep, O.J.; Lapidaire, W.; McCance, A.; Neubauer, S.; Leeson, P.; Lewandowski, A.J. Association of systolic blood pressure elevation with disproportionate left ventricular remodeling in very preterm-born young adults: The preterm heart and elevated blood pressure. JAMA Cardiol. 2021, 6, 821–829. [Google Scholar] [CrossRef]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Birthweight and mortality in adulthood: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 647–661. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Y.; Rich-Edwards, J.W.; Florio, A.A.; Shan, Z.; Wang, S.; Manson, J.A.; Mukamal, K.J.; Rimm, E.B.; Chavarro, J.E. Associations of birth weight and later life lifestyle factors with risk of cardiovascular disease in the USA: A prospective cohort study. EClinicalMedicine 2022, 51, 101570. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Godfrey, K.M.; Fall, C.; Osmond, C.; Winter, P.D.; Shaheen, S.O. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. Br. Med. J. 1991, 303, 671–675. [Google Scholar] [CrossRef]
- Dyson, A.; Kent, A.L. The effect of preterm birth on renal development and renal health outcome. Neoreviews 2019, 20, e725–e736. [Google Scholar] [CrossRef] [PubMed]
- Bonadies, L.; Cavicchiolo, M.E.; Priante, E.; Moschino, L.; Baraldi, E. Prematurity and BPD: What general practitioners should know. Eur. J. Pediatr. 2023, 182, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V.; Lupton, H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. QJM 1923, 16, 62. [Google Scholar] [CrossRef]
- Bye, A.; Røsjø, H.; Aspenes, S.T.; Condorelli, G.; Omland, T.; Wisløff, U. Circulating MicroRNAs and Aerobic Fitness—The HUNT-Study. PLoS ONE 2013, 8, e57496. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Kaminsky, L.A.; Arena, R.; Blair, S.N.; Franklin, B.A.; Myers, J.; Ross, R. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: Advances since 2009. Prog. Cardiovasc. Dis. 2017, 60, 11–20. [Google Scholar] [CrossRef]
- Edwards, M.O.; Kotecha, S.J.; Lowe, J.; Watkins, W.J.; Henderson, A.J.; Kotecha, S. Effect of preterm birth on exercise capacity: A systematic review and meta-analysis. Pediatr. Pulmonol. 2015, 50, 293–301. [Google Scholar] [CrossRef]
- Gostelow, T.; Stöhr, E.J. The effect of preterm birth on maximal aerobic exercise capacity and lung function in healthy adults: A systematic review and meta-analysis. Sports Med. 2022, 52, 2627–2635. [Google Scholar] [CrossRef]
- Agusti, A.; Faner, R. Lung function trajectories in health and disease. Lancet Respir. Med. 2019, 7, 358–364. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef]
- di Prampero, P.E. Factors limiting maximal performance in humans. Eur. J. Appl. Physiol. 2003, 90, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Clemm, H.H.; Vollsæter, M.; Røksund, O.D.; Eide, G.E.; Markestad, T.; Halvorsen, T. Exercise capacity after extremely preterm birth: Development from adolescence to adulthood. Ann. Am. Thorac. Soc. 2014, 11, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Strang-Karlsson, S.; Hovi, P.; Räikkönen, K.; Pesonen, A.K.; Heinonen, K.; Järvenpää, A.L.; Eriksson, J.G.; Andersson, S. Adults born at very low birth weight exercise less than their peers born at term. J. Pediatr. 2010, 157, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Engan, M.; Vollsæter, M.; Øymar, K.; Markestad, T.; Eide, G.E.; Halvorsen, T.; Juliusson, P.; Clemm, H. Comparison of physical activity and body composition in a cohort of children born extremely preterm or with extremely low birth weight to matched term-born controls: A follow-up study. BMJ Paediatr. Open 2019, 3, e000481. [Google Scholar] [CrossRef] [PubMed]
- Welsh, L.; Kirkby, J.; Lum, S.; Odendaal, D.; Marlow, N.; Derrick, G.; Stocks, J. The EPICure study: Maximal exercise and physical activity in school children born extremely preterm. Thorax 2010, 65, 165–172. [Google Scholar] [CrossRef]
- University of York, National Institute for Health and Care Research (2023). PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 22 June 2023).
