Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography
Abstract
1. Introduction
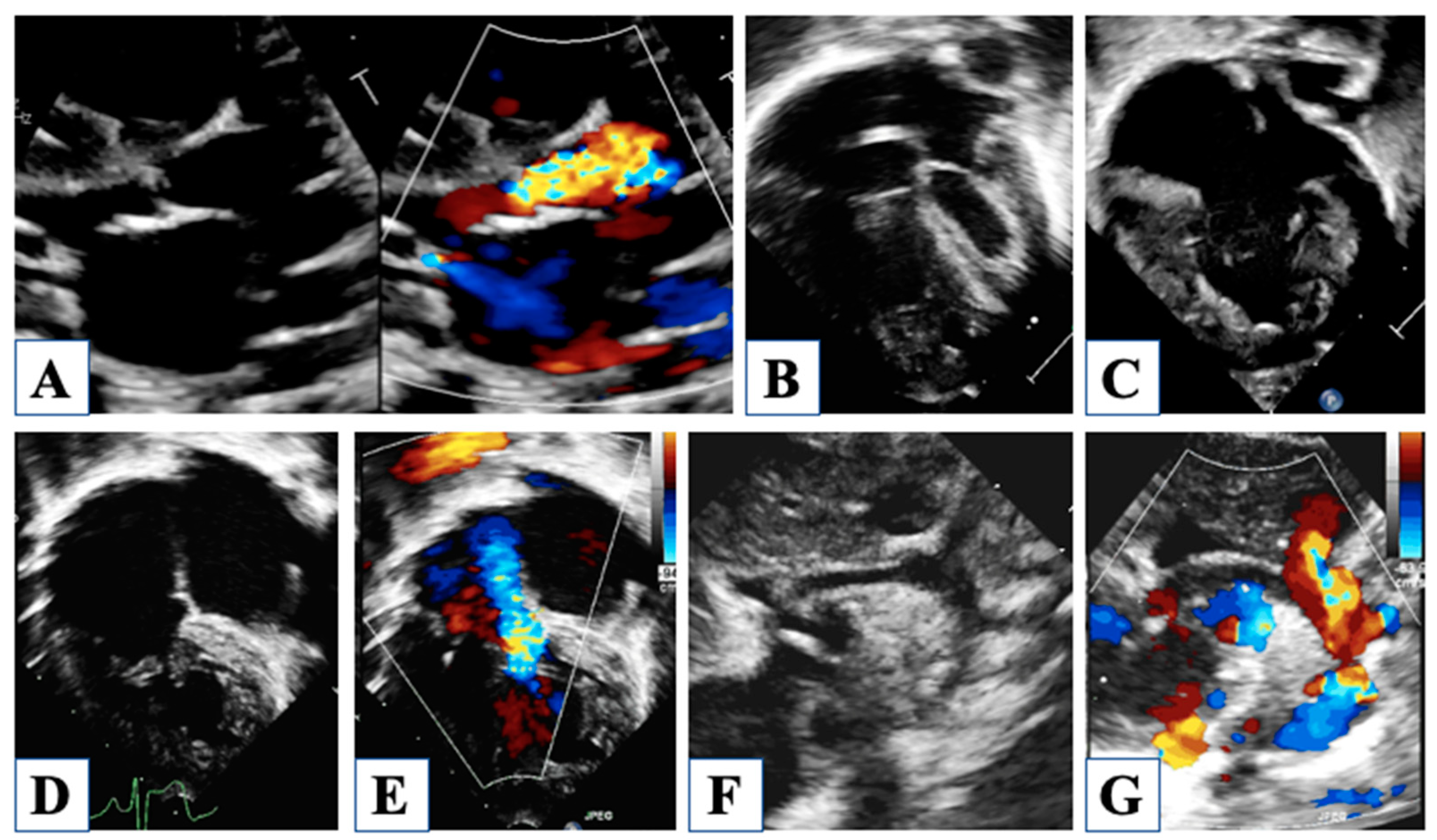
2. Fetal Life
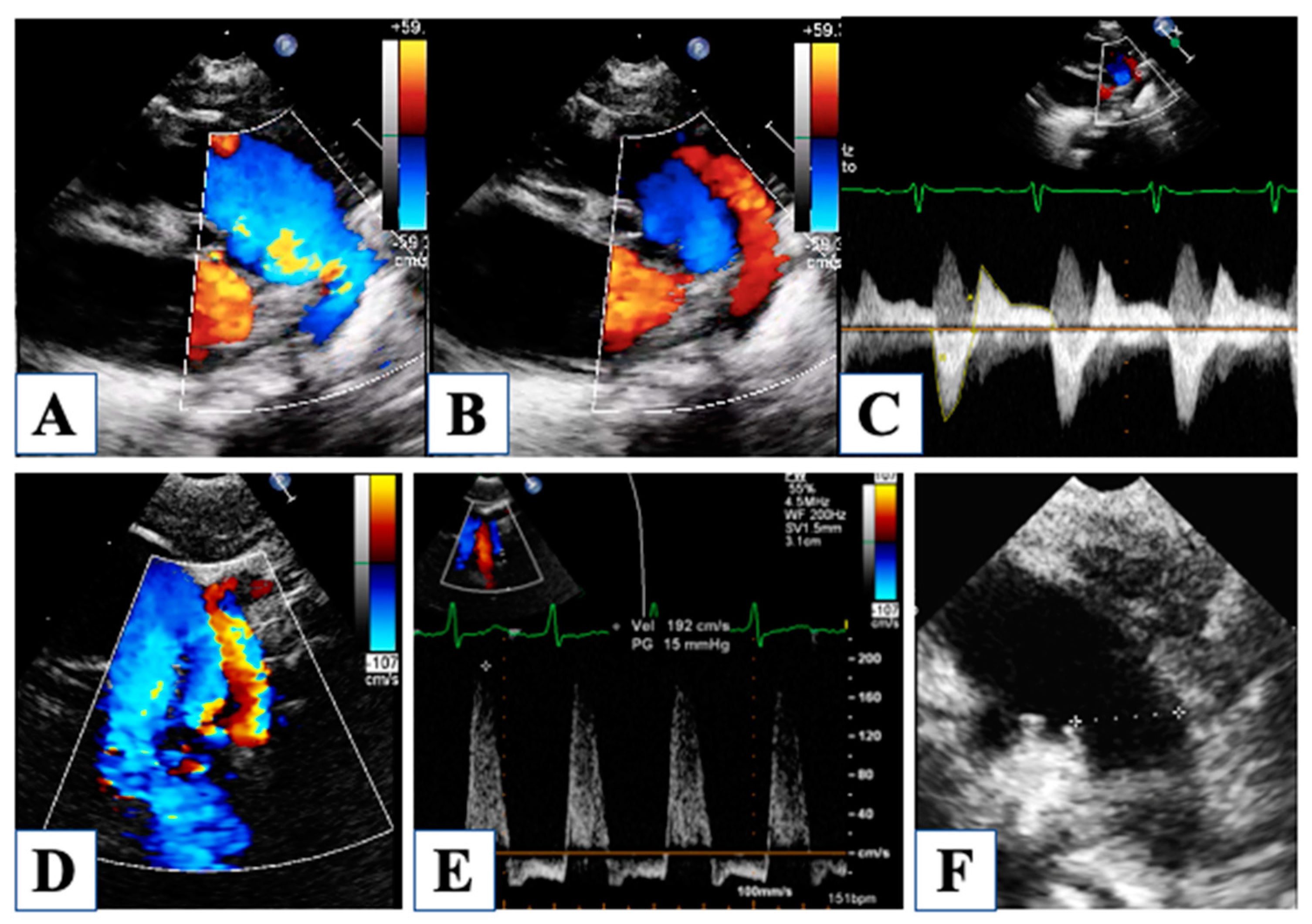
3. At Birth
4. Morphological Insights: The Borderline LV
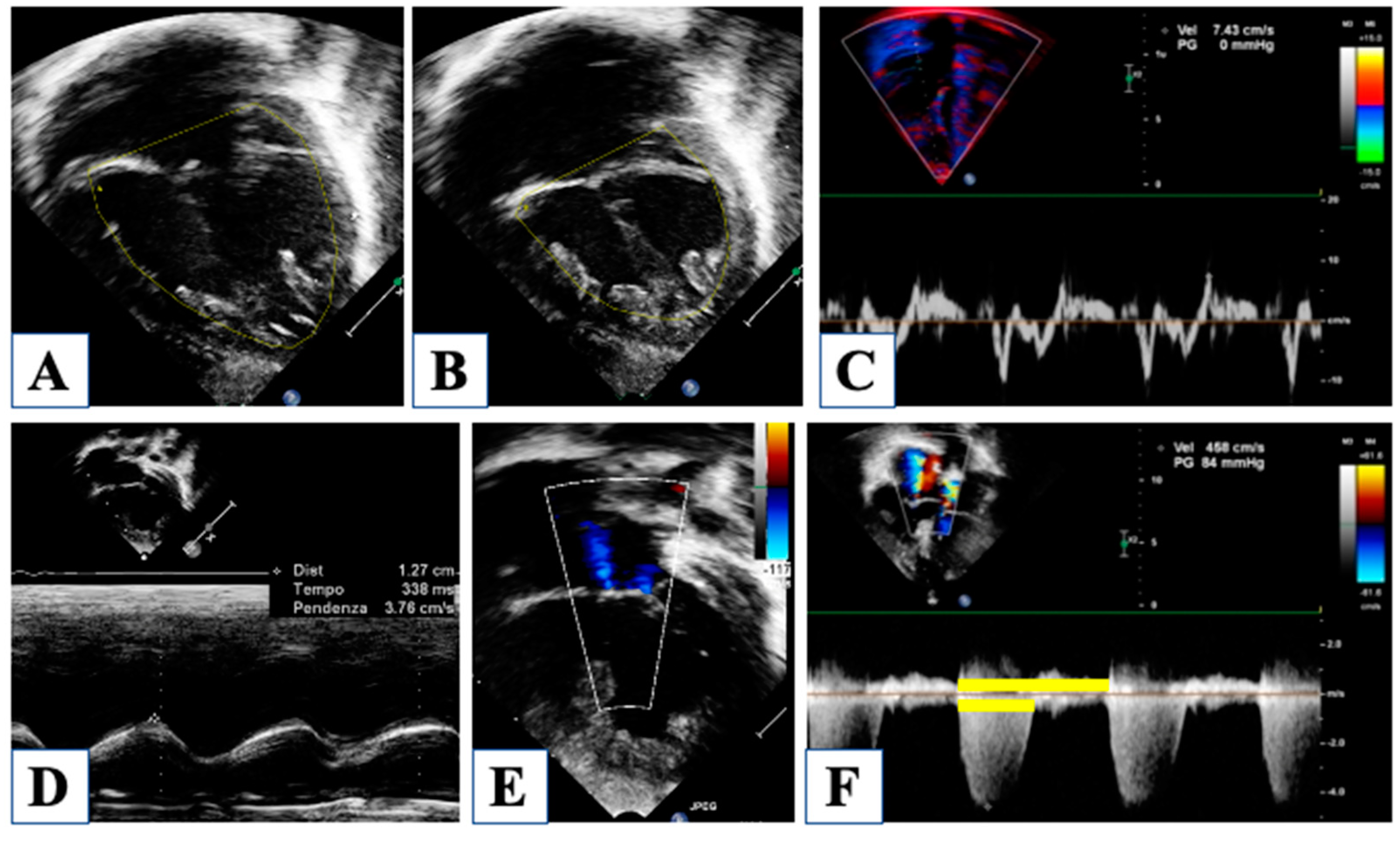
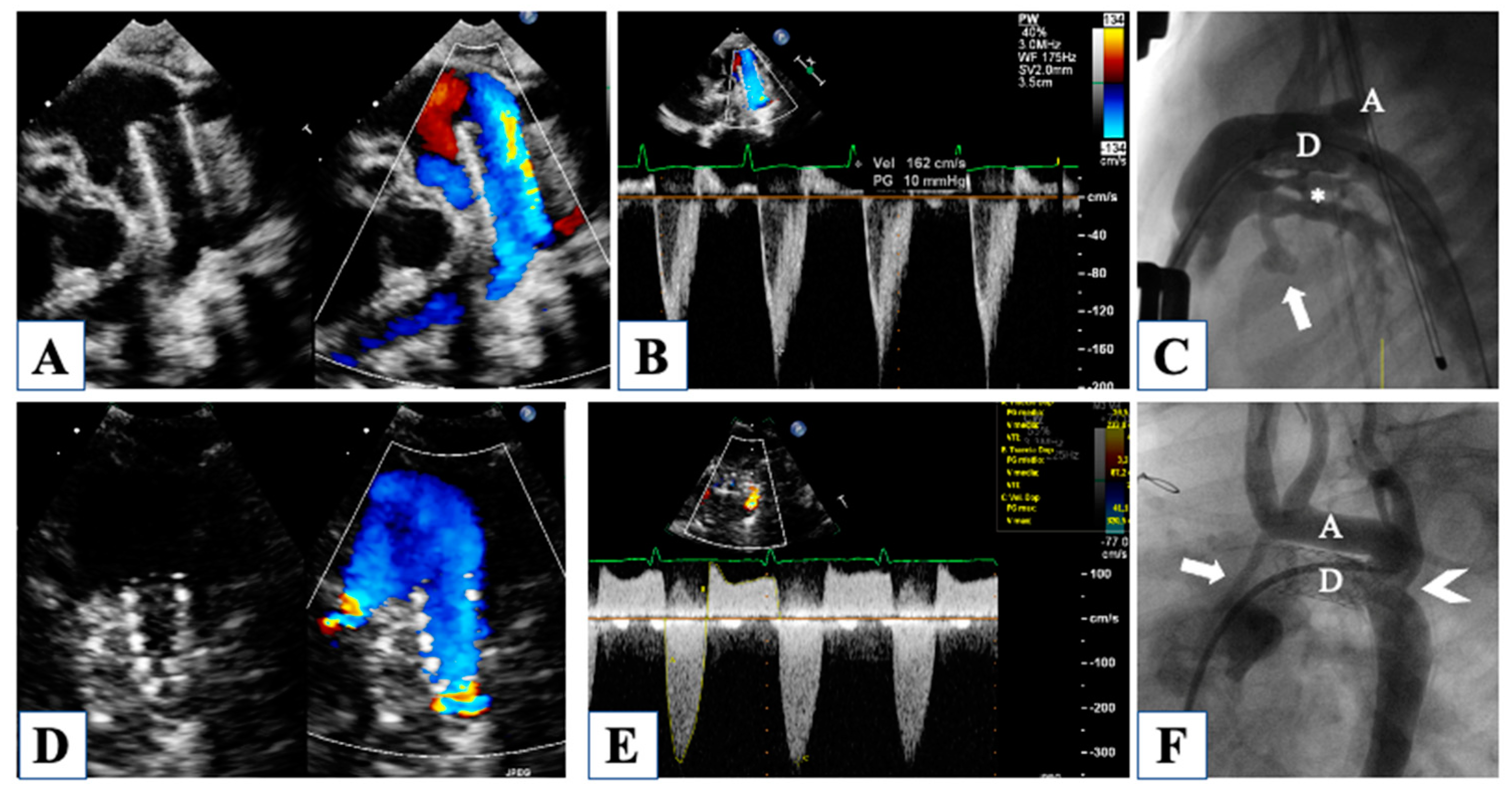
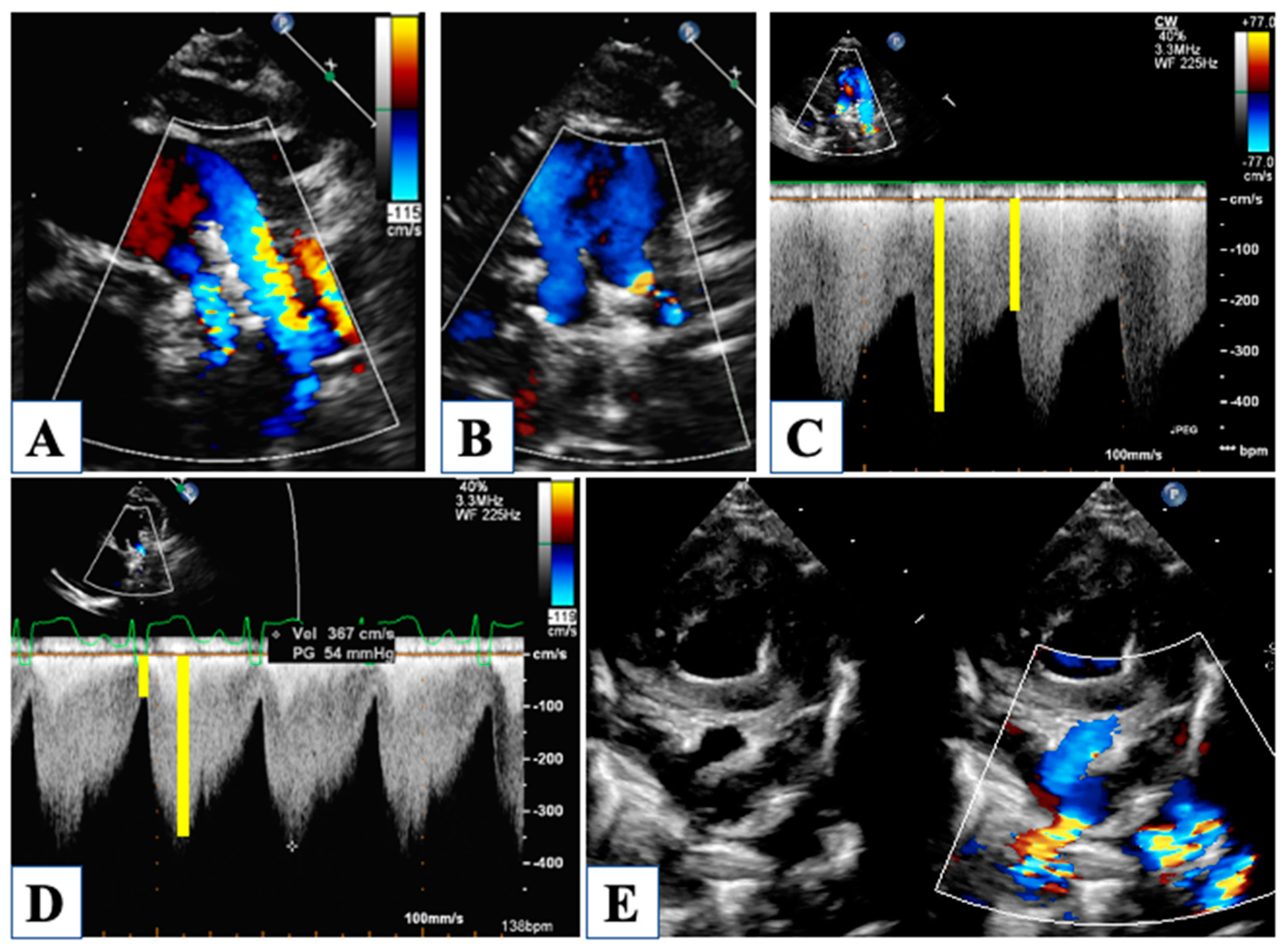
5. After Hybrid Procedure: The Interstage Period
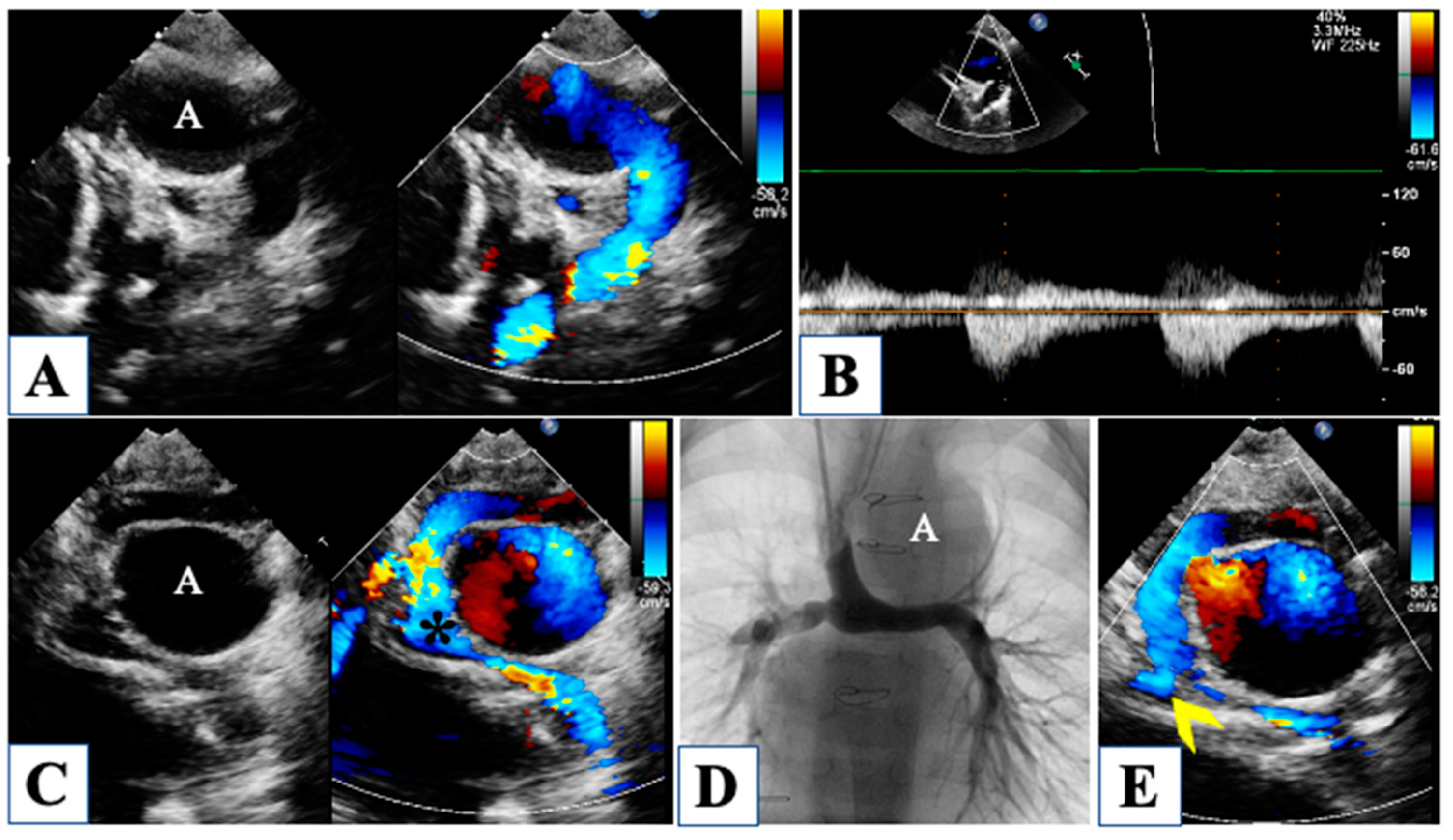
6. After Comprehensive Stage 2 Operation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galantowicz, M.; Cheatham, J.P.; Phillips, A.; Cua, C.L.; Hoffman, T.M.; Hill, S.L.; Rodeman, R. Hybrid approach for hypoplastic left heart syndrome: Intermediate results after the learning curve. Ann. Thorac. Surg. 2008, 85, 2063–2070; discussion 2070–2061. [Google Scholar] [CrossRef] [PubMed]
- Akinturk, H.; Michel-Behnke, I.; Valeske, K.; Mueller, M.; Thul, J.; Bauer, J.; Hagel, K.J.; Schranz, D. Hybrid transcatheter-surgical palliation: Basis for univentricular or biventricular repair: The Giessen experience. Pediatr. Cardiol. 2007, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ceneri, N.M.; Desai, M.H.; Tongut, A.; Ozturk, M.; Ramakrishnan, K.; Staffa, S.J.; Zurakowski, D.; Donofrio, M.T.; Downing, T.; d’Udekem, Y.; et al. Hybrid strategy in neonates with ductal-dependent systemic circulation and multiple risk factors. J. Thorac. Cardiovasc. Surg. 2022, 164, 1291–1303 e1296. [Google Scholar] [CrossRef]
- Bellsham-Revell, H. Noninvasive Imaging in Interventional Cardiology: Hypoplastic Left Heart Syndrome. Front. Cardiovasc. Med. 2021, 8, 637838. [Google Scholar] [CrossRef] [PubMed]
- Michelfelder, E.; Gomez, C.; Border, W.; Gottliebson, W.; Franklin, C. Predictive value of fetal pulmonary venous flow patterns in identifying the need for atrial septoplasty in the newborn with hypoplastic left ventricle. Circulation 2005, 112, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Boucek, M.M.; Mashburn, C.; Kunz, E.; Chan, K.C. Ductal anatomy: A determinant of successful stenting in hypoplastic left heart syndrome. Pediatr. Cardiol. 2005, 26, 200–205. [Google Scholar] [CrossRef]
