Indirect Neonatal Hyperbilirubinemia and the Role of Fenofibrate as an Adjuvant to Phototherapy
Abstract
1. Introduction
2. Patients and Methods
2.1. Subjects
- Preterm (<37 weeks gestational age).
- Conjugated bilirubin levels above 2 mg/dL or more than 15% of TSB.
- Congenital anomalies.
- Sepsis or exchange transfusion.
- Respiratory distress.
- Cephalohematoma or subgaleal bleeding.
2.2. The Study Groups
- Group A—the intervention group consisted of 50 newborns who were given conventional phototherapy in addition to a single dose of oral fenofibrate suspension administered at a dose of 10 mg/kg.
- Group B—standard phototherapy was the sole treatment that any of the 50 newborns in the control group received.
- The neonates were not assigned to the different study groups in a random fashion. We considered a group of newborns who were only given conventional phototherapy to be a control when that group’s characteristics and measurements were almost identical to those of the intervention group’s newborns who were given the same number of assignments to be in that group. This procedure was repeated until there were fifty newborns in each of the groups.
- Guidelines established by the American Academy of Pediatrics (AAP) for term and near-term infants have been centered on when phototherapy should begin and end [24]. It was determined that a BT-400 (Korea) Phototherapy device would be utilized. This device is a floor-standing, mobile phototherapy light that produces a narrow band of high-intensity blue light via blue light-emitting diodes (LEDs). Blue LEDs produce light with a wavelength that falls between (400 and 550) nanometers (the peak wavelength falls between 450 and 475 nanometers), and it is anticipated that the light will continue to function as intended for roughly 20,000 h. This spectrum, which corresponds to the spectral absorption of light by bilirubin, is therefore regarded as the most efficient and secure for the destruction of bilirubin. The baby’s vital signs were monitored throughout the process, and a gap of around 40 cm was maintained between the newborn and the photo lamp. Eye pads and diapers were used to protect both the eyes and the genital area, respectively.
2.3. Data Collection
2.4. Statistical Analysis
3. Results
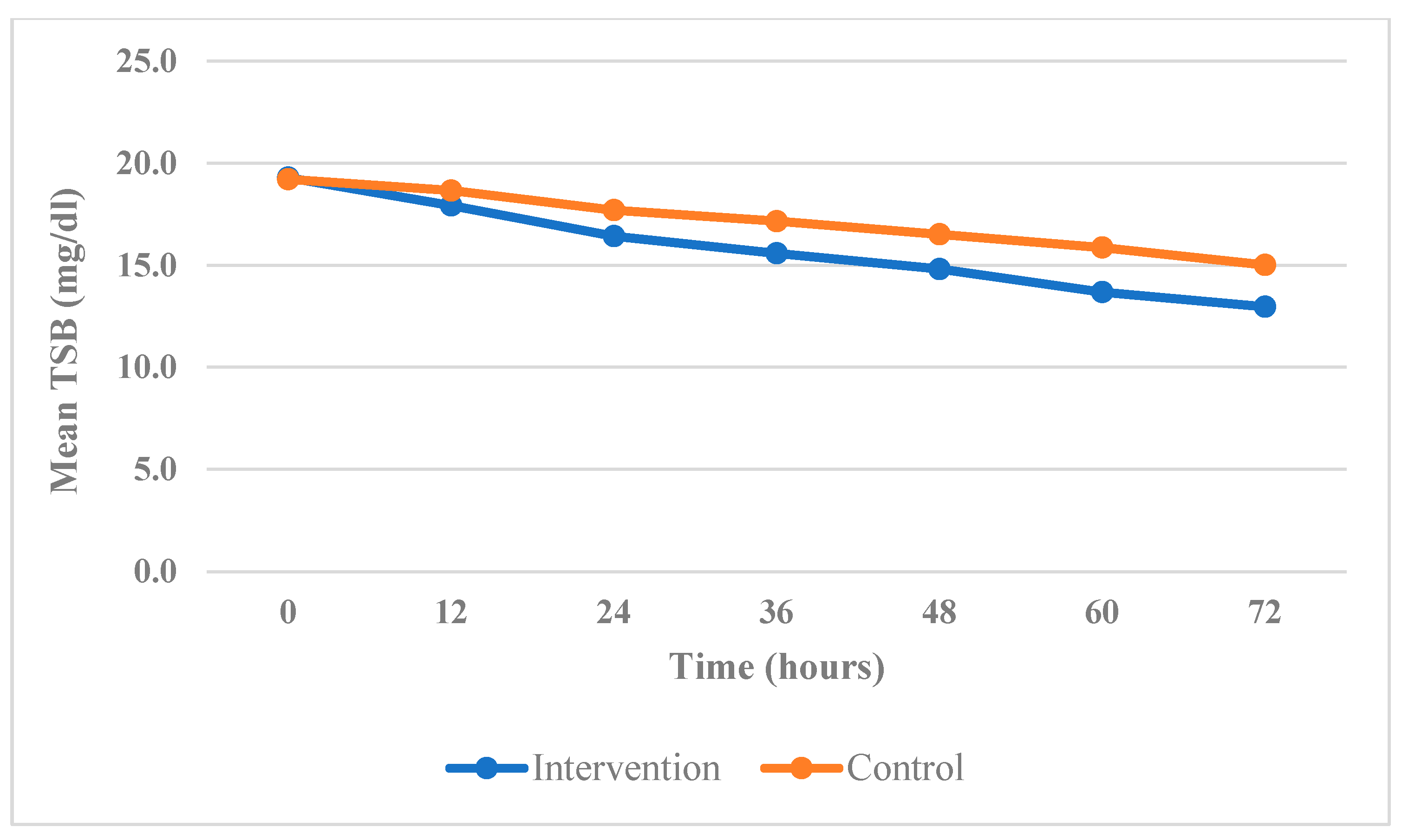
- At 24 h, the mean TSB in the group that received treatment was 16.42 mg/dL, while the mean TSB in the control group was 17.69 mg/dL.
- At 48 h, the mean TSB value in the group that received fenofibrate was 14.81 mg/dL, while the value in the control group was 16.51 mg/dL.
