Human Milk Feeding Is Associated with Decreased Incidence of Moderate-Severe Bronchopulmonary Dysplasia in Extremely Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population
2.3. Variables
2.4. Ethics Requirements
2.5. Statistical Analysis
3. Results
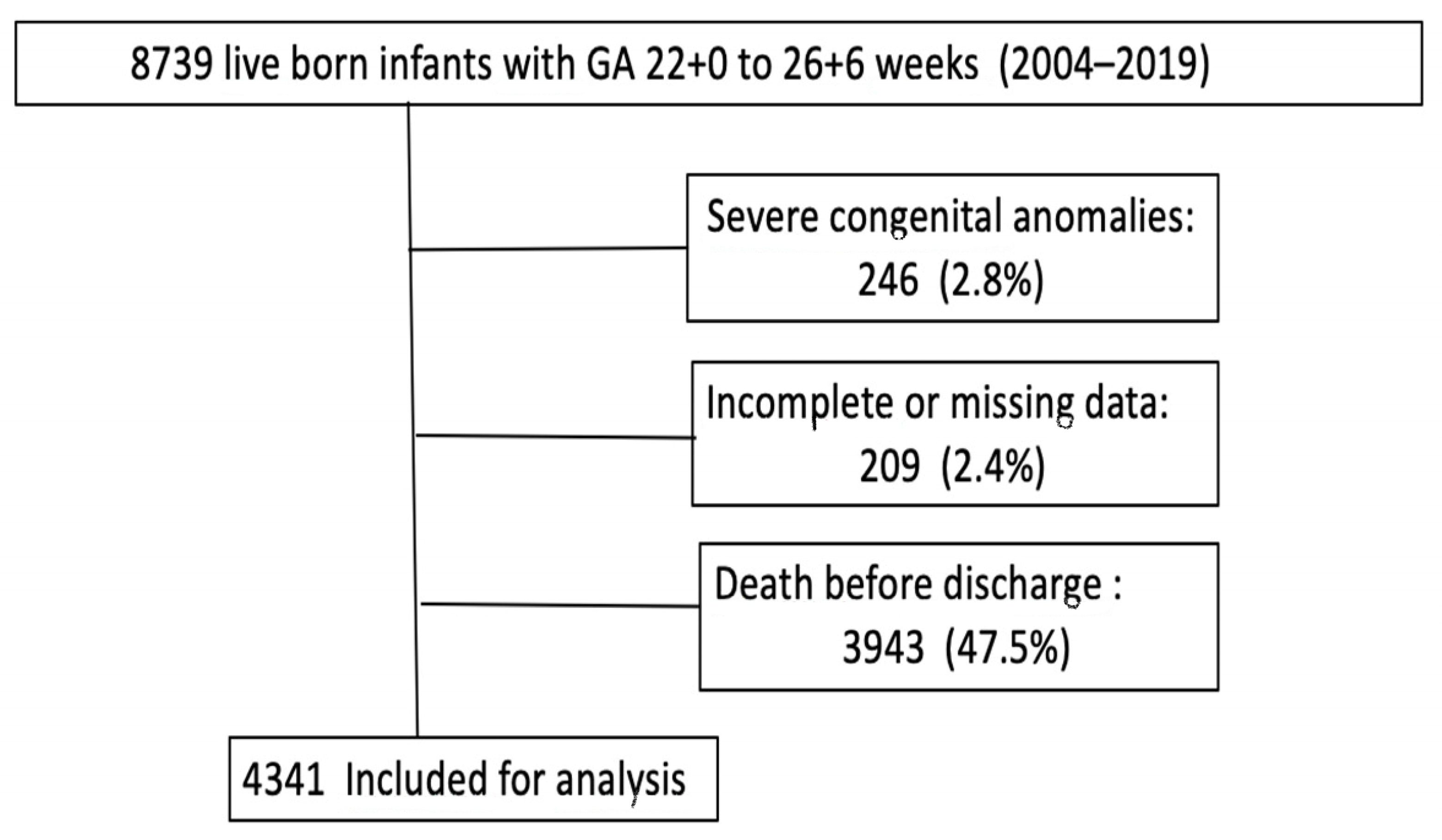
3.1. Selection of Study Cohort
3.2. Infants’ Characteristics
4. Discussion
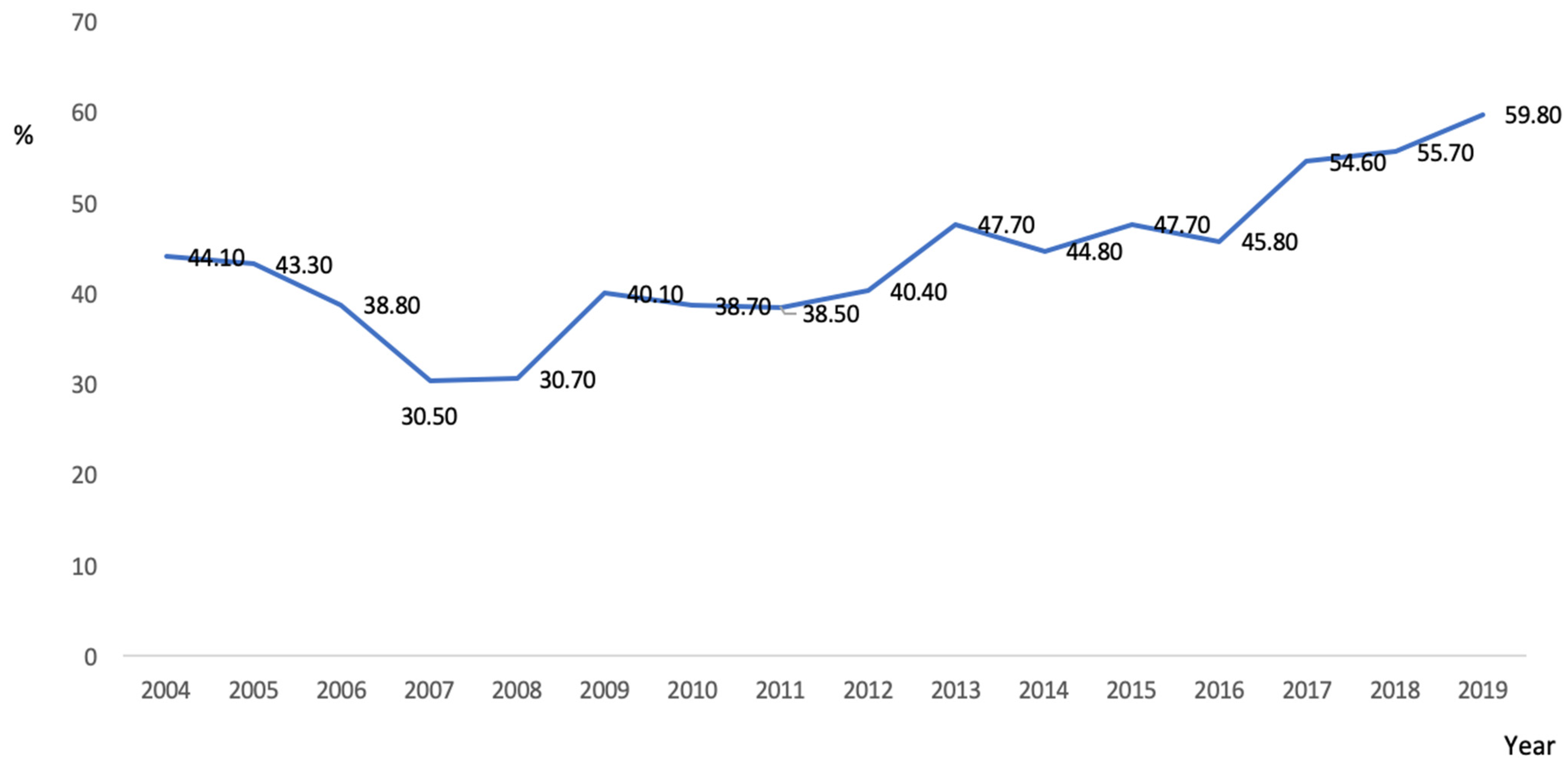
4.1. The Changing Epidemiology of BPD over the Study Period
4.2. Other Likely Mechanisms Underlying the Effects of HM Feeding on BPD
4.3. Lower Risk of BPD among HM-Fed Preterm Infants
4.3.1. Limitations
4.3.2. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Principi, N.; Di Pietro, G.M.; Esposito, S. Bronchopulmonary dysplasia: Clinical aspects and preventive and therapeutic strategies. J. Transl. Med. 2018, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.C.; Griffin, R.; Gist, K.M.; Segar, J.L.; Raina, R.; Guillet, R.; Nesargi, S.; Menon, S.; Anderson, N.; Askenazi, D.J.; et al. Neonatal Kidney Collaborative Research Committee. Association of Fluid Balance with Short- and Long-term Respiratory Outcomes in Extremely Premature Neonates. A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2248826. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, CD001145. [Google Scholar] [CrossRef]
- Avila-Alvarez, A.; Zozaya, C.; Pértega-Diaz, S.; Sanchez-Luna, M.; Iriondo-Sanz, M.; Elorza, M.D.; García-Muñoz Rodrigo, F.-M. Temporal trends in respiratory care and bronchopulmonary dysplasia in very preterm infants over a 10-year period in Spain. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 143–149. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Smith, V.C.; Zupancic, J.A.; McCormick, M.C.; Croen, L.A.; Greene, J.; Escobar, G.J.; Richardson, D.K. Trends in severe bronchopulmonary dysplasia rates between 1994 and 2002. J. Pediatr. 2005, 146, 469–473. [Google Scholar] [CrossRef]
- Berger, T.M.; Bachmann, I.I.; Adams, M.; Schubiger, G. Impact of improved survival of very low-birth-weight infants on incidence and severity of bronchopulmonary dysplasia. Biol. Neonatol. 2004, 86, 124–130. [Google Scholar] [CrossRef]
- Patel, A.L.; Johnson, T.J.; Robin, B.; Bigger, H.R.; Buchanan, A.; Christian, E.; Nandhan, V.; Shroff, A.; Schoeny, M.; Engstrom, J.L.; et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F256–F261. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Z.; Li, Q.; Zhou, J.; Yin, X.; Ma, Y.; Yin, Y.; Jiang, S.; Zhu, R.; Wu, Y.; et al. Dose-dependent effect of human milk on Bronchopulmonary dysplasia in very low birth weight infants. BMC Pediatr. 2020, 20, 522. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 238. [Google Scholar] [CrossRef]
