Congenital Syphilis—An Illustrative Review
Abstract
1. Introduction
2. Epidemiology of Syphilis
2.1. Factors Associated with the Increase in Congenital Syphilis in the US
2.2. Factors Associated with the Global Increase in Congenital Syphilis Infection
3. Perinatal Syphilis—Clinical Features, Diagnosis, and Management
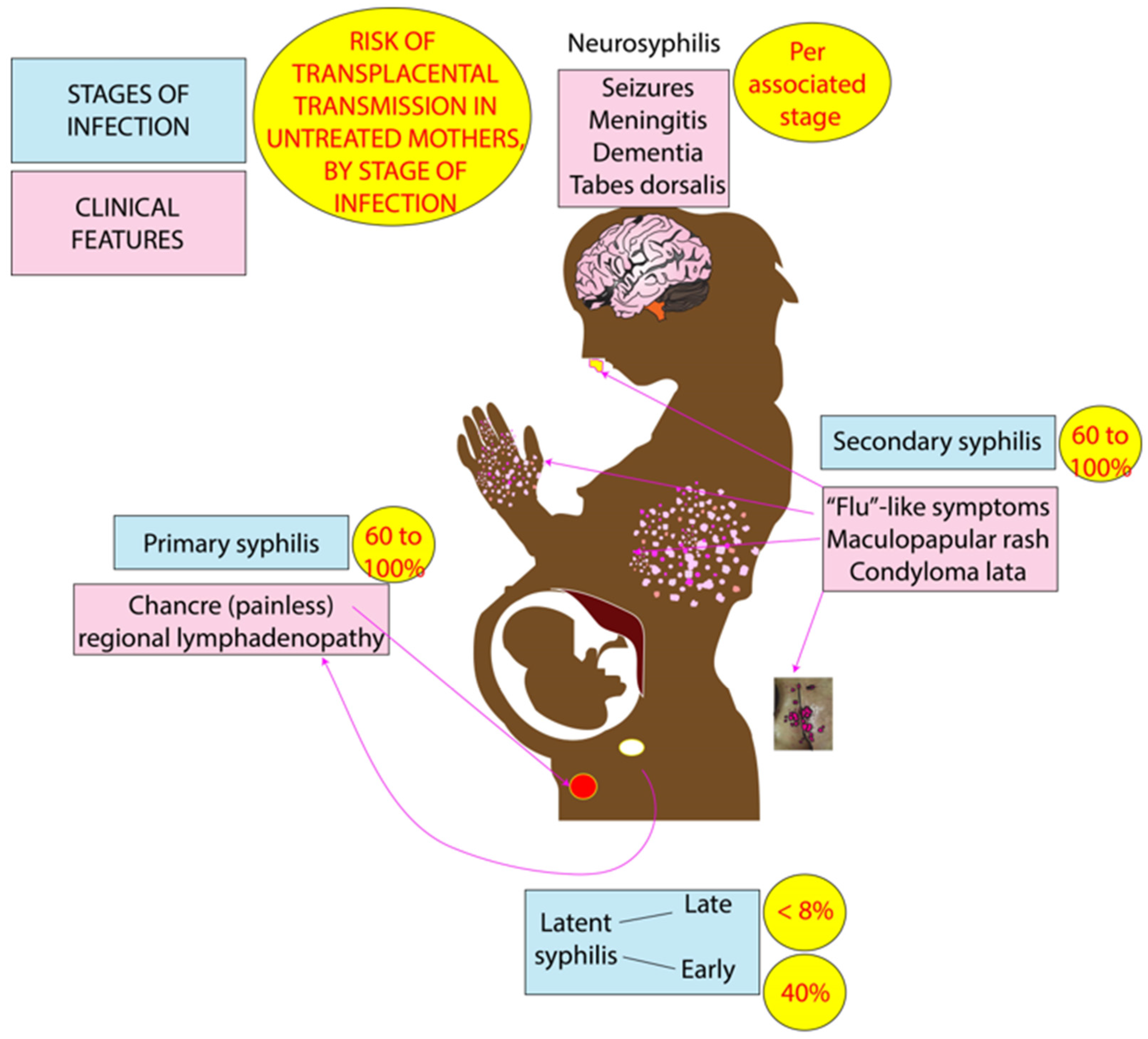
3.1. Clinical Features
3.2. Maternal-to-Fetal Transmission of Syphilis
3.3. Screening (Testing) and Management of Perinatal Syphilis
4. Congenital Syphilis: Clinical Features, Diagnosis, and Management
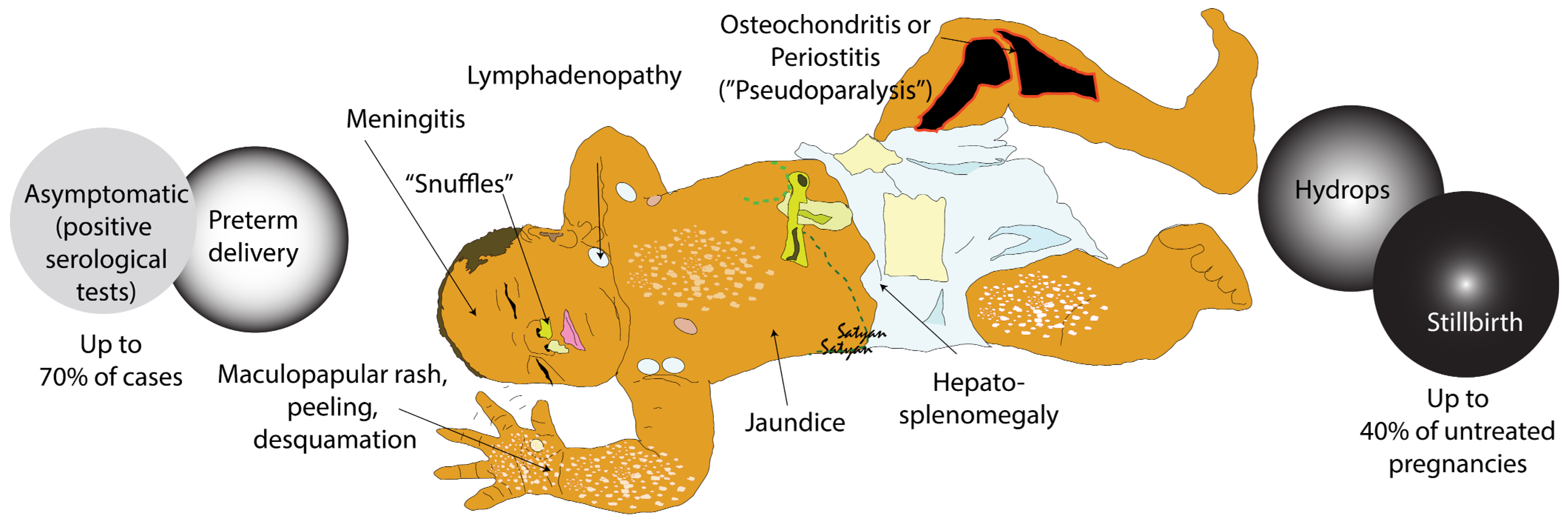
4.1. Clinical Features and Classification
4.2. Diagnosis and Management (Figure 3, Table 1)
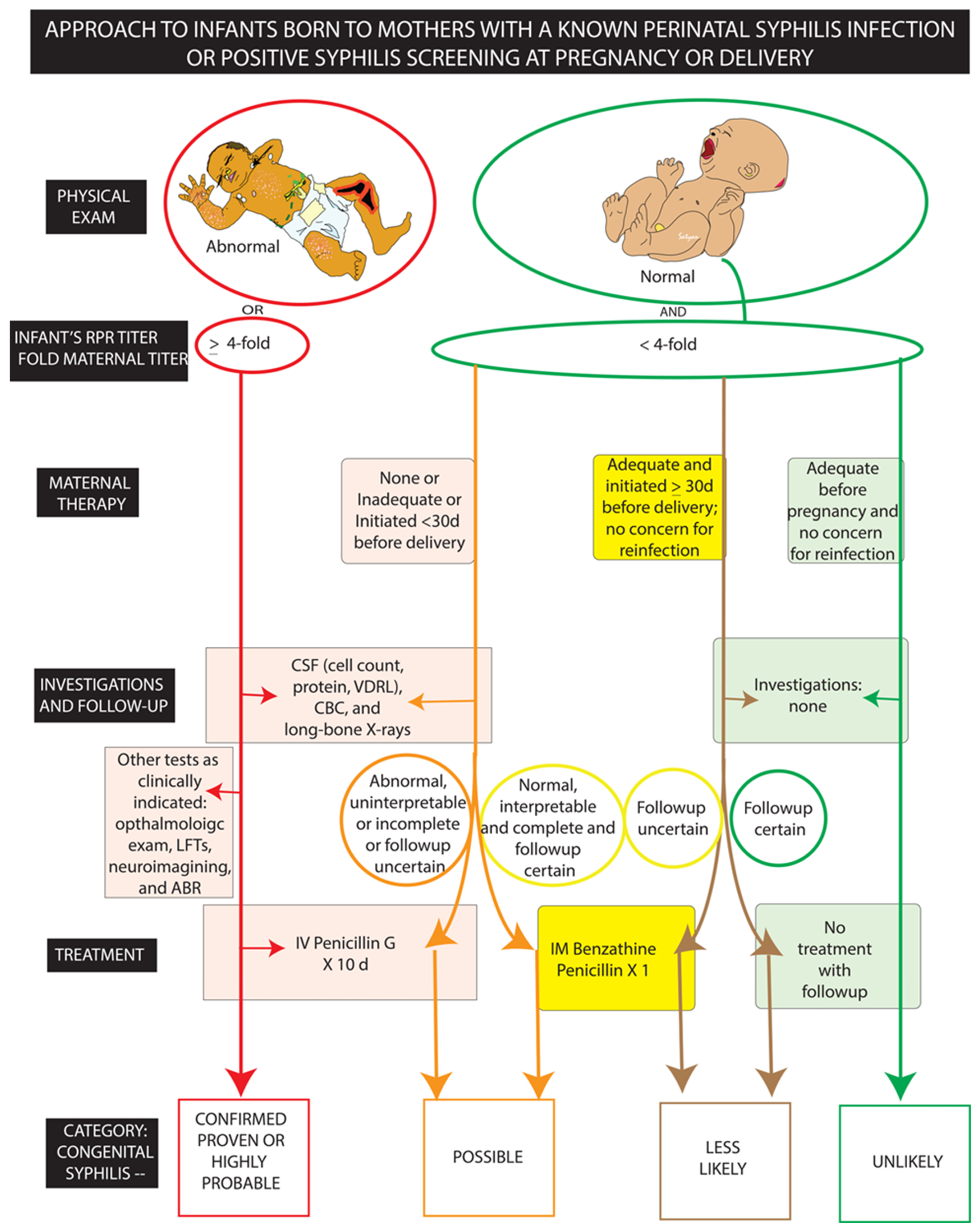
| Scenario (CDC) Risk Category (AAP) | Clinical History and Examination | Evaluation | Treatment |
|---|---|---|---|
| Scenario 1, Category: Confirmed proven or highly probable congenital syphilis | Abnormal physical exam OR RPR titer ≥ 4-fold maternal titer | CSF analysis (cell count, protein, VDRL), CBC, Long bone radiographs Other * | Intravenous Penicillin G × 10 days (regardless of evaluation results) |
| Scenario 2, Category: Possible congenital syphilis | Normal physical exam AND RPR titer < 4-fold maternal titer AND Maternal treatment none/unknown/inadequate or initiated <30 days before delivery | CSF analysis cell count, protein, VDRL, CBC, long-bone radiographs | Penicillin G × 10 days (if evaluation is abnormal, uninterpretable, incomplete, or followup uncertain OR Intramuscular Benzathine Penicillin × 1 (if evaluation and followup certain) |
| Scenario 3, Category: less likely congenital syphilis | Normal physical exam AND RPR titer < 4-fold maternal titer AND Maternal treatment: adequate and initiated ≥30 days before delivery and no concern for reinfection | None | Intramuscular Benzathine Penicillin × 1 (if followup uncertain) OR No treatment with follow-up titers (followup certain) |
| Scenario 4, Category: unlikely congenital syphilis | Normal physical exam AND RPR titer < 4-fold maternal titer AND Maternal treatment adequate before pregnancy | None | No treatment with followup RPR titers (if infant RPR positive) OR Intramuscular Benzathine Penicillin × 1 (if followup uncertain) |
