Effects of Physical Activity and Nutrition Education on the Gut Microbiota in Overweight and Obese Children
Abstract
1. Introduction
2. Materials and Methods
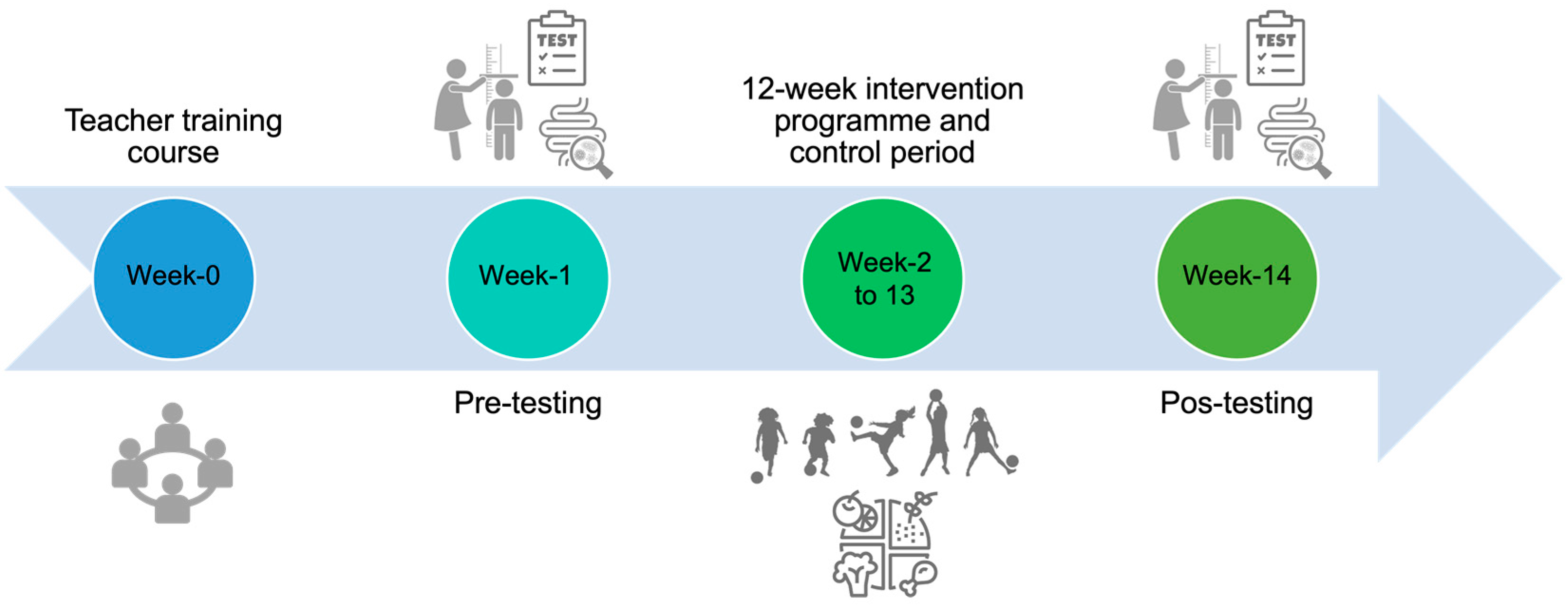
2.1. Study Design and Participants
2.2. “Football and Nutrition for Health” Intervention Program
2.3. Anthropometric Measurements and Body Composition
2.4. Physical Activity
2.5. Dietary Assessment
2.6. Faecal Sample Collection and DNA Extraction
2.7. Microbial 16S rRNA Sequence Analysis
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Results
3.1. Anthropometric, Body Composition, and Physical Activity
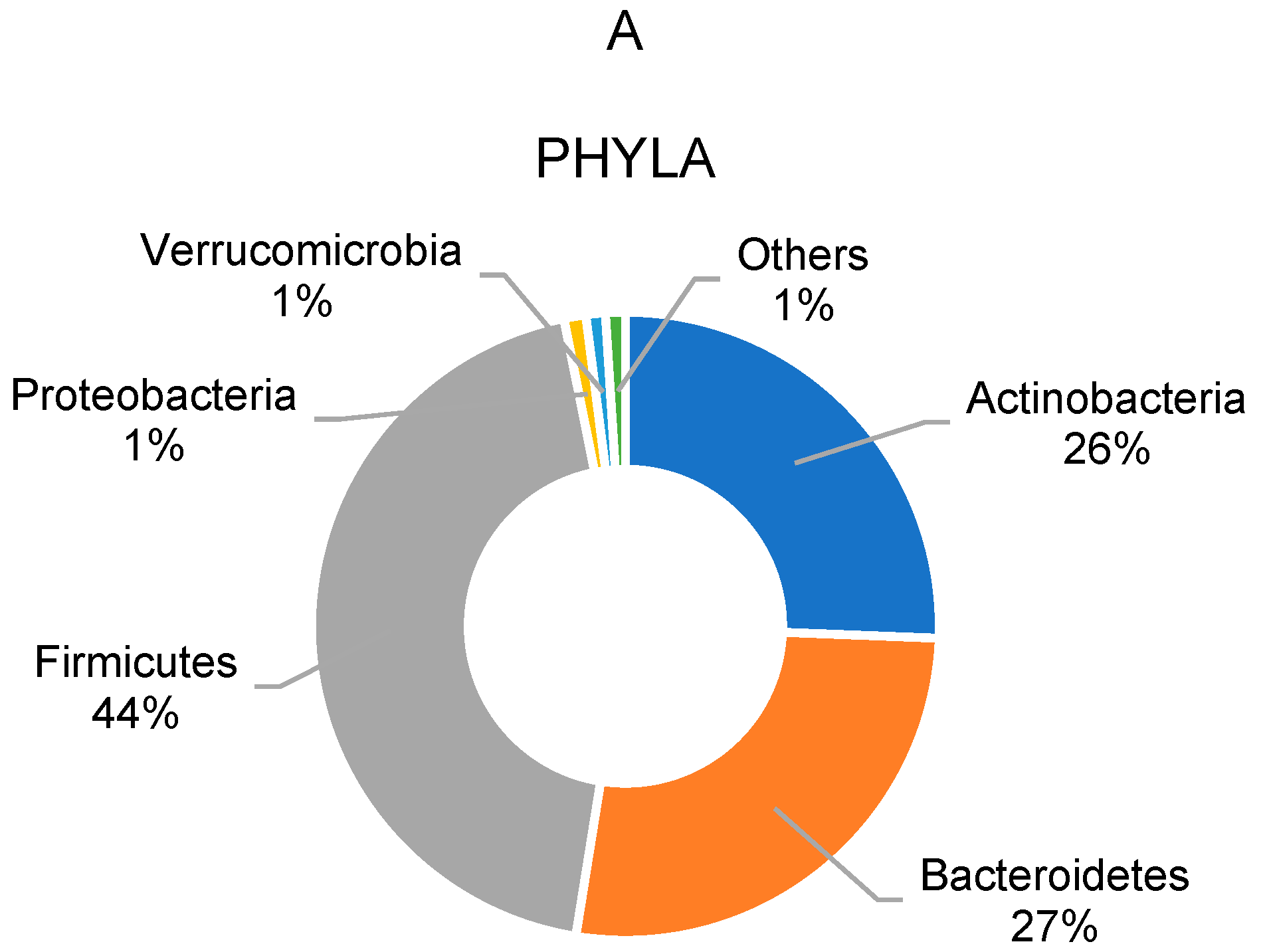
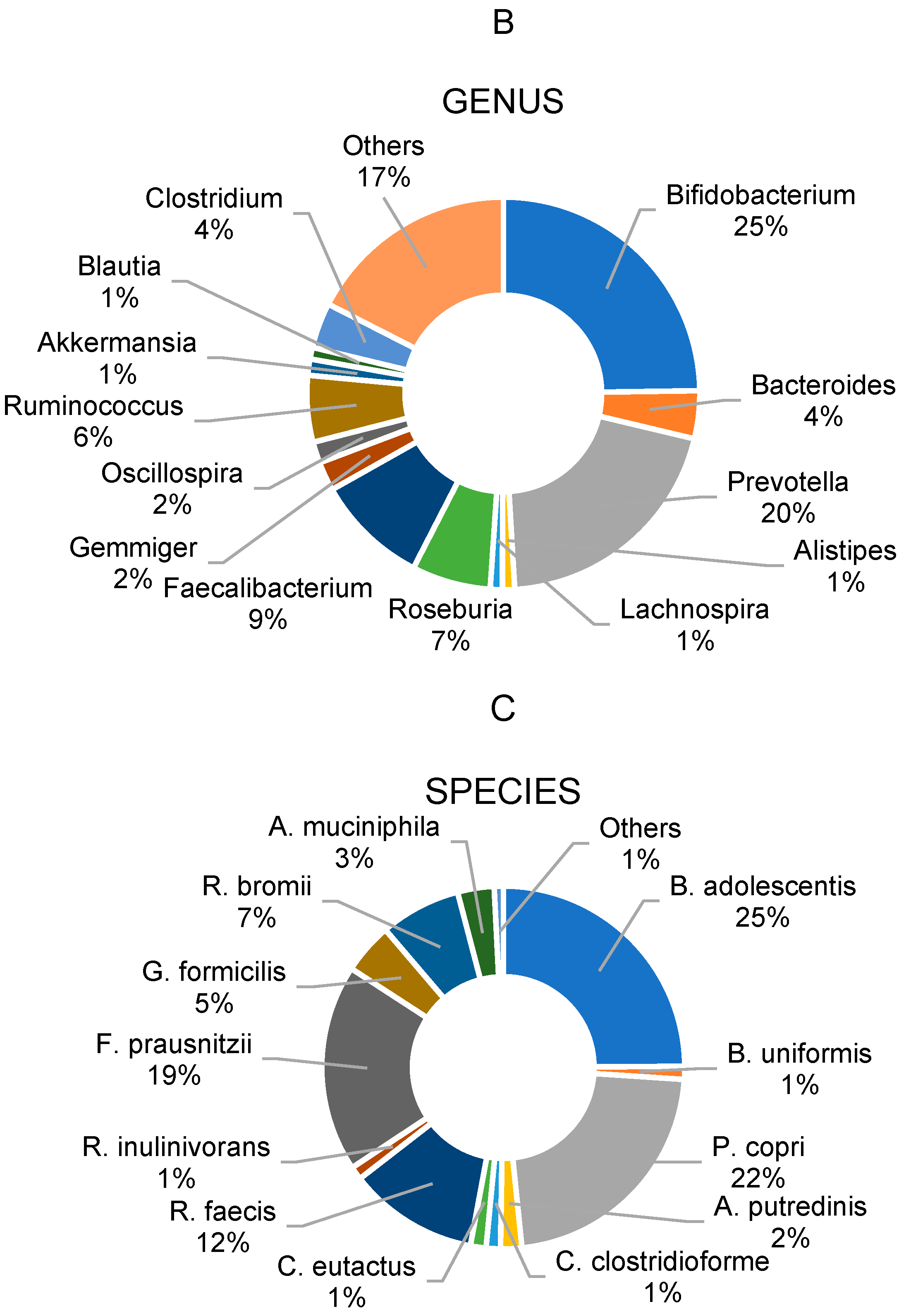
3.2. Gut Microbiota Profile of Overweight and Obese School Children
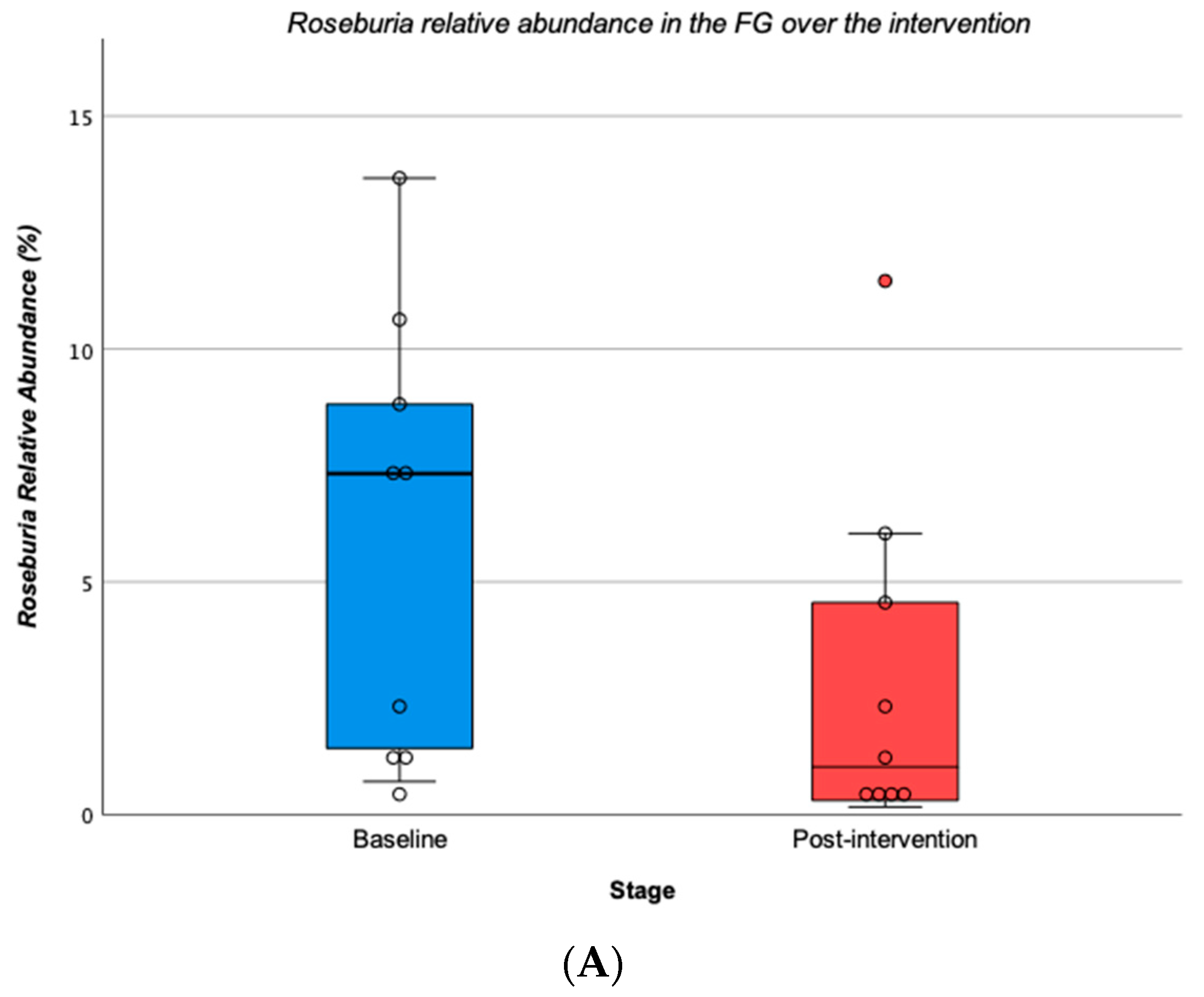
3.3. Changes in Gut Microbiota Profile over the Intervention
3.3.1. Overall Participants
3.3.2. Football Group vs. Football and Nutrition Group
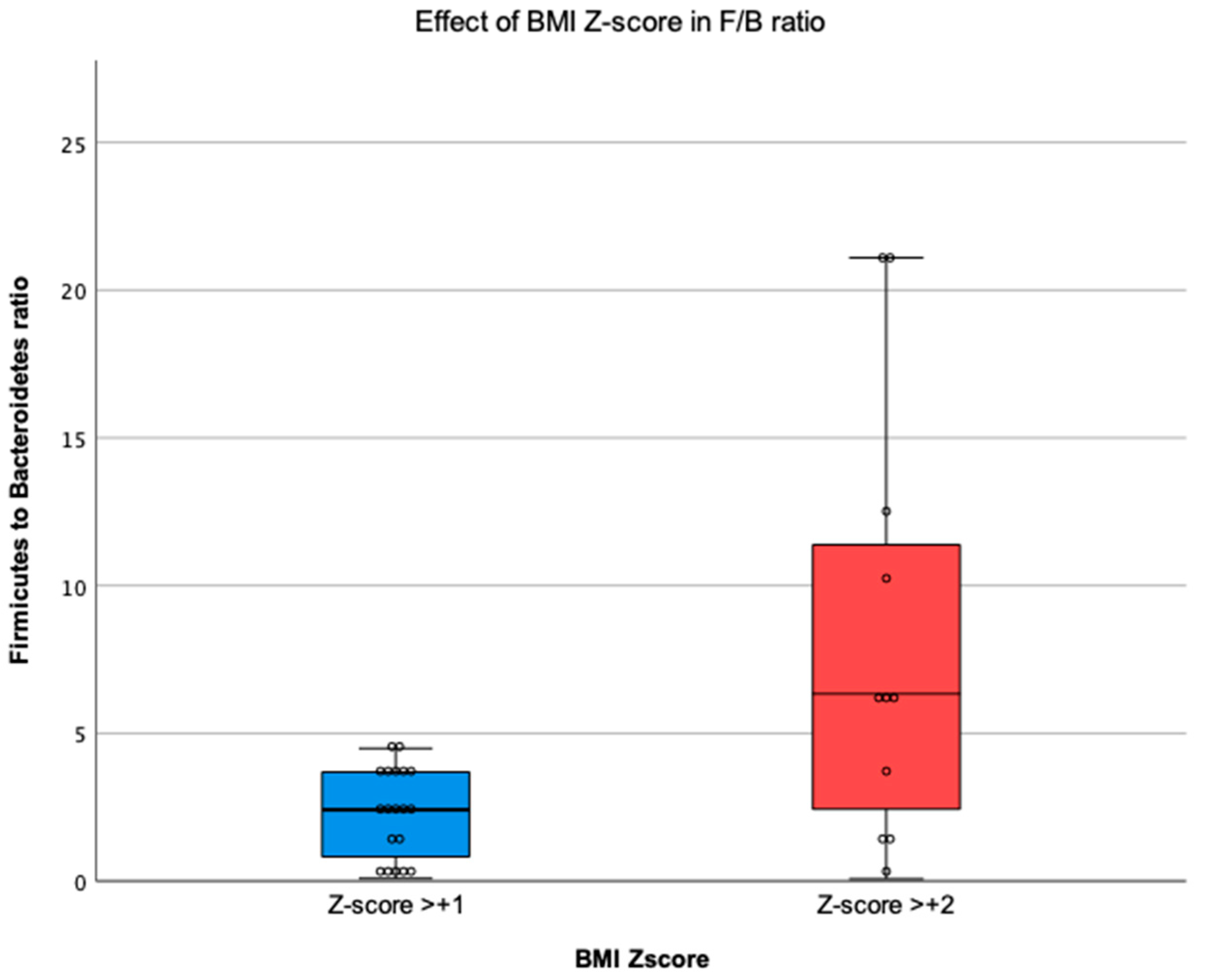
3.4. Relationship between BMI Z-Score and the Gut Microbiota
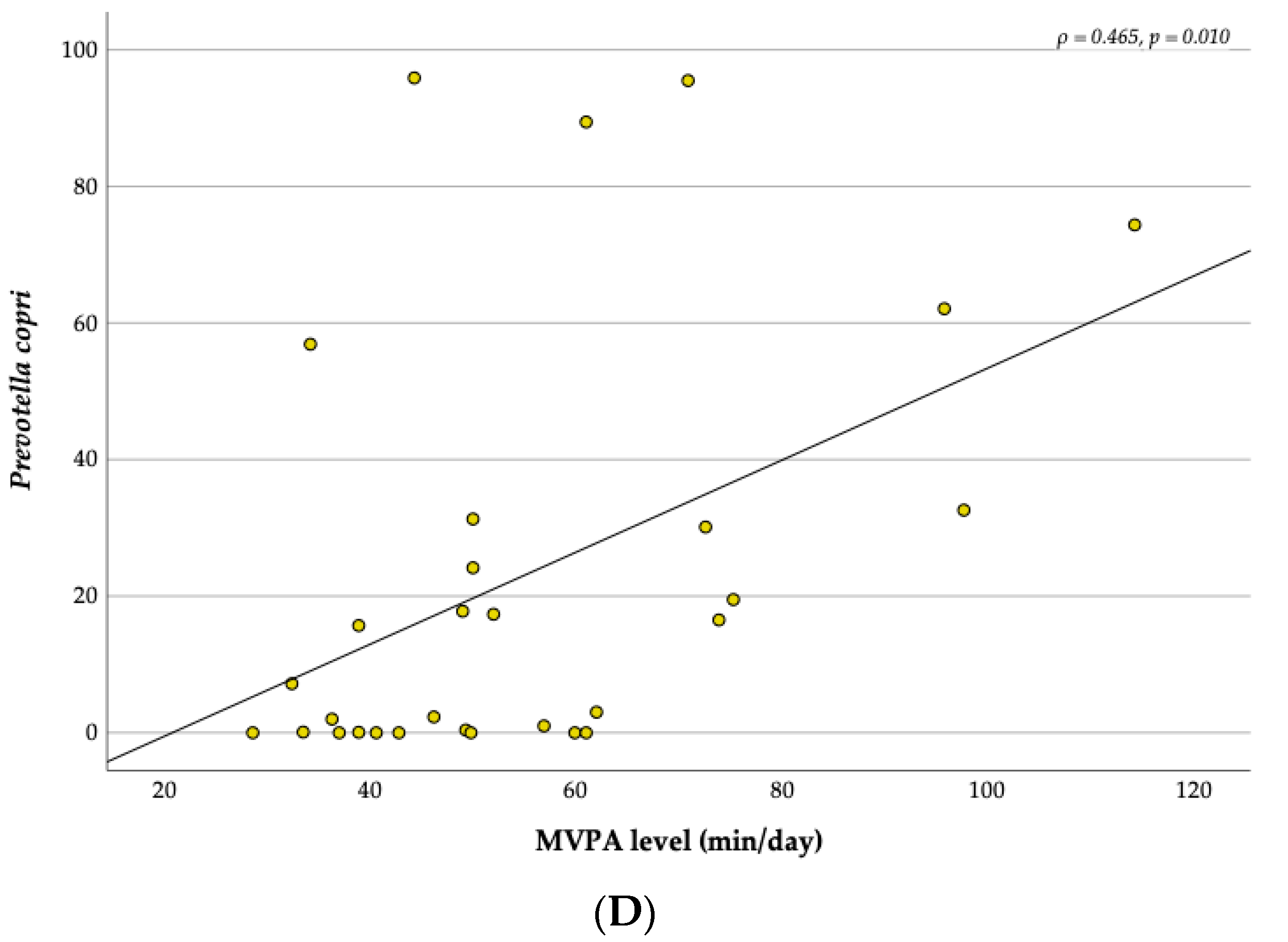
3.5. Relationship between the MVPA and the Gut Microbiota
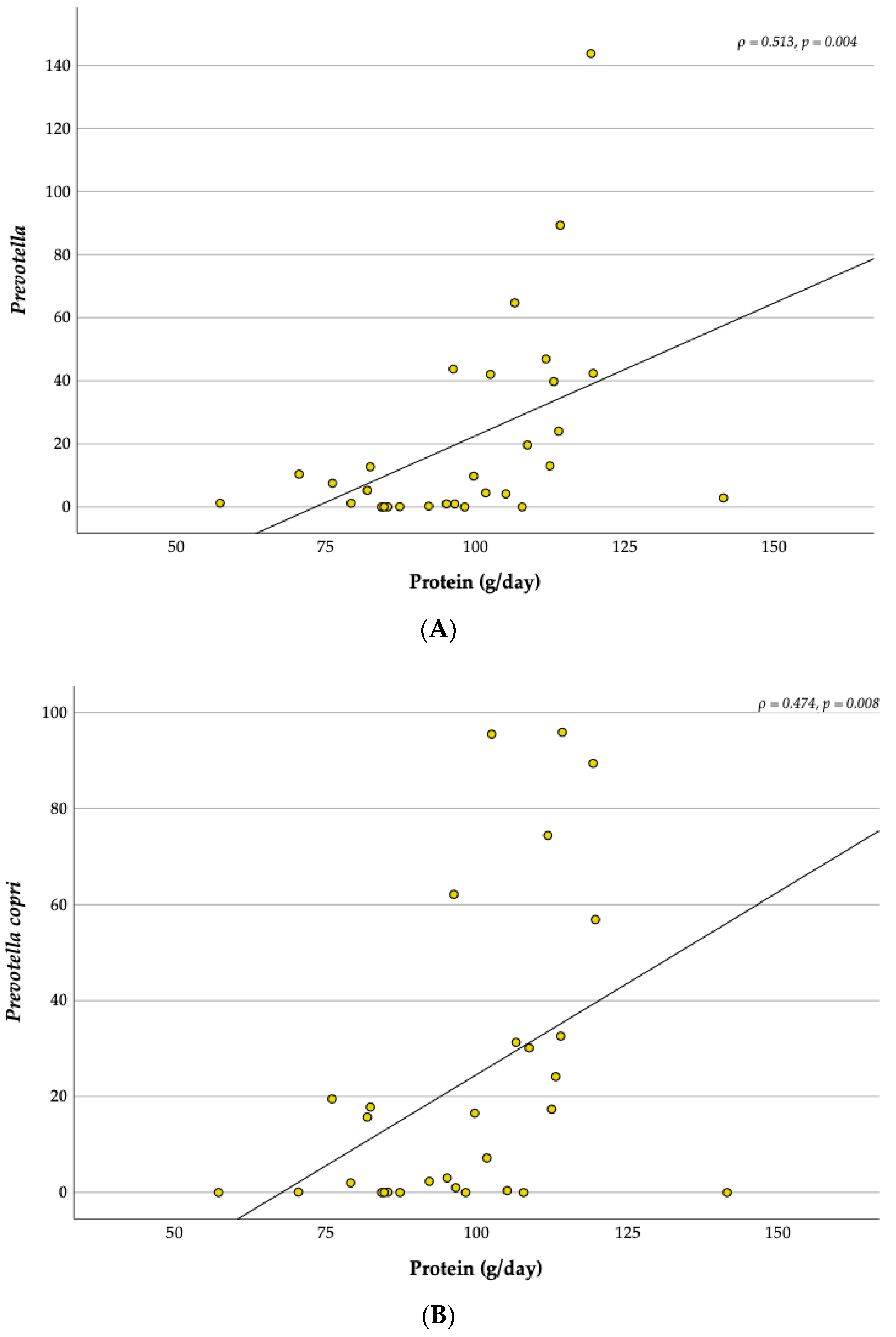
3.6. Relationship between the Dietary Intake and the Gut Microbiota
4. Discussion
4.1. Anthropometric and Body Composition
4.2. Gut Microbiota Composition in Overweight and Obese Children