- Hwang, J.S.; Rehan, V.K. Recent Advances in Bronchopulmonary Dysplasia: Pathophysiology, Prevention, and Treatment. Lung 2018, 196, 129–138. [Google Scholar] [CrossRef]
- Ofman, G.; Tipple, T.E. Antioxidants & bronchopulmonary dysplasia: Beating the system or beating a dead horse? Free Radic. Biol. Med. 2019, 142, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, W. Oxidative stress and bronchopulmonary dysplasia. Gene 2018, 678, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Asociación Española de Bancos de Leche. Available online: https://www.aeblh.org/banco-de-leche/bancos-de-leche-en-espana (accessed on 7 April 2023).
- San Feliciano, L.; Moro, M.; Figueras, J.; Tamayo, T.S.; Zozaya, C.; García-Muñoz Rodrigo, F.M.; Vento, M. The Spanish Neonatal Network SEN1500: Updated information. Pediatr. Med. 2022. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.N.; Chorioamnionitis Workshop Participants. Evaluation and Management of Women and Newborns with a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Ezz-Eldin, Z.M.; Hamid, T.A.; Youssef, M.R.; Nabil, H.-D. Clinical Risk Index for Babies (CRIB II) Scoring System in Prediction of Mortality in Premature Babies. J. Clin. Diagn. Res. 2015, 9, SC08-11. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 g. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Garmer, A. An international classification of retinopathy of prematurity. Pediatrics 1984, 74, 127–133. [Google Scholar]
- López Maestro, M.; Melgar Bonis, A.; de la Cruz-Bertolo, J.; Perapoch López, J.; Mosqueda Peña, R.; Pallás Alonso, C. Developmental centered care. Situation in Spanish neonatal units. An. Pediatr. 2014, 81, 232–240. [Google Scholar] [CrossRef]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Porta, R.; García-Muñoz Rodrigo, F.; Avila-Alvarez, A.; Ventura, P.S.; Izquierdo Renau, M.; Ginovart, G.; SEN1500 Network of the Spanish Society of Neonatology, Spain. Active approach in delivery room and survival of infants born between 22 and 26 gestational weeks are increasing in Spain. Acta Paediatr. 