Brain Volumes and Cognition in Patients with Sickle Cell Anaemia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Critical Appraisal
2.3. Meta-Analysis
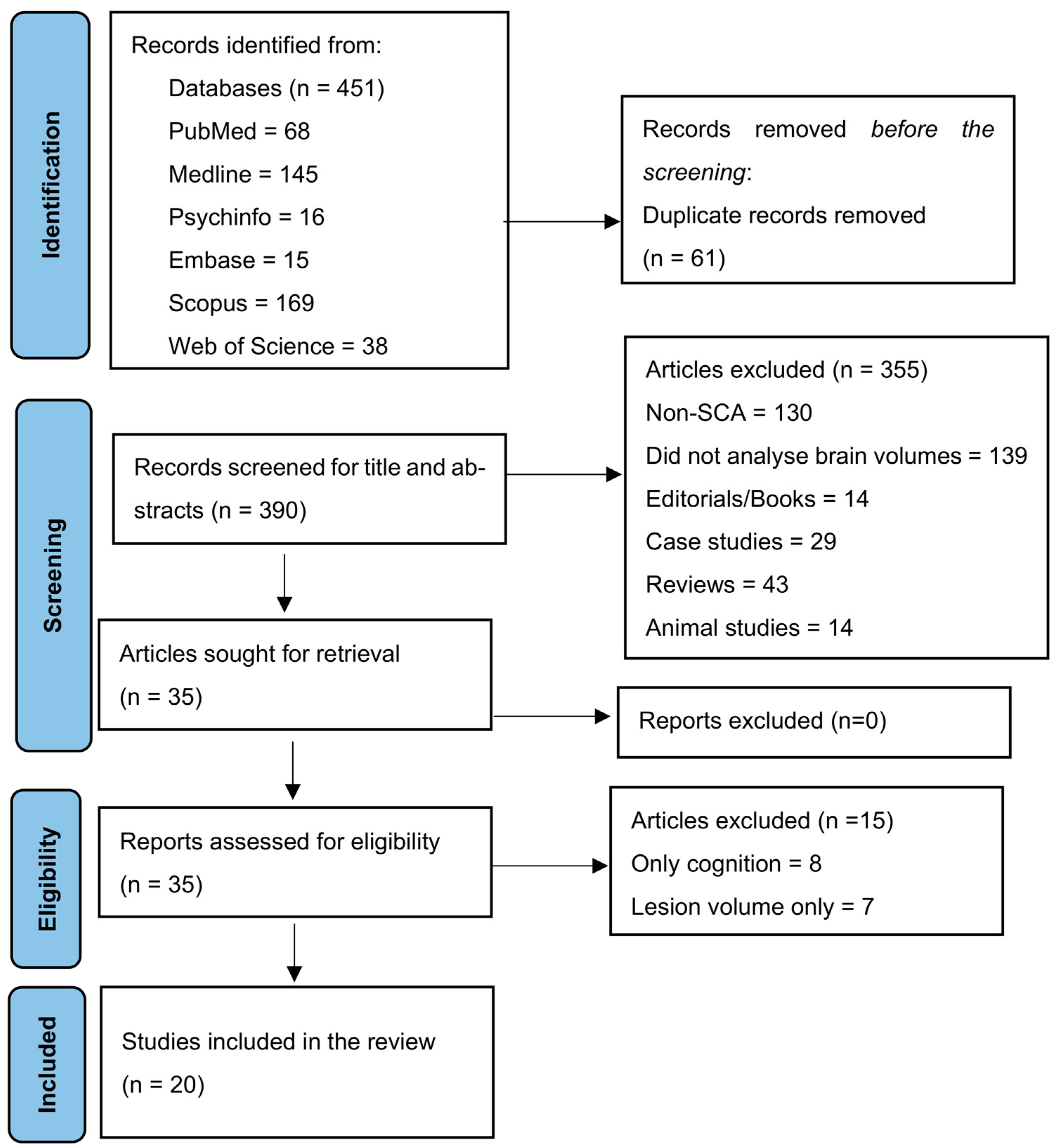
2.4. PRISMA Statement
3. Results
3.1. Characteristics of the Study
3.2. Critical Appraisal
3.3. Meta-Analysis
3.4. Outcomes
- A.
- Grey Matter Volumes
- B.
- White Matter Volumes
- C.
- Subcortical Volumes
- D.