- Chin, A.J.; Weinberg, P.M.; Barber, G. Subcostal two-dimensional echocardiographic identification of anomalous attachment of septum primum in patients with left atrioventricular valve underdevelopment. J. Am. Coll. Cardiol. 1990, 15, 678–681. [Google Scholar] [CrossRef]
- Seliem, M.A.; Chin, A.J.; Norwood, W.I. Patterns of anomalous pulmonary venous connection/drainage in hypoplastic left heart syndrome: Diagnostic role of Doppler color flow mapping and surgical implications. J. Am. Coll. Cardiol. 1992, 19, 135–141. [Google Scholar] [CrossRef]
- Bellsham-Revell, H.R.; Simpson, J.M.; Miller, O.I.; Bell, A.J. Subjective evaluation of right ventricular systolic function in hypoplastic left heart syndrome: How accurate is it? J. Am. Soc. Echocardiogr. 2013, 26, 52–56. [Google Scholar] [CrossRef]
- Friedberg, M.K.; Silverman, N.H. The systolic to diastolic duration ratio in children with hypoplastic left heart syndrome: A novel Doppler index of right ventricular function. J. Am. Soc. Echocardiogr. 2007, 20, 749–755. [Google Scholar] [CrossRef]
- Carrillo, S.A.; Texter, K.M.; Phelps, C.; Tan, Y.; McConnell, P.I.; Galantowicz, M. Tricuspid Valve and Right Ventricular Function Throughout the Hybrid Palliation Strategy for Hypoplastic Left Heart Syndrome and Variants. World J. Pediatr. Congenit. Heart Surg. 2021, 12, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Petko, C.; Uebing, A.; Furck, A.; Rickers, C.; Scheewe, J.; Kramer, H.H. Changes of right ventricular function and longitudinal deformation in children with hypoplastic left heart syndrome before and after the Norwood operation. J. Am. Soc. Echocardiogr. 2011, 24, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Bellsham-Revell, H.R.; Tibby, S.M.; Bell, A.J.; Miller, O.I.; Razavi, R.; Greil, G.F.; Simpson, J.M. Tissue Doppler time intervals and derived indices in hypoplastic left heart syndrome. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 400–407. [Google Scholar] [CrossRef]