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- The Cochrane Collaberation. Literature Search Filters for Neonatal Reviews. 2023. Available online: https://neonatal.cochrane.org/Literature-Search-Filters-for-Neonatal-Reviews (accessed on 26 June 2023).
- Whipp, B.J.; Ward, S.A. Physiological determinants of pulmonary gas exchange kinetics during exercise. Med. Sci. Sports Exerc. 1990, 22, 62–71. [Google Scholar] [CrossRef]
- Review Manager Web (RevMan Web). Version (5.4). The Cochrane Collaboration. Available online: Revman.cochrane.org (accessed on 1 August 2023).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment form for Cohort Studies; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014. [Google Scholar]
- Sidwell, K. Newcastle—Ottawa Quality Assessment Scale Case Control Studies. Available online: https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed on 27 June 2023).
- Vrijlandt, E.J.; Gerritsen, J.; Boezen, H.M.; Grevink, R.G.; Duiverman, E.J. Lung function and exercise capacity in young adults born prematurely. Am. J. Respir. Crit. Care Med. 2006, 173, 890–896. [Google Scholar] [CrossRef]
- Evensen, K.A.; Steinshamn, S.; Tjønna, A.E.; Stølen, T.; Høydal, M.A.; Wisløff, U.; Brubakk, A.M.; Vik, T. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum. Dev. 2009, 85, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Narang, I.; Bush, A.; Rosenthal, M. Gas transfer and pulmonary blood flow at rest and during exercise in adults 21 years after preterm birth. Am. J. Respir. Crit. Care Med. 2009, 180, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Sipola-Leppänen, M.; Hovi, P.; Andersson, S.; Wehkalampi, K.; Vääräsmäki, M.; Strang-Karlsson, S.; Järvenpää, A.L.; Mäkitie, O.; Eriksson, J.G.; Kajantie, E. Resting energy expenditure in young adults born preterm--the Helsinki study of very low birth weight adults. PLoS ONE 2011, 6, e17700. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.; Laurie, S.; Elliott, J.; Beasley, K.; Yang, X.; Gust, C.; Mangum, T.S.; Goodman, R.D.; Hawn, J.A.; Gladstone, I.M. Normal pulmonary gas exchange efficiency and absence of exercise-induced arterial hypoxemia in adults with bronchopulmonary dysplasia. J. Appl. Physiol. 2013, 115, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.W.; Lewandowski, A.J.; Abman, S.H.; Lovering, A.T. Physiological aspects of cardiopulmonary dysanapsis on exercise in adults born preterm. J. Physiol. 2022, 600, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Saarenpää, H.K.; Tikanmäki, M.; Sipola-Leppänen, M.; Hovi, P.; Wehkalampi, K.; Siltanen, M.; Vääräsmäki, M.; Järvenpää, A.L.; Eriksson, J.G.; Andersson, S.; et al. Lung function in very low birth weight adults. Pediatrics 2015, 136, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.T.; Bates, M.L.; Pegelow, D.F.; Palta, M.; Eickhoff, J.C.; O’Brien, M.J.; Eldridge, M.W. Pulmonary gas exchange and exercise capacity in adults born preterm. Ann. Am. Thorac. Soc. 2015, 12, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Caskey, S.; Gough, A.; Rowan, S.; Gillespie, S.; Clarke, J.; Riley, M.; Megamy, J.; Nicholls, P.; Patterson, C.; Halliday, H.L.; et al. Structural and functional lung impairment in adult survivors of bronchopulmonary dysplasia. Ann. Am. Thorac. Soc. 2016, 13, 1262–1270. [Google Scholar] [CrossRef]