2023, 112, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Ventura, P.S.; Ginovart, G.; García-Muñoz, F.; Ávila-Alvarez, A.; Izquierdo, M.; Figueras, J.; Pérez, A.; Aguilera, R.; Campos, A.M.; et al. Changes in perinatal management and outcomes in infants born at 23 weeks of gestational age during the last decade in Spain. J. Matern. Fetal Neonatal Med. 2022, 35, 10296–10304. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.S.; Zeitlin, J.; Källén, K.; Draper, E.S.; Maršál, K.; Norman, M.; Serenius, F.; van Buuren, S.; Johnson, S.; Benhammou, V.; et al. Birth outcomes between 22 and 26 weeks’ gestation in national population-based cohorts from Sweden, England and France. Acta Paediatr. 2022, 111, 59–75. [Google Scholar] [CrossRef]
- Sucasas Alonso, A.; Pértega Diaz, S.; Sáez Soto, R.; Avila-Alvarez, A. Epidemiology and risk factors for bronchopulmonary dysplasia in preterm infants born at or less than 32 weeks of gestation. An. Pediatr. 2022, 96, 242–251. [Google Scholar] [CrossRef]
- Rysavy, M.A.; Mehler, K.; Oberthür, A.; Ågren, J.; Kusuda, S.; McNamara, P.J.; Giesinger, R.E.; Kribs, A.; Normann, E.; Carlson, S.J.; et al. An Immature Science: Intensive Care for Infants Born at ≤23 Weeks of Gestation. J. Pediatr. 2021, 233, 16–25.e1. [Google Scholar] [CrossRef]
- Fanczal, E.; Berecz, B.; Szijártó, A.; Gasparics, Á.; Varga, P. The Prognosis of Preterm Infants Born at the Threshold of Viability: Fog Over the Gray Zone-Population-Based Studies of Extremely Preterm Infants. Med. Sci. Monit. 2020, 26, e926947. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, S.; Deng, X.; Luo, Z.; Chen, A.; Yu, R. Effects of Antioxidants in Human Milk on Bronchopulmonary Dysplasia Prevention and Treatment: A Review. Front. Nutr. 2022, 9, 924036. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.; Buczynski, B.W.; O’Reilly, M.A. Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells. Am. J. Respir. Cell Mol. Biol. 2014, 50, 757–766. [Google Scholar] [CrossRef]
- Matos, C.; Ribeiro, M.; Guerra, A. Breastfeeding: Antioxidative properties of breast milk. J. Appl. Biomed. 2015, 13, 169–180. [Google Scholar] [CrossRef]
- Karbasi, S.; Bahrami, A.; Asadi, Z.; Shahbeiki, F.; Naseri, M.; Zarban, A.; Ferns, G.A. The association of maternal dietary quality and the antioxidant-proxidant balance of human milk. Int. Breastfeed. J. 2022, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Gianni, M.L.; Roggero, P.; Colnaghi, M.R.; Piemontese, P.; Amato, O.; Orsi, A.; Morlacchi, L.; Mosca, F. The role of nutrition in promoting growth in pre-term infants with bronchopulmonary dysplasia: A prospective non-randomised interventional cohort study. BMC Pediatr. 2014, 14, 235. [Google Scholar] [CrossRef]
