Nutritional Value of Meals Designed for a School-Based Food Aid Program and Comparison with Similar Commercial Products: An Example of Good Practice from the DIATROFI Program
Abstract
1. Introduction
2. Materials and Methods
2.1. DIATROFI’s Program Design
2.2. DIATROFI School Meals
2.3. Collection of DIATROFI Meal Samples’ Food Labels during Two School Years
2.4. Collection of DIATROFI Meal Samples for Laboratory Analysis
2.5. Laboratory Analyses: Sample Preparation & Analytical Methods (2016–2017)
2.6. Evaluation of Nutrient Content of DIATROFI Meals through National and International Databases
2.7. Collection of Commercial Meal Samples
3. Results
3.1. DIATROFI’s Meals’ Nutrient Content
3.2. Comparison between DIATROFI Meals and Similar Commercially Available Products (Food Labels and Food Databases)
3.2.1. Traditional Pies, including Vegetables and/or Cheese and/or Chicken
3.2.2. Bakery Products
3.2.3. Whole Grain Bread and Dough Products
3.2.4. Sandwiches
3.3. Comparison between DIATROFI Meals and Similar Commercially Available Products (Laboratory Analysis Results 2016–2017)
3.4. Laboratory Analyses, Food Labels, and Food Databases Results
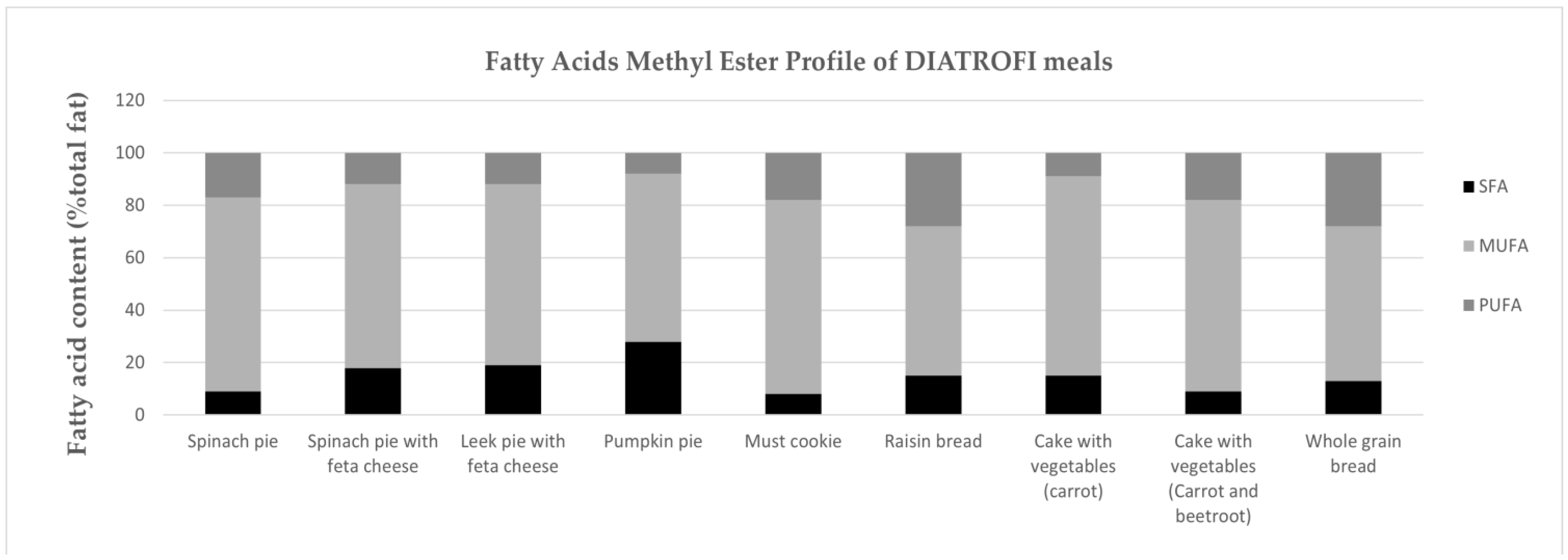
3.5. Fatty Acids Methyl Ester Profile
4. Discussion
4.1. DIATROFI Meals
4.2. Macronutrients Profile in DIATROFI Meals
4.2.1. FAME Profile
4.2.2. Added Sugars
4.2.3. Dietary Fibers
4.3. Micronutrients in DIATROFI Meals
Sodium/Salt
4.4. List of Ingredients for Meal Preparation
4.4.1. Processed Meat/Meat Products & Processed Cheese
4.4.2. Preservatives and Other Additives
4.4.3. Sweeteners
4.5. DIATROFI Meal Adaptations: Success and Challenges
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care. Available online: https://www.who.int/publications-detail-redirect/9789240009226 (accessed on 5 July 2023).