- Kaseva, N.; Wehkalampi, K.; Strang-Karlsson, S.; Salonen, M.; Pesonen, A.K.; Räikkönen, K.; Tammelin, T.; Hovi, P.; Lahti, J.; Heinonen, K.; et al. Lower conditioning leisure-time physical activity in young adults born preterm at very low birth weight. PLoS ONE 2012, 7, e32430. [Google Scholar] [CrossRef]
- Haraldsdottir, K.; Watson, A.M.; Pegelow, D.F.; Palta, M.; Tetri, L.H.; Levin, T.; Brix, M.D.; Centanni, R.M.; Goss, K.N.; Eldridge, M.M. Blunted cardiac output response to exercise in adolescents born preterm. Eur. J. Appl. Physiol. 2020, 120, 2547–2554. [Google Scholar] [CrossRef]
- Yang, J.; Epton, M.J.; Harris, S.L.; Horwood, J.; Kingsford, R.A.; Troughton, R.; Greer, C.; Darlow, B.A. Reduced exercise capacity in adults born at very low birth weight a population-based cohort study. Am. J. Respir. Crit. Care Med. 2022, 205, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.W.; Elliott, J.E.; Laurie, S.S.; Beasley, K.M.; Mangum, T.S.; Hawn, J.A.; Gladstone, I.M.; Lovering, A.T. Pulmonary gas exchange efficiency during exercise breathing normoxic and hypoxic gas in adults born very preterm with low diffusion capacity. J. Appl. Physiol. 2014, 117, 381–473. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.L.; Olsen, J.E.; Konstan, T.; Mainzer, R.M.; Hickey, L.M.; Spittle, A.J.; Wark, J.D.; Cheung, M.M.; Garland, S.M.; Duff, J.; et al. Growth from infancy to adulthood and associations with cardiometabolic health in individuals born extremely preterm. Lancet Reg. Health Wet. Pac. 2023, 34, 100717. [Google Scholar] [CrossRef]
- Huckstep, O.J.; Burchert, H.; Williamson, W.; Telles, F.; Tan, C.M.; Bertagnolli, M.; Arnold, L.; Mohamed, A.; McCormick, K.; Hanssen, H.; et al. Impaired myocardial reserve underlies reduced exercise capacity and heart rate recovery in preterm-born young adults. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 572–580. [Google Scholar] [CrossRef]
- Jarvis, D. The European Community Respiratory Health Survey II. Eur. Respir. J. 2002, 20, 1071–1079. [Google Scholar]
- Harris, C.; Lunt, A.; Peacock, J.; Greenough, A. Lung function at 16–19 years in males and females born very prematurely. Pediatr. Pulmonol. 2023, 58, 2035–2041. [Google Scholar] [CrossRef]
- Bassett, D.R. Scientific contributions of A. V. Hill: Exercise physiology pioneer. J. Appl. Physiol. 2002, 93, 1567–1582. [Google Scholar] [CrossRef]
- Flegal, K.M.; Shephard, J.A.; Looker, A.C.; Graubard, B.I.; Borrud, L.G.; Ogden, C.L.; Harris, T.B.; Everhart, J.E.; Schenker, N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am. J. Clin. Nutr. 2009, 89, 500–508. [Google Scholar] [CrossRef]
- Akindele, M.O.; Phillips, J.S.; Igumbor, E.U. The relationship between body fat percentage and body mass index in overweight and obese individuals in an urban African setting. J. Public Health Africa 2016, 7, 515. [Google Scholar] [CrossRef]