- Finally, at 72 h, the mean TSB value in the group that was given fenofibrate was 12.97 mg/dL, while the value in the control group was 15.01 mg/dL.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pathak, N.N.; Anupama, D.; Bidyut, S. Efficacy of Oral Fenofibrate in Management of Unconjugated Hyperbilirubinemia in the Neonate. Int. J. Health Res. Med. Leg. Pract. 2020, 6, 13. [Google Scholar] [CrossRef]
- Rennie, J.; Burman-Roy, S.; Murphy, M.S. Neonatal Jaundice: Summary of NICE Guidance. BMJ 2010, 340, c2409. [Google Scholar] [CrossRef] [PubMed]
- Diala, U.M.; Usman, F.; Appiah, D.; Hassan, L.; Ogundele, T.; Abdullahi, F.; Satrom, K.M.; Bakker, C.J.; Lee, B.W.; Slusher, T.M. Global Prevalence of Severe Neonatal Jaundice among Hospital Admissions: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3738. [Google Scholar] [CrossRef] [PubMed]
- Erdeve, O. Management of Neonatal Jaundice in Low-Income and Middle-Income Countries. BMJ Paediatr. Open 2020, 4, e000845. [Google Scholar] [CrossRef]
- Islam, T.; Ghosh, U.K.; Rahmanr, M.; Haqueh, M.M.; Akhter, N.; Silvana, K. Effect of Single Dose Fenofibrate as an Adjunct to Phototherapy on Unconjugated Neonatal Hyperbilirubinemia: A RCT. Bangladesh J. Child Health 2022, 45, 89–93. [Google Scholar] [CrossRef]
- Dennery, P.A.; Seidman, D.S.; Stevenson, D.K. Neonatal Hyperbilirubinemia. N. Engl. J. Med. 2001, 344, 581–590. [Google Scholar] [CrossRef]
- Erdeve, O.; Okulu, E.; Olukman, O.; Ulubas, D.; Buyukkale, G.; Narter, F.; Tunc, G.; Atasay, B.; Gultekin, N.D.; Arsan, S.; et al. The Turkish Neonatal Jaundice Online Registry: A National Root Cause Analysis. PLoS ONE 2018, 13, e0193108. [Google Scholar] [CrossRef]
- Kumar, B.; Agarwal, P.; Chorishi, A.; Dhaneria, M. Fenofibrate: A Novel Approach in Treating Uncomplicated Neonatal Hyperbilirubinemia? People J. Sci. Res. 2012, 5, 5–8. [Google Scholar]
- Muchowski, K.E. Evaluation and Treatment of Neonatal Hyperbilirubinemia. Am. Fam. Physician 2014, 89, 873–878. [Google Scholar]
- Chamorro, E.; Bonnin-Arias, C.; Pérez-Carrasco, M.J.; De Luna, J.M.; Vázquez, D.; Sánchez-Ramos, C. Effects of Light-Emitting Diode Radiations on Human Retinal Pigment Epithelial Cells In Vitro. Photochem. Photobiol. 2013, 89, 468–473. [Google Scholar] [CrossRef]
- Bhutani, V.K.; Zipursky, A.; Blencowe, H.; Khanna, R.; Sgro, M.; Ebbesen, F.; Bell, J.; Mori, R.; Slusher, T.M.; Fahmy, N.; et al. Neonatal Hyperbilirubinemia and Rhesus Disease of the Newborn: Incidence and Impairment Estimates for 2010 at Regional and Global Levels. Pediatr. Res. 2013, 74, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.E.; Blencowe, H.; Oza, S.; You, D.; Lee, A.C.; Waiswa, P.; Lalli, M.; Bhutta, Z.; Barros, A.J.; Christian, P.; et al. Every Newborn: Progress, Priorities, and Potential beyond Survival. Lancet 2014, 384, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Cuperus, F.J.; Hafkamp, A.; Hulzebos, C.; Verkade, H. Pharmacological Therapies for Unconjugated Hyperbilirubinemia. Curr. Pharm. Des. 2009, 15, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour-kacho, M.; Zahed Pasha, Y.; Ranjbar, B.; Pouramir, M.; Hajian, K.; Pounasrollah, M. The Effect of Oral Zinc Sulfate on Serum Bilirubine Level in Term Neonates with Jaundice. Int. J. Pediatr. 2017, 5, 5053–5060. [Google Scholar] [CrossRef]