4.3. Comparing the Gut Microbiota Composition between the Time Points
4.4. Firmicutes to Bacteroidetes Ratio and BMI Z-Score
4.5. Correlations with Physical Activity and Diet
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization, Regional Office for Europe. WHO European Regional Obesity Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Rutter, H. The Complex Systems Challenge of Obesity. Clin. Chem. 2018, 64, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Ammaniti, M.; Lucarelli, L.; Cimino, S.; Ambruzzi, A.M.; D’Olimpio, F. Malnutrition and Dysfunctional Mother-Child Feeding Interactions: Clinical Assessment and Research Implications. J. Am. Coll. Nutr. 2004, 23, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Du, M.; Khandpur, N.; Rossato, S.L.; Lo, C.H.; Vanevery, H.; Kim, D.Y.; Zhang, F.F.; Chavarro, J.E.; et al. Maternal Consumption of Ultra-Processed Foods and Subsequent Risk of Offspring Overweight or Obesity: Results from Three Prospective Cohort Studies. BMJ 2022, 379, e071767. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H. Overweight in Childhood and Adolescence. N. Engl. J. Med. 2004, 350, 855–857. [Google Scholar] [CrossRef]
- Petraroli, M.; Castellone, E.; Patianna, V.; Esposito, S. Gut Microbiota and Obesity in Adults and Children: The State of the Art. Front. Pediatr. 2021, 9, 657020. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef]