- UNICEF Programme Guidance for Early Life Prevention of Non-Communicable Diseases. 2019. Available online: https://www.unicef.org/media/61431/file (accessed on 5 July 2023).
- WHO Assessing the Existing Evidence Base on School Food and Nutrition Policies: A Scoping Review. Available online: https://www.who.int/publications-detail-redirect/9789240025646 (accessed on 5 July 2023).
- Yannakoulia, M.; Lykou, A.; Kastorini, C.M.; SarantiPapasaranti, E.; Petralias, A.; Veloudaki, A.; Linos, A. DIATROFI Program Research Team Socio-Economic and Lifestyle Parameters Associated with Diet Quality of Children and Adolescents Using Classification and Regression Tree Analysis: The DIATROFI Study. Public Health Nutr. 2016, 19, 339–347. [Google Scholar] [CrossRef]
- Alexy, U.; Sichert-Hellert, W.; Rode, T.; Kersting, M. Convenience Food in the Diet of Children and Adolescents: Consumption and Composition. Br. J. Nutr. 2008, 99, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.F.; Buzzard, I.M.; Gebhardt, S.E. Procedures for Estimating Nutrient Values for Food Composition Databases. J. Food Compos. Anal. 1997, 10, 102–114. [Google Scholar] [CrossRef]
- Kanzler, S.; Manschein, M.; Lammer, G.; Wagner, K.-H. The Nutrient Composition of European Ready Meals: Protein, Fat, Total Carbohydrates and Energy. Food Chem. 2015, 172, 190–196. [Google Scholar] [CrossRef]
- European Commission Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 Official Journal of the European Union 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 5 July 2023).
- Diamantis, D.V.; Stavropoulou, I.; Katsas, K.; Mugford, L.; Linos, A.; Kouvari, M. Assessing Quality of Life in First- and Second-Generation Immigrant Children and Adolescents; Highlights from the DIATROFI Food Aid and Healthy Nutrition Promotion Program. Int. J. Environ. Res. Public. Health 2023, 20, 2471. [Google Scholar] [CrossRef]
- EFSA Sets European Dietary Reference Values for Nutrient Intakes. Available online: https://www.efsa.europa.eu/en/press/news/nda100326 (accessed on 5 July 2023).
- USDA Nutrition Standards for School Meals. Available online: https://www.fns.usda.gov/cn/nutrition-standards-school-meals (accessed on 5 July 2023).
- WHO. Food and Nutrition Policy for Schools: A Tool for the Development of School Nutrition Programmes in the European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2006.