5. Public Health Interventions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penner, J.; Hernstadt, H.; Burns, J.E.; Randell, P.; Lyall, H. Stop, think SCORTCH: Rethinking the traditional ‘TORCH’ screen in an era of re-emerging syphilis. Arch. Dis. Child. 2021, 106, 117–124. [Google Scholar] [CrossRef]
- Force, U.P.S.T. Screening for Syphilis Infection in Nonpregnant Adolescents and Adults: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2022, 328, 1243–1249. [Google Scholar] [CrossRef]
- Salomè, S.; Cambriglia, M.D.; Scarano, S.M.; Capone, E.; Betts, I.; Pacella, D.; Sansone, M.; Mazzarelli, L.L.; Lo Vecchio, A.; Ranucci, G.; et al. Congenital syphilis in the twenty-first century: An area-based study. Eur. J. Pediatr. 2023, 182, 41–51. [Google Scholar] [CrossRef]
- Gilmour, L.S.; Walls, T. Congenital Syphilis: A Review of Global Epidemiology. Clin. Microbiol. Rev. 2023, 36, e0012622. [Google Scholar] [CrossRef]
- Paixao, E.S.; Ferreira, A.J.; Dos Santos, I.O.; Rodrigues, L.C.; Fiaccone, R.; Salvi, L.; de Oliveira, G.L.; Santana, J.G.; Cardoso, A.M.; Teles, C.; et al. Mortality in children under 5 years of age with congenital syphilis in Brazil: A nationwide cohort study. PLoS Med. 2023, 20, e1004209. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, S.; Matin, N.; Broutet, N.; Low, N. Effectiveness of interventions to improve screening for syphilis in pregnancy: A systematic review and meta-analysis. Lancet Infect. Dis. 2011, 11, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Knell, R.J. Syphilis in renaissance Europe: Rapid evolution of an introduced sexually transmitted disease? Proc. Biol. Sci. 2004, 271 (Suppl. 4), S174–S176. [Google Scholar] [CrossRef] [PubMed]
- Pesapane, F.; Marcelli, S.; Nazzaro, G. Hieronymi Fracastorii: The Italian scientist who described the “French disease”. An. Bras. Dermatol. 2015, 90, 684–686. [Google Scholar] [CrossRef]
- Douglas, J.M. Penicillin Treatment of Syphilis. JAMA 2009, 301, 769–771. [Google Scholar] [CrossRef]
- Kojima, N.; Klausner, J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Rep. 2018, 5, 24–38. [Google Scholar] [CrossRef]
- Clement, M.E.; Hicks, C.B. Syphilis on the Rise: What Went Wrong? JAMA 2016, 315, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Syphilis—CDC Detailed Fact Sheet. Available online: https://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm (accessed on 23 June 2023).