- Total Cortical Atrophy
4. Discussion
4.1. Grey Matter Volumes
4.2. White Matter Volumes
4.3. Subcortical Volumes
4.4. Influence of Cerebral Haemodynamic
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldeweg, T.; Hogan, A.M.; Saunders, D.E.; Telfer, P.; Gadian, D.G.; Vargha-Khadem, F.; Kirkham, F.J. Detecting white matter injury in sickle cell disease using voxel-based morphometry. Ann. Neurol. 2006, 59, 662–672. [Google Scholar] [CrossRef]
- Manfre, L.; Giarratano, E.; Maggio, A.; Banco, A.; Vaccaro, G.; Lagalla, R. MR imaging of the brain: Findings in asymptomatic patients with thalassemia intermedia and sickle cell-thalassemia disease. AJR. Am. J. Roentgenol. 1999, 173, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Moser, F.G.; Miller, S.T.; Bello, J.A.; Pegelow, C.H.; Zimmerman, R.A.; Wang, W.C.; Ohene-Frempong, K.; Schwartz, A.; Vichinsky, E.P.; Gallagher, D.; et al. The spectrum of brain MR abnormalities in sickle-cell disease: A report from the Cooperative Study of Sickle Cell Disease. AJNR. Am. J. Neuroradiol. 1996, 17, 965–972. [Google Scholar] [PubMed]
- Kawadler, J.M.; Clayden, J.D.; Clark, C.A.; Kirkham, F.J. Intelligence quotient in paediatric sickle cell disease: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2016, 58, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kung, W.M.; Yuan, S.P.; Lin, M.S.; Wu, C.C.; Islam, M.M.; Atique, S.; Touray, M.; Huang, C.Y.; Wang, Y.C. Anemia and the Risk of Cognitive Impairment: An Updated Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 777. [Google Scholar] [CrossRef]
- Kral, M.C.; Brown, R.T. Transcranial Doppler ultrasonography and executive dysfunction in children with sickle cell disease. J. Pediatr. Psychol. 2004, 29, 185–195. [Google Scholar] [CrossRef][Green Version]
- Hogan, A.M.; Kirkham, F.J.; Prengler, M.; Telfer, P.; Lane, R.; Vargha-Khadem, F.; Haan, M. An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anaemia. Br. J. Haematol. 2006, 132, 99–107. [Google Scholar] [CrossRef]
- Bakker, M.J.; Hofmann, J.; Churches, O.F.; Badcock, N.A.; Kohler, M.; Keage, H.A. Cerebrovascular function and cognition in childhood: A systematic review of transcranial Doppler studies. BMC Neurol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Prussien, K.V.; Salihu, A.; Abdullahi, S.U.; Galadanci, N.A.; Bulama, K.; Belonwu, R.O.; Kirkham, F.J.; Yarboi, J.; Bemis, H.; DeBaun, M.R.; et al. Associations of transcranial Doppler velocity, age, and gender with cognitive function in children with sickle cell anemia in Nigeria. Child Neuropsychol. 2019, 25, 705–720. [Google Scholar] [CrossRef]
- Sahu, T.; Pande, B.; Sinha, M.; Sinha, R.; Verma, H.K. Neurocognitive Changes in Sickle Cell Disease: A Comprehensive Review. Ann. Neurosci. 2022, 29, 255–268. [Google Scholar] [CrossRef]
- Stotesbury, H.; Hales, P.W.; Hood, A.M.; Koelbel, M.; Kawadler, J.M.; Saunders, D.E.; Sahota, S.; Rees, D.C.; Wilkey, O.; Layton, M.; et al. Individual Watershed Areas in Sickle Cell Anemia: An Arterial Spin Labeling Study. Front. Physiol. 2022, 13, 865391. [Google Scholar] [CrossRef] [PubMed]
- Benites, B.; Silva, C.M.; Campos, B.; Campos, P.M.; Medina, S.S.; Cendes, F.; Olalla-Saad, S.T. Progressive cognitive decline in adults with Sickle Cell Disease (SCD): The effects of the sociocultural background and cerebral atrophy. Hematol. Transfus. Cell Ther. 2020, 42, 52–53. [Google Scholar] [CrossRef]
- Darbari, D.S.; Eigbire-Molen, O.; Ponisio, M.R.; Milchenko, M.V.; Rodeghier, M.J.; Casella, J.F.; McKinstry, R.C.; DeBaun, M.R. Progressive loss of brain volume in children with sickle cell anemia and silent cerebral infarct: A report from the silent cerebral infarct transfusion trial. Am. J. Hematol. 2018, 93, E406–E408. [Google Scholar] [CrossRef]
- Mackin, R.S.; Insel, P.; Truran, D.; Vichinsky, E.P.; Neumayr, L.D.; Armstrong, F.D.; Gold, J.I.; Kesler, K.; Brewer, J.; Weiner, M.W. Neuroimaging abnormalities in adults with sickle cell anemia: Associations with cognition. Neurology 2014, 82, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Hamdule, S.; Kölbel, M.; Stotesbury, H.; Murdoch, R.; Clayden, J.D.; Sahota, S.; Hood, A.M.; Clark, C.A.; Kirkham, F.J. Effects of regional brain volumes on cognition in sickle cell anemia: A developmental perspective. Front. Neurol. 2023, 14, 1101223. [Google Scholar] [CrossRef]