- Massaro, G.D.; Radaeva, S.; Clerch, L.B.; Massaro, D. Lung alveoli: Endogenous programmed destruction and regeneration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L305–L309. [Google Scholar] [CrossRef]
- Jeppesen, D.L.; Hasselbalch, H.; Lisse, I.M.; Ersboll, A.K.; Engelmann, M.D. T-lymphocyte subsets, thymic size and breastfeeding in infancy. Pediatr. Allergy Immunol. 2004, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Tang, J.; Shi, J.; Qu, Y.; Xiong, T.; Mu, D. Human milk as a protective factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F128–F136. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Zhu, J.; Jiang, C.; Yu, Z.; Su, A. Effect of First Mother’s Own Milk Feeding Time on the Risk of Moderate and Severe Bronchopulmonary Dysplasia in Infants with Very Low Birth Weight. Front. Pediatr. 2022, 10, 887028. [Google Scholar] [CrossRef]
- Peng, W.; Han, J.; Li, S.; Zhang, L.; Yang, C.; Guo, J.; Cao, Y. The Association of Human Milk Feeding with Short-Term Health Outcomes Among Chinese Very/Extremely Low Birth Weight Infants. J. Hum. Lact. 2022, 38, 670–677. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, Z.; Zhao, X.Y.; Huang, L.H.; Wang, L.; Zhang, X.L.; Lin, X.Z. Short-term effects of fresh mother’s own milk in very preterm infants. Matern. Child Nutr. 2023, 19, e13430. [Google Scholar] [CrossRef] [PubMed]
- Briere, C.E.; McGrath, J.M.; Cong, X.; Brownell, E.; Cusson, R. Direct-Breastfeeding Premature Infants in the Neonatal Intensive Care Unit. J. Hum. Lact. 2015, 31, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Fleurant, E.; Schoeny, M.; Hoban, R.; Asiodu, I.V.; Riley, B.; Meier, P.P.; Bigger, H.; Patel, A.L. Barriers to Human Milk Feeding at Discharge of Very-Low-Birth-Weight Infants: Maternal Goal Setting as a Key Social Factor. Breastfeed. Med. 2017, 12, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Kumar, M.; Tripathi, S.; Singh, S.N.; Singh, V.K. Breastfeeding rates at discharge for very low birthweight neonates and their determinants: An observational study from a tertiary care neonatal intensive care unit in India. J. Paediatr. Child Health 2022, 58, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jiang, H. Factors associated with post NICU discharge exclusive breastfeeding rate and duration amongst first time mothers of preterm infants in Shanghai: A longitudinal cohort study. Int. Breastfeed. J. 2022, 17, 34. [Google Scholar] [CrossRef]