- Tham, E.B.; Smallhorn, J.F.; Kaneko, S.; Valiani, S.; Myers, K.A.; Colen, T.M.; Kutty, S.; Khoo, N.S. Insights into the evolution of myocardial dysfunction in the functionally single right ventricle between staged palliations using speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 314–322. [Google Scholar] [CrossRef]
- Michielon, G.; DiSalvo, G.; Fraisse, A.; Carvalho, J.S.; Krupickova, S.; Slavik, Z.; Bartsota, M.; Daubeney, P.; Bautista, C.; Desai, A.; et al. In-hospital interstage improves interstage survival after the Norwood stage 1 operation. Eur. J. Cardiothorac. Surg. 2020, 57, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Oreto, L.; Mandraffino, G.; Calaciura, R.E.; Poli, D.; Gitto, P.; Saitta, M.B.; Bellanti, E.; Carerj, S.; Zito, C.; Iorio, F.S.; et al. Hybrid Palliation for Hypoplastic Borderline Left Ventricle: One More Chance to Biventricular Repair. Children 2023, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Fenstermaker, B.; Berger, G.E.; Rowland, D.G.; Hayes, J.; Hill, S.L.; Cheatham, J.P.; Galantowicz, M.; Cua, C.L. Interstage echocardiographic changes in patients undergoing hybrid stage I palliation for hypoplastic left heart syndrome. J. Am. Soc. Echocardiogr. 2008, 21, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.J.; Hill, S.L.; Boettner, B.L.; Holzer, R.J.; Phillips, A.B.; Galantowicz, M.; Cheatham, J.P.; Kovalchin, J.P. Predictors of retrograde aortic arch obstruction after hybrid palliation of hypoplastic left heart syndrome. Pediatr. Cardiol. 2011, 32, 67–75. [Google Scholar] [CrossRef]
- Birnbaum, B.; Berger, G.; Fenstermaker, B.; Rowland, D.G.; Boettner, B.; Olshove, V.; Galantowicz, M.; Cheatham, J.P.; Cua, C.L. Echocardiographic parameters that predict outcome in aortic atresia patients undergoing comprehensive stage II procedure. Congenit. Heart Dis. 2010, 5, 409–415. [Google Scholar] [CrossRef]