- Kiarie, J.; Mishra, C.K.; Temmerman, M.; Newman, L. Accelerating the dual elimination of mother-to-child transmission of syphilis and HIV: Why now? Int. J. Gynaecol. Obstet. 2015, 130 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 24 June 2023).
- Fang, J.; Silva, R.M.; Tancredi, D.J.; Pinkerton, K.E.; Sankaran, D. Examining associations in congenital syphilis infection and socioeconomic factors between California’s small-to-medium and large metro counties. J. Perinatol. 2022, 42, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Table 21. Congenital Syphilis—Reported Cases and Rates of Reported Cases by Year of Birth, by State/Territory* and Region in Alphabetical Order, United States, 2017–2021. 2023. Available online: https://www.cdc.gov/std/statistics/2021/tables/21.htm (accessed on 23 June 2023).
- Harville, E.W.; Giarratano, G.P.; Buekens, P.; Lang, E.; Wagman, J. Congenital syphilis in East Baton Rouge parish, Louisiana: Providers’ and women’s perspectives. BMC Infect. Dis. 2021, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Prevention and Control of Sexually Transmitted Infections in the United States. Sexually Transmitted Infections: Adopting a Sexual Health Paradigm; Crowley, J.S., Geller, A.B., Vermund, S.H., Eds.; National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- Sankaran, D.; Lakshminrusimha, S.; Manja, V. Methamphetamine: Burden, mechanism and impact on pregnancy, the fetus, and newborn. J. Perinatol. 2022, 42, 293–299. [Google Scholar] [CrossRef]
- Kimball, A.; Torrone, E.; Miele, K.; Bachmann, L.; Thorpe, P.; Weinstock, H.; Bowen, V. Missed Opportunities for Prevention of Congenital Syphilis—United States, 2018. Morb. Mortal. Wkly. Rep. 2020, 69, 661–665. [Google Scholar] [CrossRef]
- Newman, L.; Kamb, M.; Hawkes, S.; Gomez, G.; Say, L.; Seuc, A.; Broutet, N. Global estimates of syphilis in pregnancy and associated adverse outcomes: Analysis of multinational antenatal surveillance data. PLoS Med. 2013, 10, e1001396. [Google Scholar] [CrossRef]
- Joseph Davey, D.; Shull, H.; Billings, J.; Wang, D.; Adachi, K.; Klausner, J. Prevalence of Curable Sexually Transmitted Infections in Pregnant Women in Low- and Middle-Income Countries From 2010 to 2015: A Systematic Review. Sex. Transm. Dis. 2016, 43, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Coffin, L.S.; Newberry, A.; Hagan, H.; Cleland, C.M.; Des Jarlais, D.C.; Perlman, D.C. Syphilis in drug users in low and middle income countries. Int. J. Drug Policy 2010, 21, 20–27. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Q.; Lin, Y.; Zhou, Y.; Lan, L.; Yang, S.; Wu, J. Global burden and trends of sexually transmitted infections from 1990 to 2019: An observational trend study. Lancet Infect. Dis. 2022, 22, 541–551. [Google Scholar] [CrossRef]