- Ranasinghe, C.; Gamage, P.; Katulanda, P.; Andraweera, N.; Thilakarathne, S.; Tharanga, P. Relationship between body mass index (bmi) and body fat percentage, estimated by bioelectrical impedance, in a group of Sri Lankan adults: A cross sectional study. BMC Public Health 2013, 13, 797. [Google Scholar] [CrossRef]
- Shete, A.N.; Bute, S.S.; Deshmukh, P.R. A study of VO2 max and body fat percentage in female athletes. J. Clin. Diagn. Res. 2014, 8, bc01–bc03. [Google Scholar] [PubMed]
- Goran, M.I.; Fields, D.A.; Hunter, G.R.; Herd, S.L.; Weinsier, R.L. Total body fat does not influence maximal aerobic capacity. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.O.; Pichard, C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur. J. Clin. Nutr. 2001, 55, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Scribbans, T.D.; Vecsey, S.; Hankinson, P.B.; Foster, W.S.; Gurd, B.J. The effect of training intensity on VO2max in young healthy adults: A meta-regression and meta-analysis. Int. J. Exerc. Sci. 2016, 9, 230–247. [Google Scholar] [PubMed]
- Crowley, E.; Powell, C.; Carson, B.P.; Davies, R.W. The effect of exercise training intensity on VO2 max in healthy adults: An overview of systematic reviews and meta-analyses. Trans. Sports Med. 2022, 2022, 9310710. [Google Scholar]
- Duke, J.W.; Lovering, A.T. Respiratory and cardiopulmonary limitations to aerobic exercise capacity in adults born preterm. J. Appl. Physiol. 2020, 129, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.W.; Gladstone, I.M.; Sheel, A.W.; Lovering, A.T. Premature birth affects the degree of airway dysanapsis and mechanical ventilatory constraints. Exp. Physiol. 2018, 103, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.N.; Beshish, A.G.; Barton, G.P.; Haraldsdottir, K.; Levin, T.S.; Tetri, L.H.; Battiola, T.J.; Mulchrone, A.M.; Pegelow, D.F.; Palta, M.; et al. Early pulmonary vascular disease in young adults born preterm. Am. J. Respir. Crit. Care Med. 2018, 198, 1549–1558. [Google Scholar] [CrossRef]
- Laurie, S.S.; Elliott, J.E.; Beasley, K.M.; Mangum, T.S.; Goodman, R.D.; Duke, J.W.; Gladstone, I.M.; Lovering, A.T. Exaggerated increase in pulmonary artery pressure during exercise in adults born preterm. Am. J. Respir. Crit. Care Med. 2018, 197, 821–823. [Google Scholar] [CrossRef]
- Parkinson, J.R.; Hyde, M.J.; Gale, C.; Santhakumaran, S.; Modi, N. Preterm birth and the metabolic syndrome in adult life: A systematic review and meta-analysis. Pediatrics 2013, 131, e1240–e1263. [Google Scholar] [CrossRef]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.K. Preterm birth and risk of heart failure up to early adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, M.; Tian, H.; Liu, Z.; Yin, X.; Xi, B. Preterm birth and risk of type 1 and type 2 diabetes: Systematic review and meta-analysis. Obes. Rev. 2014, 15, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Vinogradova, Y.; Robson, J.; Brindle, P. Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: A validation study. Heart 2008, 94, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Burchert, H.; Lapidaire, W.; Williamson, W.; McCourt, A.; Dockerill, C.; Woodward, W.; Tan, C.M.J.; Bertagnolli, M.; Mohamed, A.; Alsharqi, M.; et al. Aerobic exercise training response in preterm born young adults with elevated blood pressure and stage 1 hypertension: A randomised clinical trial. Am. J. Respir. Crit. Care Med. 2023, 207, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, C.; Sampaio, J.; Reis, V.; Sousa, N.; Duarte, J. Physiological responses to treadmill and cycle exercise. Int. J. Sports Med. 2012, 33, 26–30. [Google Scholar] [CrossRef]
- Hermansen, L.; Saltin, B. Oxygen uptake during maximal treadmill and bicycle exercise. J. Appl. Physiol. 1969, 26, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S. Experimental studies of physical fitness in relation to age. Arbeitsphysiologie 1938, 10, 251–323. [Google Scholar] [CrossRef]
- Astrand, I. Aerobic work capacity in men and women with special reference to age. Acta Physiol. Scand Suppl. 1960, 49, 1–92. [Google Scholar]
- Hawkins, S.; Wiswell, R. Rate and mechanism of maximal oxygen consumption decline with aging: Implications for exercise training. Sports Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- Hoover, J.C.; Alenazi, A.M.; Alothman, S.; Alshehri, M.M.; Rucker, J.; Kluding, P. Recruitment for exercise or physical activity interventions: A protocol for systematic review. BMJ Open 2018, 8, e019546. [Google Scholar] [CrossRef]

| Author | Country | Study Design | Number of VLBW Infants | Number of Control Subjects | Age at Follow Up (Years) | Outcome Measures |
|---|---|---|---|---|---|---|
| Vrijlandt et al., 2006 [36] | Netherlands | Prospective cohort study | 42 | 48 | 18–22 |
|
| Evensen et al., 2009 [37] | Norway | Prospective cohort study | 32 | 51 | 18 |
|
| Narang et al., 2009 [38] | UK | Prospective cohort study | 57 | 50 | 20–25 |
|
| Sipola-Leppanen et al., 2011 [39] | Finland | Prospective cohort study | 116 | 118 | 20–28 |
|
| Lovering et al., 2013 [40] | USA | Prospective cohort study | 12 | 12 | 18–27 |
|
| Clemm et al., 2014 [24] | Norway | Prospective cohort study | 34 | 33 | 24–25 |
|
| Duke et al., 2014 [48] | USA | Prospective cohort study | 13 | 14 | 20–25 |
|
| Saarenpaa et al., 2015 [42] | Finland | Prospective cohort study | 160 | 162 | 20–25 |
|
| Farrell et al., 2015 [43] | USA | Prospective cohort study | 14 | 16 | 20–23 |
|
| Caskey et al., 2016 [44] | UK | Prospective cohort study | 20 | 24 | 23–30 |
|
| Kasaeva et al., 2012 [45] | Finland | Prospective cohort study | 94 | 101 | 21–27 |
|
| Haraldsdottir et al., 2020 [46] | USA | Prospective cohort study | 12 | 12 | 24–28 |
|
| Huckstep et al., 2018 [8] | UK | Prospective cohort study | 47 | 54 | 20–26 |
|
| Yang et al., 2022 [47] | New Zealand | Prospective cohort study | 202 | 93 | 26–30 |
|
| Cheong et al., 2023 [49] | Australia | Prospective cohort study | 128 | 126 | 25 |
|
| Study | Birth Weight VLBW Infants (g) | Birth Weight Control Subjects (g) | Age at Follow Up (years) | Weight VLBW Adults (kg) | Weight Control Group (kg) | VO2 Max/Peak Measurement | VO2 Measurement in VLBW Group (mL/kg/min) | VO2 Measurement in Control Group (mL/kg/min) | VO2 Measurement in BPD Group (mL/kg/min) |