- Kastorini, C.-M.; Critselis, E.; Zota, D.; Coritsidis, A.L.; Nagarajan, M.K.; Papadimitriou, E.; Belogianni, K.; Benetou, V.; Linos, A.; on behalf of the Greek National Dietary Guidelines Scientific Team. National Dietary Guidelines of Greece for Children and Adolescents: A Tool for Promoting Healthy Eating Habits. Public Health Nutr. 2019, 22, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Chromý, V.; Vinklárková, B.; Šprongl, L.; Bittová, M. The Kjeldahl Method as a Primary Reference Procedure for Total Protein in Certified Reference Materials Used in Clinical Chemistry. I. A Review of Kjeldahl Methods Adopted by Laboratory Medicine. Crit. Rev. Anal. Chem. 2015, 45, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Dodds, E.D.; McCoy, M.R.; Rea, L.D.; Kennish, J.M. Gas Chromatographic Quantification of Fatty Acid Methyl Esters: Flame Ionization Detection vs. Electron Impact Mass Spectrometry. Lipids 2005, 40, 419–428. [Google Scholar] [CrossRef]
- Ackman, R.G.; Sipos, J.C. Application of Specific Response Factors in the Gas Chromatographic Analysis of Methyl Esters of Fatty Acids with Flame Ionization Detectors. J. Am. Oil Chem. Soc. 1964, 41, 377–378. [Google Scholar] [CrossRef]
- McCleary, B.V.; DeVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Okuma, K. Determination of Insoluble, Soluble, and Total Dietary Fiber (CODEX Definition) by Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study. J. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef]
- Xu, W.; Liang, L.; Zhu, M. Determination of Sugars in Molasses by HPLC Following Solid-Phase Extraction. Int. J. Food Prop. 2015, 18, 547–557. [Google Scholar] [CrossRef]
- Robinson, J.W. Determination of Sodium by Atomic Absorption Spectroscopy. Anal. Chim. Acta 1960, 23, 458–461. [Google Scholar] [CrossRef]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in Quality in the Analytical Laboratory. I. Traceability and Measurement Uncertainty of Analytical Results. TrAC Trends Anal. Chem. 2004, 23, 480–490. [Google Scholar] [CrossRef]
- Yiannopoulos, S.; Nicolaidou-Kanari, P.; Ioannou Kakouri, E.; Christodoulidou, M.; Anastasi, A. Food Composition Tables, 3rd ed.; Press and Information Office: Nicosia, Cyprus, 2013.
- McCance. Widdowson Composition of Foods Integrated Dataset (CoFID). Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 6 July 2023).
- USDA National Nutrient Database for Standard Reference, Legacy Release|Ag Data Commons. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 6 July 2023).
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra Virgin Olive Oil: More than a Healthy Fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids, Olive Oil and Health Status: A Systematic Review and Meta-Analysis of Cohort Studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Semma, M. Trans Fatty Acids: Properties, Benefits and Risks. J. Health Sci. 2002, 48, 7–13. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Michael, A.; Bolt, H.M.; Siméon, B.; Andrea, H.; Nils, H.; Christine, K.; Angela, M.; Gloria, P.; Daniel, R.; et al. The Role of Endogenous versus Exogenous Sources in the Exposome of Putative Genotoxins and Consequences for Risk Assessment. Arch. Toxicol. 2022, 96, 1297–1352. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for Human Health Related to the Presence of 3- and 2-monochloropropanediol (MCPD), and Their Fatty Acid Esters, and Glycidyl Fatty Acid Esters in Food. EFSA J. 2016, 14, e04426. [Google Scholar]
- Muriel, P.; López-Sánchez, P.; Ramos-Tovar, E. Fructose and the Liver. Int. J. Mol. Sci. 2021, 22, 6969. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Added Sugars and Risk Factors for Obesity, Diabetes and Heart Disease. Int. J. Obes. 2016, 40 (Suppl. S1), S22–S27. [Google Scholar] [CrossRef]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef]
- Miguel, Â.S.M.; Martins-Meyer, T.S.; Figueiredo, É.V.d.C.; Lobo, B.W.P.; Dellamora-Ortiz, G.M.; Miguel, Â.S.M.; Martins-Meyer, T.S.; Figueiredo, É.V.d.C.; Lobo, B.W.P.; Dellamora-Ortiz, G.M. Enzymes in Bakery: Current and Future Trends. In Food Industry; IntechOpen: London, UK, 2013; ISBN 978-953-51-0911-2. [Google Scholar]