- Fu, L.; Sun, Y.; Han, M.; Wang, B.; Xiao, F.; Zhou, Y.; Gao, Y.; Fitzpatrick, T.; Yuan, T.; Li, P.; et al. Incidence Trends of Five Common Sexually Transmitted Infections Excluding HIV From 1990 to 2019 at the Global, Regional, and National Levels: Results From the Global Burden of Disease Study 2019. Front. Med. 2022, 9, 851635. [Google Scholar] [CrossRef]
- Kahn, J.G.; Jiwani, A.; Gomez, G.B.; Hawkes, S.J.; Chesson, H.W.; Broutet, N.; Kamb, M.L.; Newman, L.M. The cost and cost-effectiveness of scaling up screening and treatment of syphilis in pregnancy: A model. PLoS ONE 2014, 9, e87510. [Google Scholar] [CrossRef]
- Hawkes, S.J.; Gomez, G.B.; Broutet, N. Early antenatal care: Does it make a difference to outcomes of pregnancy associated with syphilis? A systematic review and meta-analysis. PLoS ONE 2013, 8, e56713. [Google Scholar] [CrossRef]
- Sunny, M.P.; Krishnan, C.; Abdulla, P.S.; Geeta, M.G. Congenital syphilis: Need for intensification of antenatal screening and clinician awareness. Trop. Doct. 2022, 52, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Gong, X.; Li, J.; Zhang, J. Epidemiological trends and features of syphilis in China, 2014–2019. Chin. J. Dermatol. 2021, 10, 668–672. [Google Scholar]
- Gao, J.; Chen, X.; Yang, M.; Wu, Y.; Liang, T.; Li, H.; Xie, W. Adverse pregnancy outcomes and associated risk factors among pregnant women with syphilis during 2013–2018 in Hunan, China. Front. Med. 2023, 10, 1207248. [Google Scholar] [CrossRef]
- Wang, H.; Ying, X.; Lin, D.; Uwimana, M.M.P.; Zhang, X. Towards the elimination of mother to child transmission of syphilis 2015–2020: Practice and progress in Zhejiang province, eastern China. BMC Pregnancy Childbirth 2023, 23, 99. [Google Scholar] [CrossRef]
- Qin, J.B.; Feng, T.J.; Yang, T.B.; Hong, F.C.; Lan, L.N.; Zhang, C.L.; Liu, X.L.; Yang, Y.Z.; Xiao, S.Y.; Tan, H.Z. Synthesized prevention and control of one decade for mother-to-child transmission of syphilis and determinants associated with congenital syphilis and adverse pregnancy outcomes in Shenzhen, South China. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2183–2198. [Google Scholar] [CrossRef]