- Lemler, M.S.; Zellers, T.M.; Harris, K.A.; Ramaciotti, C. Coarctation index: Identification of recurrent coarctation in infants with hypoplastic left heart syndrome after the Norwood procedure. Am. J. Cardiol. 2000, 86, 697–699. [Google Scholar] [CrossRef]
- Rychik, J. Hypoplastic left heart syndrome: From in-utero diagnosis to school age. Semin. Fetal Neonatal Med. 2005, 10, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Miller, M.; Gonzalez, J.; Nardell, K.; Galas, J.; John, J.B.; Timofeev, S.; Lambou, R.; Douglas, K.; Marx, G. Echocardiography of hypoplastic left heart syndrome. Cardiol. Young 2011, 21 (Suppl. S2), 28–37. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafane, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic left heart syndrome: Current considerations and expectations. J. Am. Coll. Cardiol. 2012, 59 (Suppl. S1), S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Cui, W.; Nguyen, N.V.; Husayni, T.S.; Van Bergen, A.H.; Sajan, I.; El-Zein, C.; Polimenakos, A.; Ilbawi, M.N.; Roberson, D.A. Ventriculocoronary artery connections with the hypoplastic left heart: A 4-year prospective study: Incidence, echocardiographic and clinical features. Pediatr. Cardiol. 2010, 31, 1176–1185. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Marshall, A.C.; Wilkins-Haug, L.E.; Brown, D.W.; Benson, C.B.; Silva, V.; Marx, G.R.; Mizrahi-Arnaud, A.; Lock, J.E.; Tworetzky, W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation 2009, 120, 1482–1490. [Google Scholar] [CrossRef]
- Altmann, K.; Printz, B.F.; Solowiejczky, D.E.; Gersony, W.M.; Quaegebeur, J.; Apfel, H.D. Two-dimensional echocardiographic assessment of right ventricular function as a predictor of outcome in hypoplastic left heart syndrome. Am. J. Cardiol. 2000, 86, 964–968. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef]