- Vichinsky, E.P.; Neumayr, L.D.; Gold, J.I.; Weiner, M.W.; Rule, R.R.; Truran, D.; Kasten, J.; Eggleston, B.; Kesler, K.; McMahon, L.; et al. Neuropsychological Dysfunction and Neuroimaging Abnormalities in Neurologically Intact Adults With Sickle Cell Anemia. Jama-J. Am. Med. Assoc. 2010, 303, 1823–1831. [Google Scholar] [CrossRef]
- Kawadler, J.M.; Clayden, J.D.; Kirkham, F.J.; Cox, T.C.; Saunders, D.E.; Clark, C.A. Subcortical and cerebellar volumetric deficits in paediatric sickle cell anaemia. Br. J. Haematol. 2013, 163, 373–376. [Google Scholar] [CrossRef]
- Frangou, S.; Chitins, X.; Williams, S.C.R. Mapping IQ and gray matter density in healthy young people. NeuroImage 2004, 23, 800–805. [Google Scholar] [CrossRef]
- Wilke, M.; Sohn, J.-H.; Byars, A.W.; Holland, S.K. Bright spots: Correlations of gray matter volume with IQ in a normal pediatric population. NeuroImage 2003, 20, 202–215. [Google Scholar] [CrossRef]
- CASP. Critical Appraisal Skills Programme UK. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 14 September 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Chen, R.; Pawlak, M.A.; Flynn, T.B.; Krejza, J.; Herskovits, E.H.; Melhem, E.R. Brain morphometry and intelligence quotient measurements in children with sickle cell disease. J. Dev. Behav. Pediatr. JDBP 2009, 30, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Arkuszewski, M.; Krejza, J.; Zimmerman, R.A.; Herskovits, E.H.; Melhem, E.R. A prospective longitudinal brain morphometry study of children with sickle cell disease. Am. J. Neuroradiol. 2015, 36, 403–410. [Google Scholar] [CrossRef]
- Chen, R.; Krejza, J.; Arkuszewski, M.; Zimmerman, R.A.; Herskovits, E.H.; Melhem, E.R. Brain morphometric analysis predicts decline of intelligence quotient in children with sickle cell disease: A preliminary study. Adv. Med. Sci. 2017, 62, 151–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, S.; Bush, A.M.; Borzage, M.T.; Joshi, A.A.; Mack, W.J.; Coates, T.D.; Leahy, R.M.; Wood, J.C. Hemoglobin and mean platelet volume predicts diffuse T1-MRI white matter volume decrease in sickle cell disease patients. Neuroimage Clin. 2017, 15, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; O’Neil, S.H.; Joshi, A.A.; Li, J.; Bush, A.M.; Coates, T.D.; Leahy, R.M.; Wood, J.C. Anemia predicts lower white matter volume and cognitive performance in sickle and non-sickle cell anemia syndrome. Am. J. Hematol. 2019, 94, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Kawadler, J.M.; Murdoch, R.; Ahmed, M.; Tutuba, H.; Masamu, U.; Shmueli, K.; Saunders, D.E.; Clark, C.A.; Kim, J.; et al. Brain volume in Tanzanian children with sickle cell anaemia: A neuroimaging study. Br. J. Haematol. 2023, 201, 114–124. [Google Scholar] [CrossRef]
- Kawadler, J.M.; Clark, C.A.; McKinstry, R.C.; Kirkham, F.J. Brain atrophy in paediatric sickle cell anaemia: Findings from the silent infarct transfusion (SIT) trial. Br. J. Haematol. 2017, 177, 151–153. [Google Scholar] [CrossRef]
- Santini, T.; Koo, M.; Farhat, N.; Campos, V.P.; Alkhateeb, S.; Vieira, M.A.C.; Butters, M.A.; Rosano, C.; Aizenstein, H.J.; Mettenburg, J.; et al. Analysis of hippocampal subfields in sickle cell disease using ultrahigh field MRI. Neuroimage Clin. 2021, 30, 102655. [Google Scholar] [CrossRef]
- Steen, R.G.; Emudianughe, T.; Hankins, G.M.; Wynn, L.W.; Wang, W.C.; Xiong, X.; Helton, K.J. Brain imaging findings in pediatric patients with sickle cell disease. Radiology 2003, 228, 216–225. [Google Scholar] [CrossRef]
- Steen, R.G.; Emudianughe, T.; Hunte, M.; Glass, J.; Wu, S.; Xiong, X.; Reddick, W.E. Brain volume in pediatric patients with sickle cell disease: Evidence of volumetric growth delay? Am. J. Neuroradiol. 2005, 26, 455–462. [Google Scholar]