- Luo, Z.; Ding, Y.; Yuan, J.; Wu, Q.; Tian, L.; Zhang, L.; Li, B.; Mou, J. Predictors of Seronegative Conversion After Centralized Management of Syphilis Patients in Shenzhen, China. Front. Public Health 2021, 9, 755037. [Google Scholar] [CrossRef]
- de Brito Pinto, T.K.; da Cunha-Oliveira, A.; Sales-Moioli, A.I.L.; Dantas, J.F.; da Costa, R.M.M.; Silva Moura, J.P.; Gómez-Cantarino, S.; Valentim, R.A.M. Clinical Protocols and Treatment Guidelines for the Management of Maternal and Congenital Syphilis in Brazil and Portugal: Analysis and Comparisons: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 10513. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Valentim, R.; Fernandes da Silva, L.; Fontoura de Souza, G.; Góis Farias de Moura Santos Lima, T.; Pereira de Oliveira, C.A.; Marques Dos Santos, M.; Espinosa Miranda, A.; Cunha-Oliveira, A.; Kumar, V.; et al. Use of Interrupted Time Series Analysis in Understanding the Course of the Congenital Syphilis Epidemic in Brazil. Lancet Reg. Health Am. 2022, 7, 100163. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.; Turkova, A.; Thorne, C. Syphilis, hepatitis C and HIV in Eastern Europe. Curr. Opin. Infect. Dis. 2017, 30, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Benoit, P.; Tennenhouse, L.; Lapple, A.; Hill-Carroll, G.; Shaw, S.; Bullard, J.; Plourde, P. Congenital syphilis re-emergence in Winnipeg, Manitoba. Can. Commun. Dis. Rep. 2022, 48, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Syphilis in Canada: Technical Report on Epidemiological Trends, Determinants and Interventions; Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada: Ottawa, ON, Canada, 2020; ISBN 978-0-660-34272-6. [Google Scholar]
- Committee on Infectious Diseases, American Academy of Pediatrics; Kimberlin, D.W.; Barnett, E.D.; Lynfield, R.; Sawyer, M.H. Red Book: 2021–2024 Report of the Committee on Infectious Diseases; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- Fang, J.; Partridge, E.; Bautista, G.M.; Sankaran, D. Congenital Syphilis Epidemiology, Prevention, and Management in the United States: A 2022 Update. Cureus 2022, 14, e33009. [Google Scholar] [CrossRef]
- Congenital Syphilis-CDC Fact Sheet. 2017. Available online: https://www.cdc.gov/std/syphilis/cong-syph-oct-2019.pdf (accessed on 24 June 2023).