- Whitaker, R.C.; Wright, J.A.; Pepe, M.S.; Seidel, K.D.; Dietz, W.H. Predicting Obesity in Young Adulthood from Childhood and Parental Obesity. N. Engl. J. Med. 1997, 337, 204. [Google Scholar] [CrossRef]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- World Health Organization. WHO Discussion Paper: Draft Recommendations for the Prevention and Management of Obesity over the Life Course, Including Potential Targets; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Da Silva, C.C.; Monteil, M.A.; Davis, E.M. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child. Obes. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in Gut Microbiota Composition between Obese and Lean Children: A Cross-Sectional Study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Mbakwa, C.A.; Hermes, G.D.A.; Penders, J.; Savelkoul, P.H.M.; Thijs, C.; Dagnelie, P.C.; Mommers, M.; Zoetendal, E.G.; Smidt, H.; Arts, I.C.W. Gut Microbiota and Body Weight in School-Aged Children: The KOALA Birth Cohort Study. Obesity 2018, 26, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Pate, R.R. Effects of Exercise on BMI Z-Score in Overweight and Obese Children and Adolescents: A Systematic Review with Meta-Analysis. BMC Pediatr. 2014, 14, 225. [Google Scholar] [CrossRef]
- Pedersini, P.; Turroni, S.; Villafañe, J.H. Gut Microbiota and Physical Activity: Is There an Evidence-Based Link? Sci. Total Environ. 2020, 727, 138648. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Bai, J.; Hu, Y.; Bruner, D.W. Composition of Gut Microbiota and Its Association with Body Mass Index and Lifestyle Factors in a Cohort of 7-18 Years Old Children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef]
- Cho, K.Y. Lifestyle Modifications Result in Alterations in the Gut Microbiota in Obese Children. BMC Microbiol. 2021, 21, 10. [Google Scholar] [CrossRef]
- Hollister, E.B.; Foster, B.A.; Dahdouli, M.; Ramirez, J.; Lai, Z. Characterization of the Stool Microbiome in Hispanic Preschool Children by Weight Status and Time. Child. Obes. 2018, 14, 122–130. [Google Scholar] [CrossRef]
- López-Contreras, B.E.; Morán-Ramos, S.; Villarruel-Vázquez, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; León-Mimila, P.; Vega-Badillo, J.; Sánchez-Muñoz, F.; Llanos-Moreno, L.E.; Canizalez-Román, A.; et al. Composition of Gut Microbiota in Obese and Normal-Weight Mexican School-Age Children and Its Association with Metabolic Traits. Pediatr. Obes. 2018, 13, 381–388. [Google Scholar] [CrossRef]