- Chen, G.-C.; Tong, X.; Xu, J.-Y.; Han, S.-F.; Wan, Z.-X.; Qin, J.-B.; Qin, L.-Q. Whole-Grain Intake and Total, Cardiovascular, and Cancer Mortality: A Systematic Review and Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2016, 104, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Seal, C.J.; Courtin, C.M.; Venema, K.; de Vries, J. Health Benefits of Whole Grain: Effects on Dietary Carbohydrate Quality, the Gut Microbiome, and Consequences of Processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.N.; Chuang, R.-J.; Chow, J.; Ranjit, N.; Dave, J.M.; Mathur, M.; Markham, C.; Sharma, S.V. Food Insecurity among Low-Income Households with Children Participating in a School-Based Fruit and Vegetable Co-Op. Children 2022, 9, 1250. [Google Scholar] [CrossRef]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of Lower Sodium Intake on Health: Systematic Review and Meta-Analyses. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews; Centre for Reviews and Dissemination: New York, NY, UK, 2013. [Google Scholar]
- Gketsios, I.; Tsiampalis, T.; Foscolou, A.; Vassilakou, T.; Kanellopoulou, A.; Notara, V.; Antonogeorgos, G.; Rojas-Gil, A.P.; Kornilaki, E.N.; Lagiou, A.; et al. The Association of Junk Food Consumption with Preadolescents’ Environmental Influences: A School-Based Epidemiological Study in Greece. Children 2022, 9, 1891. [Google Scholar] [CrossRef] [PubMed]
- Kwong, E.J.L.; Whiting, S.; Bunge, A.C.; Leven, Y.; Breda, J.; Rakovac, I.; Cappuccio, F.P.; Wickramasinghe, K. Population-Level Salt Intake in the WHO European Region in 2022: A Systematic Review. Public Health Nutr. 2022, 1–14. [Google Scholar] [CrossRef]
- WHO Global Sodium Benchmarks for Different Food Categories. Available online: https://www.who.int/publications-detail-redirect/9789240025097 (accessed on 6 July 2023).
- WHO. Accelerating Salt Reduction in Europe: A Country Support Package to Reduce Population Salt Intake in the WHO European Region; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2020.
- European Commission Salt Campaign. Available online: https://health.ec.europa.eu/other-pages/basic-page/salt-campaign_en (accessed on 6 July 2023).
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and Processed Meat Consumption and Mortality: Dose-Response Meta-Analysis of Prospective Cohort Studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef] [PubMed]
- WHO. IARC Monographs Evaluate Consumption of Red Meat and Processed Meat. WHO Regional Office for the Eastern Mediterranean, 2015; Available online: https://apps.who.int/iris/handle/10665/340028 (accessed on 6 July 2023).
- Hladká, K.; Randulová, Z.; Tremlová, B.; Ponížil, P.; Mančík, P.; Černíková, M.; Buňka, F. The Effect of Cheese Maturity on Selected Properties of Processed Cheese without Traditional Emulsifying Agents. LWT Food Sci. Technol. 2014, 55, 650–656. [Google Scholar] [CrossRef]
- EFSA Dietary Reference Values. Available online: https://www.efsa.europa.eu/en/topics/topic/dietary-reference-values (accessed on 6 July 2023).
- Sadighara, P.; Safta, M.; Limam, I.; Ghanati, K.; Nazari, Z.; Karami, M.; Abedini, A. Association between Food Additives and Prevalence of Allergic Reactions in Children: A Systematic Review. Rev. Environ. Health 2023, 38, 181–186. [Google Scholar] [CrossRef] [PubMed]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet Lond. Engl. 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Skypala, I.J.; Williams, M.; Reeves, L.; Meyer, R.; Venter, C. Sensitivity to Food Additives, Vaso-Active Amines and Salicylates: A Review of the Evidence. Clin. Transl. Allergy 2015, 5, 34. [Google Scholar] [CrossRef]
- Stevens, L.J.; Kuczek, T.; Burgess, J.R.; Stochelski, M.A.; Arnold, L.E.; Galland, L. Mechanisms of Behavioral, Atopic, and Other Reactions to Artificial Food Colors in Children. Nutr. Rev. 2013, 71, 268–281. [Google Scholar] [CrossRef]
- Ataseven, N.; Yüzbaşıoğlu, D.; Keskin, A.Ç.; Ünal, F. Genotoxicity of Monosodium Glutamate. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 91, 8–18. [Google Scholar] [CrossRef]
- WHO Advises Not to Use Non-Sugar Sweeteners for Weight Control in Newly Released Guideline. Available online: https://www.who.int/news/item/15-05-2023-who-advises-not-to-use-non-sugar-sweeteners-for-weight-control-in-newly-released-guideline (accessed on 6 July 2023).
- FAO/WHO. Health Effects of the Use of Non-Sugar Sweeteners: A Systematic Review and Meta-Analysis. Available online: https://www.who.int/publications-detail-redirect/9789240046429 (accessed on 6 July 2023).