- Seshadri, N.; Kim, L.Y.; McGrath-Morrow, S.A.; Collaco, J.M. Human Milk Cessation in the NICU in Infants with Bronchopulmonary Dysplasia. Am. J. Perinatol. 2021. ahead of print. [Google Scholar] [CrossRef]
- Kim, L.Y.; McGrath-Morrow, S.A.; Collaco, J.M. Impact of breast milk on respiratory outcomes in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2019, 54, 313–318. [Google Scholar] [CrossRef]

| Total Population n = 4341 | Moderate–Severe BPD n = 1897/4341 (43.7%) | No Moderate–Severe BPD n = 2444/4341 (56.3%) | p | OR (95% CI) | |
|---|---|---|---|---|---|
| Gestational age (weeks) | 25.7 (0.84) | 25.5 (0.900) | 25.8 (0.85) | <0.001 | |
| Birth weight (g) | 812.58 (151.004) | 773.69 (145.975) | 842.77 (148.007) | <0.001 | |
| Male sex | 2262/4341 (52.1) | 1072/1897 (56.5) | 1190 /2444 (48.7)) | <0.001 | 1.369 (1.214–1.545) |
| Multiple birth | 1188/4341 (27.4) | 548/1897 (28.9) | 640/2444 (26.2) | 0.048 | 1.145 (1.001–1.309) |
| IVF | 793/4046 (19.6) | 357/1783 (20.0) | 436/2263 (19.3) | 0.548 | 1.049 (0.897–1.226) |
| Antenatal steroids | 3889/4341 (89.6) | 1694/1897 (89.3) | 2195 /2444 (89.8) | 0.583 | 0.947 (0.778–1.151) |
| Complete antenatal steroids | 2846/4341 (65.6) | 1242/1897 (65.5) | 1604/2444 (65.6) | 0.877 | |
| Outborn | 195/4341 (4.5) | 95/1897 (5) | 100/2444 (4.1) | 0.148 | 1.232 (0.967–1.647) |
| Caesarean section | 2343/4341 (54) | 1043/1897 (55) | 1300 /2444 (53.2) | 0.241 | 1.075 (0.953–1.212) |
| Chorioamnionitis | 1215/3078 (39.5) | 562/1397 (40.3) | 653/1681 (38.8) | 0.437 | 1.032 (0.954–1.117) |
| Maternal hypertensive disorder | 279/3085 (9) | 136/1394 (9.8) | 143/1691 (8.5) | 0.21 | 1.170 (0.915–1.497) |
| 1 min Apgar score | 5 (4–7) a | 5 (4–7) b | 6 (4–7) c | <0.001 | |
| 5 min Apgar score | 8 (7–9) d | 8 (6–8) e | 8 (7–9) f | <0.001 | |
| CRIB score | 4 (2–7) g | 5 (4–8) h | 4 (2–6) i | <0.001 | |
| iMV | 3746/4341 (86.3) | 1753/1897 (92.4) | 1993/2444 (81.5) | <0.001 | 2.755 (2.259–3.359) |
| HFOV | 1209/4321 (28) | 856/1888 (45.3) | 353/2433 (14.5) | <0.001 | 4.887 (4.229–5.6489 |
| Duration of iMV (hours) | 440.73 (554.78) | 699.02 (677.1) | 242.42 (318.42) | <0.001 | |
| nCPAP | 3863/4333 (89.2) | 1670/1893 (88.2) | 2194/2440 (89.9) | 0.074 | 0.840 (0.693–1.017) |
| nIPPV | 2002/3473 (57.6)) | 922/1521 (60.6) | 1080/1953 (55.3) | 0.002 | 1.244 (1.086–1.426) |
| iNO | 317/4136 (7.7%) | 253/1803 (14) | 64/2333 (2.7) | <0.001 | 5.787 (4.366–7.670) |