- Soriano, B.D.; Hoch, M.; Ithuralde, A.; Geva, T.; Powell, A.J.; Kussman, B.D.; Graham, D.A.; Tworetzky, W.; Marx, G.R. Matrix-array 3-dimensional echocardiographic assessment of volumes, mass, and ejection fraction in young pediatric patients with a functional single ventricle: A comparison study with cardiac magnetic resonance. Circulation 2008, 117, 1842–1848. [Google Scholar] [CrossRef]
- Friedberg, M.K.; Su, X.; Tworetzky, W.; Soriano, B.D.; Powell, A.J.; Marx, G.R. Validation of 3D echocardiographic assessment of left ventricular volumes, mass, and ejection fraction in neonates and infants with congenital heart disease: A comparison study with cardiac MRI. Circ. Cardiovasc. Imaging 2010, 3, 735–742. [Google Scholar] [CrossRef]
- Mah, K.; Serrano Lomelin, J.; Colen, T.; Tham, E.B.; Lin, L.; Eckersley, L.; Smallhorn, J.F.; Becher, H.; Mertens, L.; Khoo, N.S. Right Ventricular Remodeling in Hypoplastic Left Heart Syndrome is Minimally Impacted by Cardiopulmonary Bypass: A Comparison of Norwood vs. Hybrid. Pediatr. Cardiol. 2021, 42, 294–301. [Google Scholar] [CrossRef]
- Kearney, D.L. The pathological spectrum of left-ventricular hypoplasia. Semin. Cardiothorac. Vasc. Anesth. 2013, 17, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Bharati, S.; Lev, M. The surgical anatomy of hypoplasia of aortic tract complex. J. Thorac. Cardiovasc. Surg. 1984, 88, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Corno, A.F. Borderline left ventricle. Eur. J. Cardiothorac. Surg. 2005, 27, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Tchervenkov, C.I. Indications, criterions, and principles for biventricular repair. Cardiol. Young 2004, 14 (Suppl. 1), 97–100. [Google Scholar] [CrossRef]
- Colan, S.D.; McElhinney, D.B.; Crawford, E.C.; Keane, J.F.; Lock, J.E. Validation and re-evaluation of a discriminant model predicting anatomic suitability for biventricular repair in neonates with aortic stenosis. J. Am. Coll. Cardiol. 2006, 47, 1858–1865. [Google Scholar] [CrossRef]
- Nadorlik, H.A.; Egan, M.J.; Hill, S.L.; Cheatham, J.P.; Galantowicz, M.; Kovalchin, J.P. Predictors of ductus arteriosus in-stent stenosis in the hybrid approach to hypoplastic left heart syndrome. Pediatr. Cardiol. 2013, 34, 656–660. [Google Scholar] [CrossRef]