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural Sweeteners: The Relevance of Food Naturalness for Consumers, Food Security Aspects, Sustainability and Health Impacts. Int. J. Environ. Res. Public. Health 2020, 17, 6285. [Google Scholar] [CrossRef]
- Gressier, M.; Sassi, F.; Frost, G. Healthy Foods and Healthy Diets. How Government Policies Can Steer Food Reformulation. Nutrients 2020, 12, 1992. [Google Scholar] [CrossRef]
- Bleich, S.N.; Economos, C.D.; Spiker, M.L.; Vercammen, K.A.; VanEpps, E.M.; Block, J.P.; Elbel, B.; Story, M.; Roberto, C.A. A Systematic Review of Calorie Labeling and Modified Calorie Labeling Interventions: Impact on Consumer and Restaurant Behavior. Obesity 2017, 25, 2018–2044. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, L.A.; Schuilenburg, L.A.; Thrivikraman, J.K.; Numans, M.E.; Kiefte-de Jong, J.C. Needs and Perceptions Regarding Healthy Eating among People at Risk of Food Insecurity: A Qualitative Analysis. Int. J. Equity Health 2019, 18, 184. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Statistical Authority Press Release Food Security 2020 Survey on Income and Living Conditions; Greece. 2021. Available online: https://www.statistics.gr/documents/20181/69024ad6-d5e5-4c8e-cd9c-35bf5306ab38 (accessed on 6 July 2023).
| Meal | Total Fat (g/100 g) | SFA (g/100 g) | Carbohydrates (g/100 g) | Total Sugars (g/100 g) | Protein (g/100 g) | Dietary Fiber (g/100 g) | Sodium (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Spinach pie | |||||||
| 2016 Laboratory Analysis | 9.9 | 0.9 | 31.0 | 1.5 | 5.7 | 3.8 | 388 |
| 2016 Food databases | 10.3 | 1.6 | 32.1 | 1.0 | 6.4 | 3.8 | - † |
| 2016 Nutrition label | 11.1 | 1.5 | 28.8 | 1.3 | 3.9 | 2.3 | 400 |
| 2016 Similar commercial products (n = 5) | 13.5 | 4.0 | 25.4 | 1.3 | 4.5 | 1.5 | 395 |
| 2023 Food databases | 6.3 | 0.9 | 23.1 | 0.6 | 6.9 | 6.2 | - † |
| 2023 Nutrition label | 6.5 | 0.9 | 21.7 | 0.7 | 4.4 | 4.4 | 360 |
| 2023 Similar commercial products (n = 4) | 17.1 | 2.9 | 26.5 | 2.6 | 6.5 | 2.5 | 343 |
| Spinach pie with feta cheese | |||||||
| 2016 Laboratory Analysis | 11.3 | 1.9 | 28.2 | 2.7 | 7.9 | 3.2 | 364 |
| 2016 Food databases | 12.5 | 3.0 | 31.7 | 1.5 | 8.1 | 3.7 | - † |
| 2016 Nutrition label | 12.0 | 2.5 | 28.0 | 1.2 | 5.2 | 2.1 | 430 |
| 2016 Similar commercial products (n = 4) | 18.7 | 5.1 | 25.6 | 1.3 | 5.9 | 1.5 | 415 |
| 2023 Food databases | 10.0 | 1.1 | 22.8 | 0.7 | 7.8 | 5,3 | - † |
| 2023 Nutrition label | 9.7 | 0.9 | 21.1 | 0.6 | 6.9 | 3.8 | 360 |
| 2023 Similar commercial products (n = 5) | 16.7 | 4.1 | 28.8 | 1.9 | 5.8 | 1.6 | 388 |
| Leek pie with feta cheese | |||||||
| 2016 Laboratory Analysis | 10.1 | 1.9 | 29.5 | 3.2 | 8.5 | 3.2 | 360 |