- Mehta, S.; Huey, S.L.; McDonald, D.; Knight, R.; Finkelstein, J.L. Nutritional Interventions and the Gut Microbiome in Children. Annu. Rev. Nutr. 2021, 41, 479–510. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC Growth Charts: United States. Adv. Data 2000, 314, 1–27. [Google Scholar]
- Ismael, S.; Silvestre, M.P.; Vasques, M.; Araújo, J.R.; Morais, J.; Duarte, M.I.; Pestana, D.; Faria, A.; Pereira-Leal, J.B.; Vaz, J.; et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Fuller, C.W.; Junge, A. Planning and Implementing a Nationwide Football-Based Health-Education Programme. Br. J. Sports Med. 2012, 46, 6–10. [Google Scholar] [CrossRef]
- Fuller, C.W.; Junge, A.; Decelles, J.; Donald, J.; Jankelowitz, R.; Dvorak, J. “Football for Health”—A Football-Based Health-Promotion Programme for Children in South Africa: A Parallel Cohort Study. Br. J. Sports Med. 2010, 44, 546–554. [Google Scholar] [CrossRef]
- Rodrigues, S.S.P.; Franchini, B.; Graça, P.; de Almeida, M.D.V. A New Food Guide for the Portuguese Population: Development and Technical Considerations. J. Nutr. Educ. Behav. 2006, 38, 189–195. [Google Scholar] [CrossRef]
- Stewart, A.D.; Marfell-Jones, M.J.; Olds, T.; Hans de Ridder, J. International Standards for Anthropometric Assessment; Engineering & Technology, Sports and Leisure: 2011; ISBN 0-620-36207-3. Available online: https://repository.openpolytechnic.ac.nz/items/cb3ad790-1a9f-4790-9616-d0651ddc6503 (accessed on 15 July 2023).