- Cindoruk, M.; Kerem, M.; Karakan, T.; Salman, B.; Akin, O.; Alper, M.; Erdem, O.; Ünal, S. Peroxisome Proliferators-Activated Alpha Agonist Treatment Ameliorates Hepatic Damage in Rats with Obstructive Jaundice: An Experimental Study. BMC Gastroenterol. 2007, 7, 44. [Google Scholar] [CrossRef]
- Gohil, J.R.; Rathod, V.S.; Rathod, B.D. Efficacy and Safety of Fenofibrate in Uncomplicated Hyperbilirubinemia in Newborn: A Randomized Trial, with a 6-Month Follow-Up. Asian J. Pediatr. Res. 2020, 4, 37–42. [Google Scholar] [CrossRef]
- Chaudhary, G.S.; Chaudhary, V.; Chaurasiya, O.S.; Chandrakant, V.; Kumar, V. Oral Fenofibrate in Neonatal Hyperbilirubinemia: A Randomized Controlled Trial. Indian J. Child Health 2016, 3, 54–58. [Google Scholar] [CrossRef]
- Ahmadpour-kacho, M.; Zahed Pasha, Y.; Moghadamnia, A.A.; Khafri, S.; Vafaeinezhad, M. Effect of Oral Fenofibrate on Serum Bilirubin Level in Term Neonates With Hyperbilirubinemia. Int. J. Pediatr. 2018, 6, 8317–8327. [Google Scholar] [CrossRef]
- Dabour, S.; Ismael, Y.; Assar, E.; Allam, M. Role of Fenofibrate in Management of Unconjugated Hyperbilirubinemia in Neonates. Int. J. Adv. Res. 2016, 4, 2505–2517. [Google Scholar] [CrossRef]
- Zamiri-Miandoab, N.; Montazeri, R.; Hassanpour, S.; Mirghafourvand, M. Effect of Fenofibrate on Neonatal Hyperbilirubinemia: A Systematic Review and Meta-Analysis. Iran. J. Neonatol. 2021, 12, 76–84. [Google Scholar] [CrossRef]
- Awad, M.H.; Amer, S.; Hafez, M.; Nour, I.; Shabaan, A. Fenofibrate as an Adjuvant to Phototherapy in Pathological Unconjugated Hyperbilirubinemia in Neonates: A Randomized Control Trial. J. Perinatol. 2021, 41, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Al-Asy, H.; El-Sharkawy, H.; Mabrouk, M.; Hamad, M. Effect of Fenofibrate on Indirect Neonatal Hyperbilirubinemia. J. Clin. Neonatol. 2015, 4, 82. [Google Scholar] [CrossRef]
- Al-Banna, S.; Riad, A.; Anis, S. The Effect of Fenofibrate and Antioxidant Vitamins [D, E and C] in Treatment of Uncomplicated Neonatal Hyperbilirubinemia. Ann. Neonatol. J. 2020, 2, 37–48. [Google Scholar] [CrossRef]
- Subcommittee on Hyperbilirubinemia. Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef]
- Saadat, S.H.; Goodarzi, R.; Gharaei, B. Oral Fenofibrate for Hyperbilirubinemia in Term Neonates: A Single-Blind Randomized Controlled Trial. J. Clin. Trans. Sci. 2023, 7, e85. [Google Scholar] [CrossRef]