| 2016 Food databases | 12.5 | 3.0 | 33.1 | 1.7 | 7.6 | 3.6 | - † |
| 2016 Nutrition label | 11.1 | 2.9 | 29.1 | 0.8 | 5.6 | 2.0 | 400 |
| 2016 Similar commercial products (n = 6) | 14.8 | 5.4 | 24.2 | 1.4 | 7.2 | 1.5 | 459 |
| 2023 Food databases | 7.7 | 1.1 | 23.7 | 1 | 5.0 | 6.7 | - † |
| 2023 Nutrition label | 7.1 | 1 | 22.4 | 1 | 4.1 | 5 | 320 |
| 2023 Similar commercial products (n = 4) | 15.4 | 4.3 | 28.4 | 1.6 | 7.1 | 1.8 | 379 |
| Pumpkin pie | |||||||
| 2016 Laboratory Analysis | 7.2 | 2.0 | 31.6 | 15.2 | 5.2 | 1.9 | 160 |
| 2016 Food databases | 7.2 | 1.7 | 32.4 | 15.0 | 5.2 | 3.0 | - † |
| 2016 Nutrition label | 7.3 | 1.6 | 30.1 | 14.4 | 5.1 | 2.4 | 141 |
| 2016 Similar commercial products (n = 0) | - | - | - | - | - | - | - |
| Zucchini and feta cheese pie * | |||||||
| 2023 Food databases | 9.6 | 1.8 | 21.1 | 0.8 | 6.3 | 2.3 | - † |
| 2023 Nutrition label | 9.5 | 1.5 | 21.5 | 0.77 | 6.3 | 2.9 | 320 |
| 2023 Similar commercial products (n = 3) | 11.4 | 3.2 | 28 | 4.5 | 6.3 | 2.1 | 197 |
| Chicken pie * | |||||||
| 2023 Food databases | 13.8 | 5.1 | 29.2 | 3.3 | 12.2 | 2.9 | - † |
| 2023 Nutrition label | 13.5 | 4.9 | 28.8 | 3.4 | 10.7 | 2.4 | 400 |
| 2023 Similar commercial products (n = 4) | 11.9 | 3.5 | 27.5 | 3.1 | 9.3 | 2 | 519 |
| Meal | Total Fat (g/100 g) | SFA (g/100 g) | Carbohydrates (g/100 g) | Total Sugars (g/100 g) | Protein (g/100 g) | Dietary Fiber (g/100 g) | Sodium (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Must cookie | |||||||
| 2016 Laboratory Analysis | 14.8 | 1.3 | 59.1 | 17.3 | 7.6 | 4.0 | 80 |
| 2016 Food databases | 14.1 | 1.0 | 59.3 | 16.9 | 7.1 | 4.2 | - † |
| 2016 Nutrition label | 15.0 | 2.7 | 59.5 | 18.3 | 6.9 | 3.9 | 91 |
| 2016 Similar commercial products (n = 3) | 17.7 | 4.4 | 58.3 | 21.0 | 6.6 | 2.2 | 84 |
| 2023 Food databases | 9.6 | 0.8 | 44.9 | 9.5 | 7.7 | 4.3 | - † |
| 2023 Nutrition label | 10.2 | 1.5 | 45 | 9.9 | 7.6 | 4 | 40 |
| 2023 Similar commercial products (n = 4) | 19.8 | 4.1 | 63.3 | 18 | 6.5 | 2.7 | 137 |
| Raisin bread | |||||||
| 2016 Laboratory Analysis | 2.9 | 0.4 | 48.4 | 11.5 | 10.3 | 4.8 | 220 |
| 2016 Food databases | 2.7 | 0.6 | 57.3 | 15.1 | 9.1 | 6.1 | - † |
| 2016 Nutrition label | 2.9 | 0.9 | 54.2 | 14.9 | 9.3 | 5.0 | 394 |
| 2016 Similar commercial products (n = 5) | 6.5 | 1.8 | 52.9 | 16.5 | 8.2 | 3.1 | 314 |
| 2023 Food databases | 2.7 | 0.6 | 57.3 | 15.1 | 9.1 | 6.1 | - † |
| 2023 Nutrition label | 2.9 | 0.9 | 54.2 | 14.9 | 9.3 | 5 | 156 |
| 2023 Similar commercial products (n = 4) | 10.9 | 3.2 | 52.7 | 14.3 | 10.3 | 3.3 | 238 |
| Cake with vegetables (carrot) | |||||||
| 2016 Laboratory Analysis | 16.8 | 2.5 | 37.5 | 10.9 | 6.5 | 2.9 | 276 |