- de Onis, M.; Lobstein, T. Defining Obesity Risk Status in the General Childhood Population: Which Cut-Offs Should We Use? Int. J. Pediatr. Obes. 2010, 5, 458–460. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar] [CrossRef]
- The National Institutes of Health, Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual; The National Institutes of Health, Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2020. [Google Scholar]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J.; Bell, S.J.; Chertow, G.M.; Chumlea, W.C.; Knox, T.A.; Kotler, D.P.; Lukaski, H.C.; Schoeller, D.A. Bioelectrical Impedance Methods in Clinical Research: A Follow-up to the NIH Technology Assessment Conference. Nutrition 1999, 15, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Trost, S.G.; Loprinzi, P.D.; Moore, R.; Pfeiffer, K.A. Comparison of Accelerometer Cut Points for Predicting Activity Intensity in Youth. Med. Sci. Sports Exerc. 2011, 43, 1360–1368. [Google Scholar] [CrossRef]
- Evenson, K.R.; Catellier, D.J.; Gill, K.; Ondrak, K.S.; McMurray, R.G. Calibration of Two Objective Measures of Physical Activity for Children. J. Sports Sci. 2008, 26, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Troiano, R.P. Large-Scale Applications of Accelerometers: New Frontiers and New Questions. Med. Sci. Sports Exerc. 2007, 39, 1501. [Google Scholar] [CrossRef]
- Kuritz, A.; Mall, C.; Schnitzius, M.; Mess, F. Physical Activity and Sedentary Behavior of Children in Afterschool Programs: An Accelerometer-Based Analysis in Full-Day and Half-Day Elementary Schools in Germany. Front. Public Health 2020, 8, 463. [Google Scholar] [CrossRef]
- Steene-johannessen, J.; Hansen, B.H.; Dalene, K.E.; Kolle, E.; Northstone, K.; Møller, N.C.; Grøntved, A.; Wedderkopp, N.; Kriemler, S.; Page, A.S.; et al. Variations in Accelerometry Measured Physical Activity and Sedentary Time across Europe—Harmonized Analyses of 47,497 Children and Adolescents. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 38. [Google Scholar] [CrossRef]
- Cooper, A.R.; Goodman, A.; Page, A.S.; Sherar, L.B.; Esliger, D.W.; van Sluijs, E.M.F.; Andersen, L.B.; Anderssen, S.; Cardon, G.; Davey, R.; et al. Objectively Measured Physical Activity and Sedentary Time in Youth: The International Children’s Accelerometry Database (ICAD). Int. J. Behav. Nutr. Phys. Act. 2015, 12, 113. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Lopes, C.; Torres, D.; Oliveira, A.; Severo, M.; Alarcão, V.; Guiomar, S.; Mota, J.; Teixeira, P.; Ramos, E.; Rodrigues, S.; et al. Inquérito Alimentar Nacional e de Atividade Física, IAN-AF 2015-2016: Relatório Metodológico; Universidade do Porto: Porto, Portugal, 2017. [Google Scholar]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D. High-Fat Diet-Induced Obesity Rat Model: A Comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Gregory Caporaso, J.; Fuhrman, J.A.; et al. Improved Bacterial 16S RRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys Crossmark Downloaded From. Msystems 2015, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.; Marques, C.; Faria, A.; Teixeira, D.; Barreiros-Mota, I.; Durão, C.; Araújo, J.; Ismael, S.; Brito, S.; Cardoso, M.; et al. Influence of Human Milk on Very Preterms’ Gut Microbiota and Alkaline Phosphatase Activity. Nutrients 2021, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Santacruz, A.; Marcos, A.; Wärnberg, J.; Martí, A.; Martin-Matillas, M.; Campoy, C.; Moreno, L.A.; Veiga, O.; Redondo-Figuero, C.; Garagorri, J.M.; et al. Interplay between Weight Loss and Gut Microbiota Composition in Overweight Adolescents. Obesity 2009, 17, 1906–1915. [Google Scholar] [CrossRef]
- Nadal, I.; Santacruz, A.; Marcos, A.; Warnberg, J.; Garagorri, M.; Moreno, L.A.; Martin-Matillas, M.; Campoy, C.; Martí, A.; Moleres, A.; et al. Shifts in Clostridia, Bacteroides and Immunoglobulin-Coating Fecal Bacteria Associated with Weight Loss in Obese Adolescents. Int. J. Obes. 2009, 33, 758–767. [Google Scholar] [CrossRef]
- Vasconcellos, F.; Seabra, A.; Cunha, F.; Montenegro, R.; Penha, J.; Bouskela, E.; Nogueira Neto, J.F.; Collett-Solberg, P.; Farinatti, P. Health Markers in Obese Adolescents Improved by a 12-Week Recreational Soccer Program: A Randomised Controlled Trial. J. Sports Sci. 2016, 34, 564–575. [Google Scholar] [CrossRef]