- Lawrence, R.M.; Lawrence, R.A. Breast milk and infection. Clin. Perinatol. 2004, 31, 501–528. [Google Scholar] [CrossRef]
- O’Connor, M.; Kleinman, S.; Goff, M. Syphilis in Pregnancy. J. Midwifery Women’s Health 2008, 53, e17–e21. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Contrindications of Breastfeeding. 2023. Available online: https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/contraindications-to-breastfeeding.html (accessed on 24 June 2023).
- Centers for Disease Control and Prevention. Syphilis during Pregnancy. 2021. Available online: https://www.cdc.gov/std/treatment-guidelines/syphilis-pregnancy.htm (accessed on 24 June 2023).
- Alexander, J.M.; Sheffield, J.S.; Sanchez, P.J.; Mayfield, J.; Wendel, G.D., Jr. Efficacy of treatment for syphilis in pregnancy. Obstet. Gynecol. 1999, 93, 5–8. [Google Scholar] [CrossRef]
- Roberts, C.P.; Raich, A.; Stafylis, C.; Klausner, J.D. Alternative Treatments for Syphilis During Pregnancy. Sex. Transm. Dis. 2019, 46, 637–640. [Google Scholar] [CrossRef]
- Aleem, S.; Walker, L.S.; Hornik, C.D.; Smith, M.J.; Grotegut, C.A.; Weimer, K.E. Severe congenital syphilis in the neonatal intensive care unit: A retrospective case series. Pediatr. Infect. Dis. J. 2022, 41, 335–339. [Google Scholar] [CrossRef]
- Kim, Y.H.; Song, J.H.; Kim, C.J.; Yang, E.M. Congenital Syphilis Presenting with Only Nephrotic Syndrome: Reemergence of a Forgotten Disease. J. Korean Med. Sci. 2017, 32, 1374–1376. [Google Scholar] [CrossRef]
- Dombrowski, K.; Crawford, D.; Khan, B.; Tyler, K. Current Rural Drug Use in the US Midwest. J. Drug Abus. 2016, 2, 22. [Google Scholar]
- California Department of Housing and Community Development. Farmworkers. 2012. Available online: https://www.hcd.ca.gov/community-development/building-blocks/housing-needs/farmworkers.shtml (accessed on 20 June 2023).
- National Center for Farmworker Health, Inc. Maternal and Child Health. 2017. Available online: http://www.ncfh.org/maternal-and-child-health.html (accessed on 20 June 2023).
- Hetrick, M. Medicaid and Migrant Farmworkers: Why the State Residency Requirement Presents a Significant Access Barrier and What States Should Do About it. Health Matrix J. Law Med. 2015, 25, 437. Available online: https://scholarlycommons.law.case.edu/cgi/viewcontent.cgi?article=1027&context=healthmatrix (accessed on 24 June 2023).