- Mosharref, M.; Rehnuma, N.; Jahan, N.; Zafreen, F. Effect of Oral Fenofibrate on Serum Bilirubin Level in Term Neonates with Unconjugated Hyperbilirubinaemia: A Randomized Control Trial. J. Armed Med. Coll. 2021, 16, 35–38. [Google Scholar] [CrossRef]
- Prabha, S.; Saravanan, S. The Efficacy of Fenofibrate as an Adjunct to Phototherapy for Neonatal Hyperbilirubinemia. Int. J. Paediatr. Geriatr. 2020, 3, 73–75. [Google Scholar] [CrossRef]
- Gowda, B.L.; Viswanathakumar, H.M.; Yamuna, B.N.; Arun, D.J. Efficacy of Oral Fenofibrate in the Management of Unconjugated Hyperbilirubinemia in Neonates—A Prospective Study. Int. J. Recent Trends Sci. 2014, 13, 235. [Google Scholar]
| Variables | Intervention Group Mean (SD) | Control Group Mean (SD) | p-Value |
|---|---|---|---|
| Age in days | 3.74 (1.139) | 3.88 (1.239) | 0.558 (NS) * |
| Gestational age in weeks | 38.58 (0.758) | 38.52 (0.995) | 0.735 (NS) * |
| Weight in kg | 3.01 (0.195) | 3.018 (0.26) | 0.795 (NS) * |
| Variables | Intervention Group Mean (SD) | Control Group Mean (SD) | p-Value |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Gender | |||
| Male | 28 (56.0) | 29 (58.0) | 1.00 (NS) ** |
| Female | 22 (44.0) | 21 (42.0) | |
| Diagnosis | |||
| ABO incompatibility | 25 (50.0) | 28 (56.0) | 0.862 (NS) ** |
| Rh incompatibility | 15 (30.0) | 13 (26.0) | |
| Exaggerated physiological jaundice | 10 (20.0) | 9 (18.0) | |
| Direct Coombs test | |||
| Positive | 16 (32.0) | 13 (26.0) | 0.66 (NS) ** |
| Negative | 34 (68.0) | 37 (74.0) |
| Time (Hours) | TSB mg/dL | p-Value | |
|---|---|---|---|
| Intervention Group Mean (SD) | Control Group Mean (SD) | ||
| 0 (on admission) | 19.28 (0.427) | 19.21 (0.518) | 0.456 (NS) |
| 12 | 17.92 (0.539) | 18.65 (0.573) | 0.001 |
| 24 | 16.42 (0.736) | 17.69 (0.621) | <0.001 |
| 48 | 14.81 (0.872) | 16.51 (0.688) | <0.001 |
| 72 | 12.97 (0.946) | 15.01 (1.02) | <0.001 |
| Average time for discharge (h) | 63.6 (8.83) | 90.9 (8.07) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabo, S.K.; Gargary, K.H.; Erdeve, O. Indirect Neonatal Hyperbilirubinemia and the Role of Fenofibrate as an Adjuvant to Phototherapy. Children 2023, 10, 1192. https://doi.org/10.3390/children10071192
Shabo SK, Gargary KH, Erdeve O. Indirect Neonatal Hyperbilirubinemia and the Role of Fenofibrate as an Adjuvant to Phototherapy. Children. 2023; 10(7):1192. https://doi.org/10.3390/children10071192
Chicago/Turabian StyleShabo, Salam K., Khalaf H. Gargary, and Omer Erdeve. 2023. "Indirect Neonatal Hyperbilirubinemia and the Role of Fenofibrate as an Adjuvant to Phototherapy" Children 10, no. 7: 1192. https://doi.org/10.3390/children10071192
APA StyleShabo, S. K., Gargary, K. H., & Erdeve, O. (2023). Indirect Neonatal Hyperbilirubinemia and the Role of Fenofibrate as an Adjuvant to Phototherapy. Children, 10(7), 1192. https://doi.org/10.3390/children10071192