- Seabra, A.; Katzmarzyk, P.; Carvalho, M.J.; Seabra, A.; Coelho-E-Silva, M.; Abreu, S.; Vale, S.; Póvoas, S.; Nascimento, H.; Belo, L.; et al. Effects of 6-Month Soccer and Traditional Physical Activity Programmes on Body Composition, Cardiometabolic Risk Factors, Inflammatory, Oxidative Stress Markers and Cardiorespiratory Fitness in Obese Boys. J. Sports Sci. 2016, 34, 1822–1829. [Google Scholar] [CrossRef]
- Seabra, A.; Brito, J.; Figueiredo, P.; Beirão, L.; Seabra, A.; Carvalho, M.J.; Abreu, S.; Vale, S.; Pedretti, A.; Nascimento, H.; et al. School-Based Soccer Practice Is an Effective Strategy to Improve Cardiovascular and Metabolic Risk Factors in Overweight Children. Prog. Cardiovasc. Dis. 2020, 63, 807–812. [Google Scholar] [CrossRef]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric Obesity Is Associated with an Altered Gut Microbiota and Discordant Shifts in Firmicutes Populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2008, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, A.; Fernandes, M.R.; Rodrigues, V.A.A.; Groppo, F.C.; Cardoso, A.L.; Avila-Campos, M.J.; Nakano, V. Correlation between Body Mass Index and Faecal Microbiota from Children. Clin. Microbiol. Infect. 2016, 22, e1–e258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, H.; Jiang, F.; Shen, Y.; Li, X.; Hu, X.; Shen, X.; Wei, P. Alteration of the Gut Microbiota Associated with Childhood Obesity by 16S RRNA Gene Sequencing. PeerJ 2020, 8, e8317. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; George, G.; Kabeerdoss, J.; Hepsiba, J.; Chandragunasekaran, A.M.S.; Ramakrishna, B.S. Quantitative Differences in Intestinal Faecalibacterium Prausnitzii in Obese Indian Children. Br. J. Nutr. 2010, 103, 335–338. [Google Scholar] [CrossRef]
- Gao, X.; Jia, R.; Xie, L.; Kuang, L.; Feng, L.; Wan, C. A Study of the Correlation between Obesity and Intestinal Flora in School-Age Children. Sci. Rep. 2018, 8, 14511. [Google Scholar] [CrossRef]
- Borgo, F.; Verduci, E.; Riva, A.; Lassandro, C.; Riva, E.; Morace, G.; Borghi, E. Relative Abundance in Bacterial and Fungal Gut Microbes in Obese Children: A Case Control Study. Child. Obes. 2017, 13, 78–84. [Google Scholar] [CrossRef]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and Function of the Healthy Pre-Adolescent Pediatric Gut Microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of Inulin on the Human Gut Microbiota: Stimulation of Bifidobacterium Adolescentis and Faecalibacterium Prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14. [Google Scholar] [CrossRef]
- Schloss, P.D.; Iverson, K.D.; Petrosino, J.F.; Schloss, S.J. The Dynamics of a Family’s Gut Microbiota Reveal Variations on a Theme. Microbiome 2014, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Hippe, B.; Remely, M.; Aumueller, E.; Pointner, A.; Magnet, U.; Haslberger, A.G. Faecalibacterium Prausnitzii Phylotypes in Type Two Diabetic, Obese, and Lean Control Subjects. Benef. Microbes 2016, 7, 511–517. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Karlsson, C.L.J.; Önnerfält, J.; Xu, J.; Molin, G.; Ahrné, S.; Thorngren-Jerneck, K. The Microbiota of the Gut in Preschool Children with Normal and Excessive Body Weight. Obesity 2012, 20, 2257–2261. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M.; et al. The Prevotella Copri Complex Comprises Four Distinct Clades Underrepresented in Westernized Populations. Cell Host Microbe 2019, 26, 666–679. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Sun, Y.; Ip, L.Y.T.; Wang, L.; Chan, F.K.L.; Miao, Y.; Ng, S.C. Prevotella Species in the Human Gut Is Primarily Comprised of Prevotella Copri, Prevotella Stercorea and Related Lineages. Sci. Rep. 2022, 12, 9055. [Google Scholar] [CrossRef]





| FG (n = 9) | NFG (n = 6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline * | Post * | %Δ | Baseline * | Post * | %Δ | ||||
| Age (y) | 8.8 | ±0.8 | 9.2 | ±0.8 | 9.4 | ±0.2 | 9.7 | ±0.2 | ||
| Height (cm) | 134.8 | ±11.2 | 138.1 | ±11.2 | 2.4 | 141.9 | ±1.4 | 144.5 | ±2.0 | 1.8 |
| Weight (kg) | 41.9 | ±11.9 | 42.7 | ±11.8 | 1.8 | 46.8 | ±6.6 | 46.6 | ±7.0 | −0.6 |
| BMI (kg/m2) | 22.6 | ±2.9 | 22.0 | ±2.7 † | −2.8 | 23.3 | ±3.3 | 22.3 | ±3.4 † | −4.1 |
| BMI Z-score | 1.8 | ±0.4 | 1.6 | ±0.4 † | −10.0 | 1.7 | ±0.5 | 1.5 | ±0.7 † | −14.7 |
| WC (cm) | 76.1 | ±11.2 | 75.7 | ±10.8 | −0.5 | 78.8 | ±7.1 | 74.5 | ±9.0 † | −5.4 |
| WtHr | 0.6 | ±0.0 | 0.5 | ±0.0 † | −2.8 | 0.6 | ±0.0 | 0.5 | ±0.1 † | −7.0 |