- Chan, E.Y.L.; Smullin, C.; Clavijo, S.; Papp-Green, M.; Park, E.; Nelson, M.; Giarratano, G.; Wagman, J.A. A qualitative assessment of structural barriers to prenatal care and congenital syphilis prevention in Kern County, California. PLoS ONE 2021, 16, e0249419. [Google Scholar] [CrossRef] [PubMed]
- Slutsker, J.S.; Hennessy, R.R.; Schillinger, J.A. Factors contributing to congenital syphilis cases—New York City, 2010–2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1088. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Abramovitz, D.; Lozada, R.; Martinez, G.; Rangel, M.G.; Staines, H.; Patterson, T.L.; Strathdee, S.A. Mujer Mas Segura (Safer Women): A combination prevention intervention to reduce sexual and injection risks among female sex workers who inject drugs. BMC Public Health 2012, 12, 653. [Google Scholar] [CrossRef]
- Smullin, C.; Wagman, J.; Mehta, S.; Klausner, J.D. A Narrative Review of the Epidemiology of Congenital Syphilis in the United States From 1980 to 2019. Sex. Transm. Dis. 2021, 48, 71–78. [Google Scholar] [CrossRef]
- Appel, P.W.; Warren, B.E.; Yu, J.; Rogers, M.; Harris, B.; Highsmith, S.; Davis, C. Implementing substance abuse intervention services in New York City sexually transmitted disease clinics: Factors promoting interagency collaboration. J. Behav. Health Serv. Res. 2017, 44, 168–176. [Google Scholar] [CrossRef]
- Youth Community Health Assessment of Resources and Trends (CHART) Project. 2015. Available online: https://www.co.fresno.ca.us/home/showdocument?id=20461 (accessed on 20 June 2023).
- Reno, H.; Fox, B.; Highfill, C.; McKee, A.; Trolard, A.; Liang, S.Y.; Stoner, B.P.; Meyerson, B.E. The Emerging Intersection Between Injection Drug Use and Early Syphilis in Nonurban Areas of Missouri, 2012–2018. J. Infect. Dis. 2020, 222, S465–S470. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Cary, M.; Duarte, G.; Jesus, G.; Alarcão, J.; Torre, C.; Costa, S.; Costa, J.; Carneiro, A.V. Effectiveness of needle and syringe Programmes in people who inject drugs–An overview of systematic reviews. BMC Public Health 2017, 17, 309. [Google Scholar] [CrossRef]
- Clement, M.E.; Hicks, C.B. RPR and the serologic diagnosis of syphilis. JAMA 2014, 312, 1922–1923. [Google Scholar] [CrossRef]
- Castro, A.R.; Esfandiari, J.; Kumar, S.; Ashton, M.; Kikkert, S.E.; Park, M.M.; Ballard, R.C. Novel point-of-care test for simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. J. Clin. Microbiol. 2010, 48, 4615–4619. [Google Scholar] [CrossRef] [PubMed]