| 2016 Food databases | 17.2 | 2.9 | 39.2 | 15.4 | 6.7 | 3.1 | - † |
| 2016 Nutrition label | 15.1 | 3.5 | 39.8 | 16.3 | 6.8 | 2.7 | 298 |
| 2016 Similar commercial products (n = 3) ^ | 19.8 | 5.1 | 50.0 | 35.0 | 4.2 | 2.0 | 312 |
| 2022 Food databases ‡ | 17.2 | 2.9 | 39.2 | 15.4 | 6.7 | 3.1 | - † |
| 2022 Nutrition label ‡ | 15.1 | 3.5 | 39.8 | 16.3 | 6.8 | 2.7 | 298 |
| 2023 Similar commercial products (n = 3) ^ | 14.3 | 6.7 | 61.5 | 36.2 | 5.2 | 2 | 276 |
| Cake with vegetables (Carrot and beetroot) | |||||||
| 2016 Laboratory Analysis | 14.1 | 1.6 | 43.3 | 13.2 | 6.7 | 3.3 | 333 |
| 2016 Food databases | 17.8 | 2.5 | 42.5 | 16.1 | 6.8 | 3.1 | - † |
| 2016 Nutrition label | 16.7 | 3.4 | 37.3 | 15.2 | 6.9 | 3.1 | 272 |
| 2016 Similar commercial products (n = 3) ^ | 19.8 | 5.1 | 50.0 | 35.0 | 4.2 | 2.0 | 312 |
| 2022 Food databases ‡ | 17.8 | 2.5 | 42.5 | 16.1 | 6.8 | 3.1 | - † |
| 2022 Nutrition label ‡ | 16.7 | 3.4 | 37.3 | 15.2 | 6.9 | 3.1 | 272 |
| 2023 Similar commercial products (n = 3) ^ | 14.3 | 6.7 | 61.5 | 36.2 | 5.2 | 2 | 276 |
| Meal | Total Fat (g/100 g) | SFA (g/100 g) | Carbohydrates (g/100 g) | Total Sugars (g/100 g) | Protein (g/100 g) | Dietary Fiber (g/100 g) | Sodium (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Whole grain bread | |||||||
| 2016 Laboratory Analysis | 3.9 | 0.6 | 48.0 | 2.0 | 10.0 | 4.6 | 430 |
| 2016 Food databases | 3.4 | 0.5 | 52.8 | 1.5 | 9.5 | 5.4 | 501 |
| 2016 Nutrition label | 2.7 | 0.3 | 48.4 | 2.4 | 10.0 | 4.6 | 500 |
| 2016 Similar commercial products (n = 5) | 5.3 | 1.6 | 40.9 | 5.2 | 10.9 | 6.1 | 562 |
| 2023 Food databases | 1.2 | 0.3 | 43.8 | 0.7 | 9.5 | 5.3 | 200 |
| 2023 Nutrition label | 1 | 0.3 | 45.7 | 0.5 | 9.1 | 6 | 160 |
| 2023 Similar commercial products (n = 5) | 4.1 | 0.7 | 42.1 | 4.5 | 10.1 | 5 | 541 |
| Whole grain pizza slice with vegetables * | |||||||
| 2023 Food databases | 10.8 | 4 | 24.5 | 3.1 | 8.6 | 2.0 | - † |
| 2023 Nutrition label | 10 | 3.9 | 24 | 2.8 | 8.5 | 1.9 | 328 |
| 2023 Similar commercial products (n = 4) | 8.5 | 3.2 | 28.5 | 2.9 | 6.2 | 1.9 | 490 |
| Whole grain pizza slice with baked chicken * | |||||||
| 2023 Food databases | 10.2 | 4.1 | 22.4 | 2.7 | 13.4 | 2.4 | - † |
| 2023 Nutrition label | 9.8 | 3.9 | 22 | 2.4 | 13 | 2.3 | 284 |
| 2023 Similar commercial products (n = 4) | 8.3 | 4 | 28.1 | 3.9 | 11.8 | 0.5 | 595 |
| Meal | Total Fat (g/100 g) | SFA (g/100 g) | Carbohydrates (g/100 g) | Total Sugars (g/100 g) | Protein (g/100 g) | Dietary Fiber (g/100 g) | Sodium (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Chicken and tomato sandwich ^ | |||||||
| 2016 Laboratory Analysis | 2.4 | 0.6 | 28.6 | 3.3 | 12.3 | 2.4 | 388 |