|---|---|---|---|---|---|---|---|---|---|
| Vrijlandt et al., 2006 [36] | 1246 ± 232 | - | 18–22 | 65 ± 10 | 72 ± 10 |
| 35.3 ± 6.9 | 20.8 ± 1.2 | - |
| Evensen et al., 2009 [37] | 1245 (800–1500) | 3700 (2670–5140) | 18 | 64.2 ± 1.7 | 69.8 ± 1.3 |
| 48.8 ± 1.4 | 48.5 ± 1.1 | - |
| Lovering et al., 2013 [40] | 1160 ± 450 | - | 21–24 | 64.7 ± 9.3 | 75.7 ± 10.4 |
| 40.6 ± 9.4 | 48.8 ± 7.6 | 40.7 ± 14.3 |
| Clemm et al., 2014 [24] | 1173 ± 163 | - | 24–25 | 71.5 ± 4.3 | 72.3 ± 5.9 |
| 40.7 ± 2.8 | 44.2 ± 3.2 | - |
| Duke et al., 2014 [48] | 1080 ± 430 | - | 20–25 | 65 ± 10 | 72 ± 12 |
| 35.0 ± 9.0 | 48.0 ± 9.0 | - |
| Farrell et al., 2015 [43] | 1027 ± 296 | >1500 | 20–23 | 76.3 ± 5.0 | 71.8 ± 5.4 |
| 39.5 ± 1.7 | 38.9 ± 1.6 | - |
| Caskey et al., 2016 [44] | 1234 ± 205 | 3569 ± 297 | 21–30 | - | - |
| 45.2 ±11.3 | 39.3 ± 8.8 | 35.6 ± 7.5 |
| Haraldsdottir et al., 2020 [46] | <1500 g | - | 24–28 | 70.1 ± 13.3 | 75.6 ± 0.7 |
| 34.88 ± 9.26 | 45.79 + 8.71 | - |
| Huckstep et al., 2021 [50] | 1916 ± 806 | 3390 ± 424 | 22–27 | - | - |
| 33.6 ± 8.6 | 40.1 ± 9.0 | - |
| Yang et al., 2022 [47] | 1131 ± 233 | 3362 ± 529 | 28–29 | 74.1 ± 18.8 | 80.8 ± 16.3 |
| 30.46 ± 8.06 | 31.45 ± 7.86 | 28.3 ± 1.1 |
| Study | Weight VLBW Infants (g) | Weight Control Subjects (g) | Age at Follow Up VLBW Infants (Years) | Age at Follow Up Control Subjects (Years) | PA Assessment Measure | Results | Summary of Impact |
|---|---|---|---|---|---|---|---|
| Vrijlandt et al., 2006 [36] | 1246 ± 232 | - | 19 ± 0.3 | 20.8 ± 1.2 |
|
| |
| Narang et al., 2009 [38] | 1440 ± 550 | 3410 ± 2390 | 21.7 ± 1.2 | 23.1 ± 2.0 |
|
| |
| Sippola-Leppanen et al., 2011 [39] | 1125 ± 223 | 3606 ± 469 | 22.3 ± 2.2 | 22.6 ± 2.2 |
|
| |
| Kaseva et al., 2012 [45] | 1157 ± 208.7 | 3608 ± 492 | 24.9 ± 2.1 | 25.1 ± 2.2 |
|
| |
| Clemm et al., 2014 [24] | 1173 ± 163 | - | 24.7 ± 1.2 | 25.1 ± 1.2 |
|
| |
| Caskey et al., 2016 [44] | 1234 ± 205 | 3569 ± 297 | 26.4 ± 3.7 | 28.3 ± 3.3 |
|
| |
| Saarenpaa et al., 2015 [42] | 1126 ± 218 | 3599 ± 466 | 22.4 ± 2.1 | 22.5 ± 2.5 |
|
| |
| Huckstep et al., 2018 [8] | 1916 ±806 | 3390 ± 424 | 22.7 ± 3.04 | 23.6 ± 3.8 |
|
| |
| Yang et al., 2022 [47] | 1131 ±233 | 3362 ± 529 | 28.3 ± 1.1 | 28.2 ± 0.9 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poole, G.; Harris, C.; Greenough, A. Exercise Capacity in Very Low Birth Weight Adults: A Systematic Review and Meta-Analysis. Children 2023, 10, 1427. https://doi.org/10.3390/children10081427
Poole G, Harris C, Greenough A. Exercise Capacity in Very Low Birth Weight Adults: A Systematic Review and Meta-Analysis. Children. 2023; 10(8):1427. https://doi.org/10.3390/children10081427
Chicago/Turabian StylePoole, Grace, Christopher Harris, and Anne Greenough. 2023. "Exercise Capacity in Very Low Birth Weight Adults: A Systematic Review and Meta-Analysis" Children 10, no. 8: 1427. https://doi.org/10.3390/children10081427
APA StylePoole, G., Harris, C., & Greenough, A. (2023). Exercise Capacity in Very Low Birth Weight Adults: A Systematic Review and Meta-Analysis. Children, 10(8), 1427. https://doi.org/10.3390/children10081427