| %BFM | 34.8 | ±4.8 | 31.8 | ±5.7 † | −8.6 | 30.5 | ±6.2 | 29.7 | ±7.3 | −2.5 |
| FFM (kg) | 27.6 | ±9.1 | 29.4 | ±9.5 † | 6.6 | 32.3 | ±2.8 | 32.3 | ±2.4 | 0.1 |
| MM (kg) | 17.9 | ±6.1 | 19.1 | ±6.3 † | 6.3 | 21.9 | ±2.0 | 21.2 | ±2.1 | −3.3 |
| MVPA (min/d) | 46.7 | ±15.1 | 58.6 | ±10.1 † | 25.5 | 59.9 | ±28.9 | 57.9 | ±30.7 | −3.5 |
| FG (n = 9) | NFG (n = 6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | %Δ | Baseline | Post | %Δ | |||||
| phylum level | ||||||||||
| Actinobacteria | 24.5 | ±26.2 | 17.3 | ±13.9 | −29.5 | 27.3 | ±19.4 | 9.3 | ±5.3 | −66.1 |
| Bacteroidetes | 26.2 | ±27.1 | 24.3 | ±24.6 | −7.3 | 28.0 | ±25.7 | 40.0 | ±31.5 | 43.2 |
| Firmicutes | 46.3 | ±24.0 | 50.3 | ±17.8 | 8.8 | 41.4 | ±17.4 | 48.3 | ±24.5 | 16.6 |
| Proteobacteria | 1.0 | ±0.9 | 1.5 | ±1.4 | 47.7 | 1.4 | ±1.6 | 1.3 | ±1.0 | −1.7 |
| F/B ratio | 5.4 | ±6.9 | 3.9 | ±2.9 | −27.7 | 5.7 | ±7.9 | 2.6 | ±2.5 | −53.1 |
| genus level | ||||||||||
| Bifidobacterium | 23.4 | ±25.2 | 13.9 | ±13.4 | −40.5 | 26.9 | ±19.5 | 9.0 | ±6.4 † | −66.4 |
| Bacteroides | 4.4 | ±4.7 | 4.3 | ±7.9 | −3.0 | 3.2 | ±2.3 | 4.7 | ±4.1 | 44.5 |
| Prevotella | 18.7 | ±29.5 | 21.3 | ±46.4 | 13.7 | 22.8 | ±26.7 | 22.4 | ±22.6 | −1.6 |
| Roseburia | 5.9 | ±4.7 | 3.0 | ±3.8 † | −49.9 | 7.3 | ±6.6 | 9.5 | ±9.3 | 31.0 |
| Faecalibacterium | 9.7 | ±8.9 | 7.1 | ±4.7 | −27.3 | 8.6 | ±3.6 | 7.1 | ±4.7 | −17.5 |
| Ruminococcus | 6.0 | ±5.4 | 3.8 | ±4.9 | −36.7 | 4.7 | ±4.4 | 4.7 | ±3.5 | 0.1 |
| species level | ||||||||||
| Bifidobacterium adolescentis | 22.2 | ±28.8 | 21.5 | ±24.8 | −3.4 | 29.0 | ±25.1 | 10.3 | ±9.9 | −64.4 |
| Prevotella copri | 23.0 | ±32.8 | 17.7 | ±28.9 | −23.1 | 21.3 | ±25.2 | 33.6 | ±41.3 | 57.3 |
| Roseburia faecis | 10.2 | ±10.2 | 7.4 | ±8.8 | −27.2 | 13.8 | ±12.2 | 20.4 | ±22.7 | 48.0 |
| Faecalibacterium prausnitzii | 19.2 | ±16.7 | 21.7 | ±13.2 | 12.8 | 17.5 | ±8.3 | 15.9 | ±12.0 | −8.9 |
| Ruminococcus bromii | 7.8 | ±8.9 | 8.2 | ±7.1 | 5.1 | 6.5 | ±6.4 | 4.6 | ±6.3 | −29.4 |
| FG (n = 9) | NFG (n = 6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | %Δ | Baseline | Post | %Δ | |||||
| Macronutrients | ||||||||||
| Energy (kcal) | 1916.2 | ±362.9 | 1816.0 | ±379.0 | −5.2 | 1909.1 | ±285.8 | 1704.3 | ±329.1 | −10.7 |
| Protein (g) | 92.6 | ±17.6 | 101.7 | ±10.8 | 9.7 | 104.1 | ±22.2 | 95.3 | ±21.1 | −8.5 |
| * Carbohydrate (g) | 259.7 | ±78.1 | 203.1 | ±61.6 | −21.8 | 247.9 | ±68.4 | 203.6 | ±46.3 | −17.9 |
| Fiber (g) | 17.3 | ±9.2 | 14.8 | ±7.3 | −14.5 | 14.6 | ±5.7 | 13.5 | ±5.2 | −7.2 |
| Sugar (g) | 89.9 | ±57.4 | 67.3 | ±36.7 | −25.1 | 81.7 | ±31.7 | 61.8 | ±17.2 | −24.4 |
| Fat (g) | 56.9 | ±14.5 | 66.7 | ±17.2 | 17.1 | 56.6 | ±13.4 | 55.5 | ±16.0 | −2.0 |
| Saturated Fat (g) | 18.9 | ±4.6 | 21.7 | ±4.6 | 14.6 | 18.5 | ±6.9 | 19.9 | ±6.1 | 7.1 |
| Food Groups | ||||||||||
| Fruits and Vegetables (g) | 653.9 | ±238.6 | 516.7 | ±430.4 | −20.9 | 364.2 | ±329.5 | 374.0 | ±262.2 | 2.7 |
| * Red Meat (g) | 72.8 | ±87.1 | 134.8 | ±94.9 | 85.2 | 60.7 | ±66.9 | 155.0 | ±90.7 | 155.4 |
| Cheese and Yoghurt (g) | 122.9 | ±123.8 | 140.1 | ±115.6 | 14.0 | 109.0 | ±115.5 | 144.8 | ±128.9 | 32.8 |
| Dairy (g) | 361.3 | ±103.6 | 408.4 | ±149.6 | 13.0 | 453.0 | ±269.9 | 476.3 | ±140.3 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgado, M.C.; Sousa, M.; Marques, C.; Coelho, A.B.; Costa, J.A.; Seabra, A. Effects of Physical Activity and Nutrition Education on the Gut Microbiota in Overweight and Obese Children. Children 2023, 10, 1242. https://doi.org/10.3390/children10071242
Morgado MC, Sousa M, Marques C, Coelho AB, Costa JA, Seabra A. Effects of Physical Activity and Nutrition Education on the Gut Microbiota in Overweight and Obese Children. Children. 2023; 10(7):1242. https://doi.org/10.3390/children10071242
Chicago/Turabian StyleMorgado, Micaela C., Mónica Sousa, Cláudia Marques, André B. Coelho, Júlio A. Costa, and André Seabra. 2023. "Effects of Physical Activity and Nutrition Education on the Gut Microbiota in Overweight and Obese Children" Children 10, no. 7: 1242. https://doi.org/10.3390/children10071242
APA StyleMorgado, M. C., Sousa, M., Marques, C., Coelho, A. B., Costa, J. A., & Seabra, A. (2023). Effects of Physical Activity and Nutrition Education on the Gut Microbiota in Overweight and Obese Children. Children, 10(7), 1242. https://doi.org/10.3390/children10071242