| 2016 Food databases | 3.0 | 0.6 | 27.2 | 2.7 | 13.5 | 2.4 | - † |
| 2016 Nutrition label | 2.4 | 0.5 | 33.1 | 2.6 | 14.9 | 2.9 | 315 |
| 2016 Similar commercial products (n = 6) | 8.4 | 2.4 | 24.2 | 2.2 | 12.5 | 1.7 | 459 |
| Cheese and cucumber or tomato sandwich ^ | |||||||
| 2016 Laboratory Analysis | 10.5 | 6.8 † | 30.3 | 2.1 | 10.3 | 3.5 | 479 |
| 2016 Food databases | 10.9 | 5.8 | 32.9 | 1.8 | 10.2 | 3.5 | - † |
| 2016 Nutrition label | 10.5 | 6.8 | 30.3 | 2.1 | 10.3 | 3.5 | 479 |
| 2016 Similar commercial products (n = 4) | 9.6 | 4.2 † | 24.0 | 2.8 | 12.6 | 1.6 | 570 |
| Whole wheat bread with roasted vegetables and cheese ^ | |||||||
| 2016 Laboratory Analysis | 10.2 | 4.9 | 19.8 | 3.7 | 18.5 | 5.7 | 352 |
| 2016 Food databases | 9.8 | 3.9 | 28.9 | 3.5 | 11.5 | 4.7 | - † |
| 2016 Nutrition label | 10.1 | 3.8 | 26.3 | 1.9 | 10.1 | 3.4 | 656 |
| 2016 Similar commercial products (n = 0) | - | - | - | - | - | - | - |
| Egg and olive-oil-based sauce sandwich ^ | |||||||
| 2016 Laboratory Analysis | 8.9 | 1.9 | 32.6 | 1.6 | 10.6 | 4.6 | 300 |
| 2016 Food databases | 9.1 | 1.8 | 33.0 | 1.9 | 11.1 | 4.7 | - † |
| 2016 Nutrition label | 9.0 | 2.8 | 31.0 | 2.7 | 11.7 | 3.2 | 717 |
| 2016 Similar commercial products (n = 5) | 10.1 | 2.0 | 21.9 | 1.8 | 10.3 | 1.9 | 313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouvari, M.; Diamantis, D.V.; Katsas, K.; Radaios, V.; Veloudaki, A.; Linos, A. Nutritional Value of Meals Designed for a School-Based Food Aid Program and Comparison with Similar Commercial Products: An Example of Good Practice from the DIATROFI Program. Children 2023, 10, 1268. https://doi.org/10.3390/children10071268
Kouvari M, Diamantis DV, Katsas K, Radaios V, Veloudaki A, Linos A. Nutritional Value of Meals Designed for a School-Based Food Aid Program and Comparison with Similar Commercial Products: An Example of Good Practice from the DIATROFI Program. Children. 2023; 10(7):1268. https://doi.org/10.3390/children10071268
Chicago/Turabian StyleKouvari, Matina, Dimitrios V. Diamantis, Konstantinos Katsas, Vasiliki Radaios, Afroditi Veloudaki, and Athena Linos. 2023. "Nutritional Value of Meals Designed for a School-Based Food Aid Program and Comparison with Similar Commercial Products: An Example of Good Practice from the DIATROFI Program" Children 10, no. 7: 1268. https://doi.org/10.3390/children10071268
APA StyleKouvari, M., Diamantis, D. V., Katsas, K., Radaios, V., Veloudaki, A., & Linos, A. (2023). Nutritional Value of Meals Designed for a School-Based Food Aid Program and Comparison with Similar Commercial Products: An Example of Good Practice from the DIATROFI Program. Children, 10(7), 1268. https://doi.org/10.3390/children10071268