- Baker, C.E.; Corsini, C.; Cosentino, D.; Dubini, G.; Pennati, G.; Migliavacca, F.; Hsia, T.Y.; Modeling of Congenital Hearts Alliance, I. Effects of pulmonary artery banding and retrograde aortic arch obstruction on the hybrid palliation of hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2013, 146, 1341–1348. [Google Scholar] [CrossRef]
- Meza, J.M.; Hickey, E.J.; Blackstone, E.H.; Jaquiss, R.D.B.; Anderson, B.R.; Williams, W.G.; Cai, S.; Van Arsdell, G.S.; Karamlou, T.; McCrindle, B.W. The Optimal Timing of Stage 2 Palliation for Hypoplastic Left Heart Syndrome: An Analysis of the Pediatric Heart Network Single Ventricle Reconstruction Trial Public Data Set. Circulation 2017, 136, 1737–1748. [Google Scholar] [CrossRef]
- Biglino, G.; Giardini, A.; Ntsinjana, H.N.; Schievano, S.; Hsia, T.Y.; Taylor, A.M.; Modeling of Congenital Hearts Alliance Collaborative Group. Ventriculoarterial coupling in palliated hypoplastic left heart syndrome: Noninvasive assessment of the effects of surgical arch reconstruction and shunt type. J. Thorac. Cardiovasc. Surg. 2014, 148, 1526–1533. [Google Scholar] [CrossRef]
- Baba, K.; Kotani, Y.; Chetan, D.; Chaturvedi, R.R.; Lee, K.J.; Benson, L.N.; Grosse-Wortmann, L.; Van Arsdell, G.S.; Caldarone, C.A.; Honjo, O. Hybrid versus Norwood strategies for single-ventricle palliation. Circulation 2012, 126, S123–S131. [Google Scholar] [CrossRef]
- Dave, H.; Rosser, B.; Knirsch, W.; Hubler, M.; Pretre, R.; Kretschmar, O. Hybrid approach for hypoplastic left heart syndrome and its variants: The fate of the pulmonary arteries. Eur. J. Cardiothorac. Surg. 2014, 46, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Stern, K.W.; McElhinney, D.B.; Gauvreau, K.; Geva, T.; Brown, D.W. Echocardiographic evaluation before bidirectional Glenn operation in functional single-ventricle heart disease: Comparison to catheter angiography. Circ. Cardiovasc. Imaging 2011, 4, 498–505. [Google Scholar] [CrossRef] [PubMed]