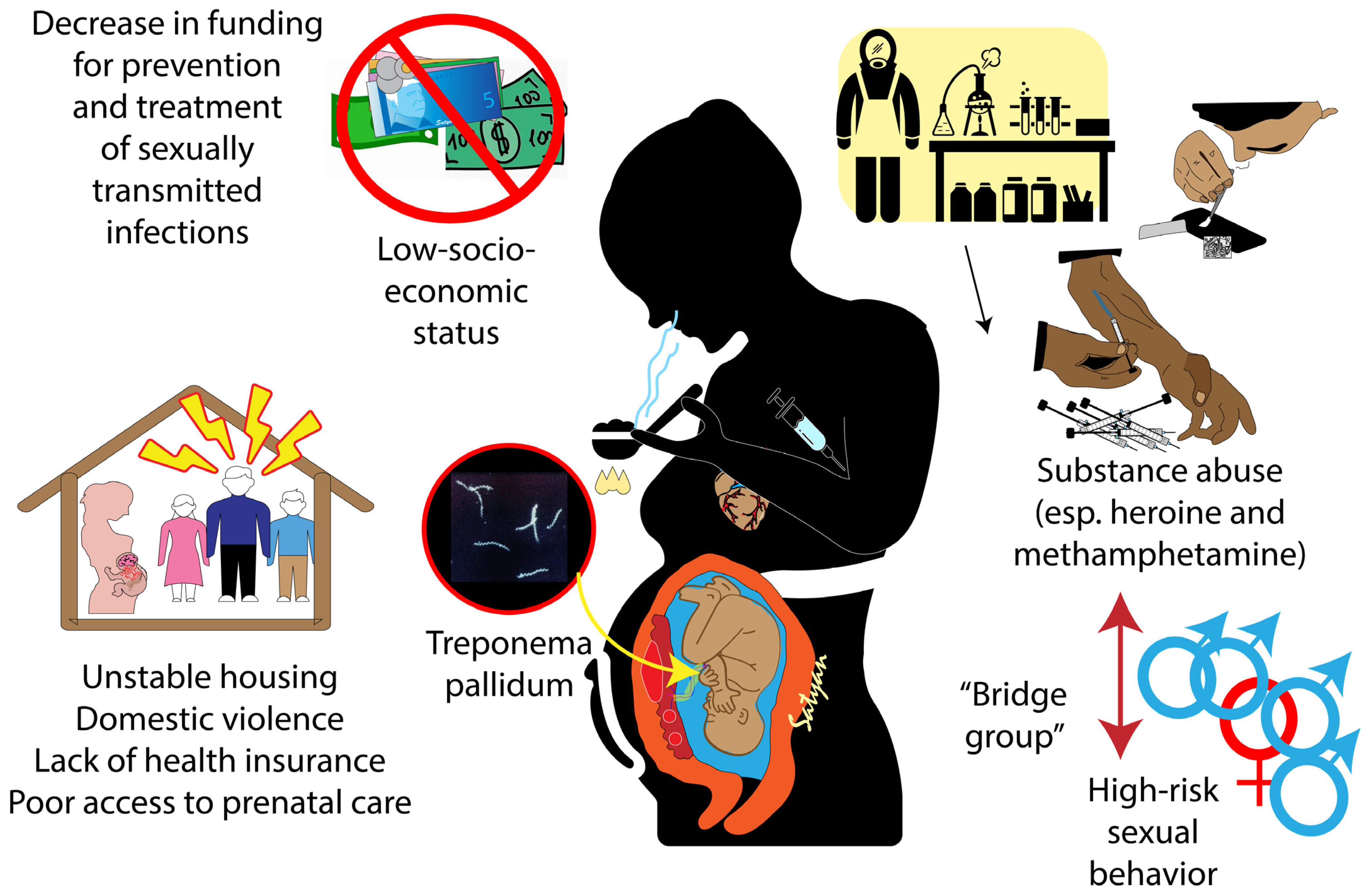
| Type of Risk Factor | Description |
|---|---|
| At individual level | High risk sexual behaviors Substance use Belonging to vulnerable groups either due to geographic location or race/ethnicity Poor health literacy, language barriers Inadequate prioritization of personal healthcare and poor utilization of available resources Stigma and fear of judgment Lack of health insurance |
| Community level | Inadequate access to healthcare Limited number of clinicians accepting Medicaid Inadequate medical knowledge on syphilis among clinicians and limited guidance Judgmental and stigmatizing approach Poor provision of sexual health education |
| System level | Poverty Structural and systemic racism Homelessness and housing insecurity Inadequate focus and resource allocation to rural and remote areas Lack of resources to ensure contact tracing and followup Lack of funding Inadequate public health infrastructure Integration of health information systems to provide information to health policy makers |
| Gaps in the current testing for perinatal syphilis |
| Healthcare delivery: Isolated care systems with inadequate infrastructure Insufficient screening during pregnancy by clinicians Inadequately implemented screening protocols Complexity of syphilis screening and diagnosis Delay in testing Testing below par in areas of high prevalence of syphilis and at-risk populations Insufficient efforts for contact tracing and followup |
| Pregnant person: Missed prenatal visits and noncompliance with testing and treatment. Mistrust in healthcare and public health system Transiency of pregnant people and relationship instability Substance use Mental health issues Complexity of testing and followup |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankaran, D.; Partridge, E.; Lakshminrusimha, S. Congenital Syphilis—An Illustrative Review. Children 2023, 10, 1310. https://doi.org/10.3390/children10081310
Sankaran D, Partridge E, Lakshminrusimha S. Congenital Syphilis—An Illustrative Review. Children. 2023; 10(8):1310. https://doi.org/10.3390/children10081310
Chicago/Turabian StyleSankaran, Deepika, Elizabeth Partridge, and Satyan Lakshminrusimha. 2023. "Congenital Syphilis—An Illustrative Review" Children 10, no. 8: 1310. https://doi.org/10.3390/children10081310
APA StyleSankaran, D., Partridge, E., & Lakshminrusimha, S. (2023). Congenital Syphilis—An Illustrative Review. Children, 10(8), 1310. https://doi.org/10.3390/children10081310