- Wang, Y.; Hardy, S.J.; Ichesco, E.; Zhang, P.; Harris, R.E.; Darbari, D.S. Alteration of grey matter volume is associated with pain and quality of life in children with sickle cell disease. Transl. Res. J. Lab. Clin. Med. 2022, 240, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain charts for the human lifespan. Nature 2022, 604, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Prussien, K.V.; Compas, B.E.; Siciliano, R.E.; Ciriegio, A.E.; Lee, C.A.; Kassim, A.A.; DeBaun, M.R.; Donahue, M.J.; Jordan, L.C. Cerebral Hemodynamics and Executive Function in Sickle Cell Anemia. Stroke 2021, 52, 1830–1834. [Google Scholar] [CrossRef]
- Stotesbury, H.; Kirkham, F.J.; Kölbel, M.; Balfour, P.; Clayden, J.D.; Sahota, S.; Sakaria, S.; Saunders, D.E.; Howard, J.; Kesse-Adu, R.; et al. White matter integrity and processing speed in sickle cell anemia. Neurology 2018, 90, e2042–e2050. [Google Scholar] [CrossRef]
- Hijmans, C.T.; Grootenhuis, M.A.; Oosterlaan, J.; Heijboer, H.; Peters, M.; Fijnvandraat, K. Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatr. Blood Cancer 2011, 57, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Katus, L.; Hayes, N.J.; Mason, L.; Blasi, A.; McCann, S.; Darboe, M.K.; De Haan, M.; Moore, S.E.; Lloyd-Fox, S.; Elwell, C.E. Implementing neuroimaging and eye tracking methods to assess neurocognitive development of young infants in low- and middle-income countries [version 2; peer review: 2 approved]. Gates Open Res. 2019, 3, 1113. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
Types of studies:
| Types of studies:
|
Methodological aspects:
| Methodological aspects:
|
| Author and Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Grade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baldweg et al., 2006 [1] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Chen et al., 2009 [22] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | - | Satisfactory |
| Chen et al., 2015 [23] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Chen et al., 2017 [24] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Choi et al., 2017 [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Choi et al., 2019 [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Hamdule et al., 2023 [15] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Jacob et al., 2023 [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Kawadler et al., 2013 [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Kawadler et al., 2017 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Mackin et al., 2014 [14] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | - | Good |
| Manfre et al., 1999 [2] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | - | Satisfactory |
| Moser et al., 1996 [3] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | Good |
| Santini et al., 2021 [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Steen et al., 2003 [30] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | Good |
| Steen et al., 2005 [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | Good |
| Vichinsky et al., 2010 [16] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | - | Good |
| Wang et al., 2022 [32] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Chen et al., 2017 [24] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Darbari et al., 2014, 2018 [13] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdule, S.; Kirkham, F.J. Brain Volumes and Cognition in Patients with Sickle Cell Anaemia: A Systematic Review and Meta-Analysis. Children 2023, 10, 1360. https://doi.org/10.3390/children10081360
Hamdule S, Kirkham FJ. Brain Volumes and Cognition in Patients with Sickle Cell Anaemia: A Systematic Review and Meta-Analysis. Children. 2023; 10(8):1360. https://doi.org/10.3390/children10081360
Chicago/Turabian StyleHamdule, Shifa, and Fenella J. Kirkham. 2023. "Brain Volumes and Cognition in Patients with Sickle Cell Anaemia: A Systematic Review and Meta-Analysis" Children 10, no. 8: 1360. https://doi.org/10.3390/children10081360
APA StyleHamdule, S., & Kirkham, F. J. (2023). Brain Volumes and Cognition in Patients with Sickle Cell Anaemia: A Systematic Review and Meta-Analysis. Children, 10(8), 1360. https://doi.org/10.3390/children10081360