| Author | Ref. Number | Stage | Echo Parameter | Measurement Required | Meaning |
|---|---|---|---|---|---|
| Michelfelder et al., 2005 | [5] | Fetal | S/D velocity and forward/reverse VTI ratio | Pulmonary venous flow pattern | Increased reverse flow correlated with restrictive ASD |
| Boucek et al., 2015 | [6] | Pre-stage I | Ductal anatomy | Orientation, length of the ductus | Suitability for stenting |
| Chin et al., 1990 | [7] | Pre-stage I Interstage Stage II | Atrial septum morphology | Atrial septal communication | Implications on interventional/surgical management |
| Seliem et al., 1992 | [8] | Pre-stage I | Connection and drainage of pulmonary veins | Bidimensional, Color flow Doppler of pulmonary veins | Identification of anomalous pulmonary venous drainage |
| Bellsham-Revell et al., 2013 | [9] | Pre-stage I | RV function | Qualitative assessment (validated against magnetic resonance) | Impact of RV dysfunction on survival |
| Friedberg et al., 2007 | [10] | All | S/D duration ratio | Tricuspid regurgitation jet | High S/D ratio correlated with worse RV function |
| Carrillo et al., 2021 | [11] | All | RVFAC, TAPSE, Tricuspid regurgitation | RV area change, annular excursion, tricuspid regurgitation jet (vena contracta) | Impact on survival at all stages |
| Petko et al., 2011 | [12] | After stage I | RVFAC, TAPSE, Tricuspid regurgitation | RV area change, annular excursion, tricuspid regurgitation jet (vena contracta) | Impact on survival at all stages |
| Bellsham-Revell et al., 2012 | [13] | All stages (Norwood) | TDI indices, Myocardial performance index, S/D duration ratio | TDI of the RV free wall, tricuspid regurgitation jet | Myocardial performance index increased compared to normal values. |
| Tham et al., 2014 | [14] | All stages (Norwood) | Longitudinal and circumferential deformation | Speckle tracking-derived longitudinal and circumferential strain and strain rate | Inability of the single RV to fully adapt to systemic pressures. |