| Surfactant therapy | 3440/4333 (79.4) | 1624/1896 (85.7) | 1816/2437 (74.5) | <0.001 | 2.042 (1.744–2.390) |
| Prophylactic indomethacin | 286/4335 (6.6) | 74/1983 (3.9) | 212/2442 (8.7) | <0.001 | 0.428 (0.326–0.562) |
| Postnatal systemic steroids | 1177/4324 (27.2) | 851/1892 (45.0) | 326/2432 (13.4) | <0.001 | 5.281 (4.556–6.122) |
| Early onset sepsis | 295/4326 (6.8) | 121/1887 (6.4) | 174/2439 (7.1) | 0.35 | 0.892 (0.701–1.134) |
| Late onset sepsis | 2773/4339 (63) | 1405/1896 (74.1) | 1328/2443 (54.4) | <0.001 | 2.403 (2.110–2.736) |
| Inotropic therapy | 1808/4139 (43.7) | 1016/1808 (56.3) | 792/2334(33.9) | <0.001 | 2.507 (2.209–2.845) |
| NEC | 513/4341 (11.8) | 277/1897 (14.6) | 236/2444 (9.7) | <0.001 | 1.600 (1.329–1.925) |
| NEC surgery | 379/4335 (8.7) | 209/1895 (11) | 170/2440 (7) | <0.001 | 1.655 (1.339–2.046) |
| PDA medical/surgical treatment | 2576/4268 (60.35) | 1336/1869 (71.5) | 1240/2399 (51.7) | <0.001 | 2.343 (2.060–2.664) |
| Major cerebral lesion | 872/4341 (20.1) | 483/1897 (25.5) | 389/2444 (15.9) | <0.001 | 1.805 (1.554–2.096) |
| ROP surgery | 674/4341 (15.5) | 405/1897 (21.3) | 269/2444 (11) | <0.001 | 2.195 (1.856–2.595) |
| ROP grade 3 or more | 685/4244 (16.1) | 428/1856 (23.1) | 257/2388 (10.8) | <0.001 | 2.485 (2.100–2.942) |
| Duration of admission in NICU (days) | 99 (30) j | 114 (32) k | 87 (22) l | <0.001 | |
| Exclusive HM feeding at discharge | 1493/4222 (35.2) | 559/1851 (30.2) | 934/2391 (39.1) | <0.001 | 0.675 (0.593–0.768) |
| Any amount of HM feeding at discharge | 2474/4242 (58.3) | 953/1851 (58.3) | 1521/2391 (63.6) | <0.001 | 0.607 (0.537–0.698) |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| ‘HM Feeding at Discharge’ Not Included | Including ‘HM Feeding at Discharge’ | Including ‘Any Amount of HM Feeding at Discharge’ | ||||
| n = 3610 | n = 3569 | n = 3569 | ||||
| Nagelkerke R-Square | 0.32 | 0.33 | 0.327 | |||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Birth weight (g) | 0.999 (0.998–0.999) | <0.001 | 0.998 (0.998–0.999) | <0.001 | 0.998 (0.998–0.999) | <0.001 |
| Male sex | 1.355 (1.154–1.591) | <0.001 | 1.356 (1.153–1.594) | <0.001 | 1.355 (1.152–1.593) | <0.001 |
| 1 min Apgar score | 1.033 (0.975–1.094) | 0.275 | 1.031 (0.973–1.092) | 0.301 | 1.033 (0.975–1.094) | 0.275 |
| 5 min Apgar score | 0.931 (0.864–1.002) | 0.057 | 0.931 (0.865–1.003) | 0.060 | 0.931 (0.864–1.002) | 0.057 |
| CRIB score | 1.011 (0.976–1.048) | 0.533 | 1.011 (0.976–1.048) | 0.543 | 1.011 (0.976–1.048) | 0.533 |
| iMV | 0.717 (0.539–0.954) | 0.022 | 0.715 (0.537–0.950) | 0.021 | 0.717 (0.539–0.954) | 0.022 |
| HFOV | 2.103 (1.728–2.560) | <0.001 | 2.128 (1.746–2.594) | <0.001 | 2.124 (1.743–2.589) | <0.001 |
| Duration of mechanical ventilation (hours) | 1.002 (1.001–1.002) | <0.001 | 1.002 (1.001–1.002) | <0.001 | 1.002 (1.001–1.002) | <0.001 |
| Surfactant therapy | 1.021 (0.808–1.290) | 0.861 | 0.999 (0.789–1.264) | 0.992 | 1.009 (0.797–1.277) | 0.94 |
| iNO | 1.655 (1.164–2.352) | 0.005 | 1.717 (1.205–2.447) | 0.003 | 1.741 (1.222–2.482) | 0.002 |
| PDA surgical/medical treatment | 1.256 (1.064–1.483) | 0.007 | 1.249 (1.056–1.447) | 0.009 | 1.261 (1.067–1.491) | 0.007 |
| Late onset sepsis | 1.646 (1.394–1.945) | <0.001 | 1.636 (1.383–1.936) | <0.001 | 1.634 (1.381–1.933) | <0.001 |
| NEC | 0.844 (0.658–1.084) | 0.184 | 0.835 (0.649–1.074) | 0.160 | 0.826 (0.642–1.063) | 0.137 |
| Exclusive HM at discharge | NA | 0.758 (0.641–0.896) | 0.001 | NA | ||
| Any amount of HM at discharge | NA | NA | 0.770 (0.656–0.904) | 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verd, S.; Porta, R.; Ginovart, G.; Avila-Alvarez, A.; García-Muñoz Rodrigo, F.; Izquierdo Renau, M.; Ventura, P.S. Human Milk Feeding Is Associated with Decreased Incidence of Moderate-Severe Bronchopulmonary Dysplasia in Extremely Preterm Infants. Children 2023, 10, 1267. https://doi.org/10.3390/children10071267
Verd S, Porta R, Ginovart G, Avila-Alvarez A, García-Muñoz Rodrigo F, Izquierdo Renau M, Ventura PS. Human Milk Feeding Is Associated with Decreased Incidence of Moderate-Severe Bronchopulmonary Dysplasia in Extremely Preterm Infants. Children. 2023; 10(7):1267. https://doi.org/10.3390/children10071267
Chicago/Turabian StyleVerd, Sergio, Roser Porta, Gemma Ginovart, Alejandro Avila-Alvarez, Fermín García-Muñoz Rodrigo, Montserrat Izquierdo Renau, and Paula Sol Ventura. 2023. "Human Milk Feeding Is Associated with Decreased Incidence of Moderate-Severe Bronchopulmonary Dysplasia in Extremely Preterm Infants" Children 10, no. 7: 1267. https://doi.org/10.3390/children10071267
APA StyleVerd, S., Porta, R., Ginovart, G., Avila-Alvarez, A., García-Muñoz Rodrigo, F., Izquierdo Renau, M., & Ventura, P. S. (2023). Human Milk Feeding Is Associated with Decreased Incidence of Moderate-Severe Bronchopulmonary Dysplasia in Extremely Preterm Infants. Children, 10(7), 1267. https://doi.org/10.3390/children10071267