| Michielon et al., 2020 | [15] | All stages (Norwood) | RVFAC, TAPSE, longitudinal deformation | RV area, annular excursion, speckle tracking-derived longitudinal strain, and strain rate | Impact on outcome at all stages |
| Oreto et al., 2023 | [16] | Pre-stage I | Left ventricle, aortic and mitral valves, long axis ratio | Aortic annulus, mitral annulus, aortic root, left ventricular/heart long axis, left ventricular volumes, and mass | Evaluation of the borderline left ventricle |
| Fenstermaker et al., 2008 | [17] | Interstage I–II | Pulmonary arteries flow velocity, pulsatility index, and systolic/diastolic ratio; | CW Doppler on pulmonary arteries | Correlation with ASD restriction, ductal obstruction, aortic arch obstruction |
| ASD mean gradient, ductus arteriosus peak velocity, retro-aortic arch peak velocity | CW Doppler through ASD, ductus and aortic arch | ||||
| Egan et al., 2011 | [18] | Interstage I–II | Smaller aortic root, higher flow velocity from the retrograde aortic arch | Linear measurement of aortic root, CW Doppler through the retrograde aortic arch | Prediction of retrograde aortic arch obstruction |
| Birnbaum et al., 2010 | [19] | Interstage-stage II | Retrograde ductal VTI, RV FAC | CW Doppler through the ductus, RV area change | Correlation with peri-operative cardiac output |
| Lemler et al., 2000 | [20] | After Norwood stage I | Coarctation index | Narrowest/widest descending aortic diameter ratio | A value < 0.7 correlates with coarctation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oreto, L.; Guccione, P.; Gitto, P.; Bruno, L.; Zanai, R.; Grasso, N.; Iannace, E.; Zito, C.; Carerj, S.; Agati, S. Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography. Children 2023, 10, 1012. https://doi.org/10.3390/children10061012
Oreto L, Guccione P, Gitto P, Bruno L, Zanai R, Grasso N, Iannace E, Zito C, Carerj S, Agati S. Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography. Children. 2023; 10(6):1012. https://doi.org/10.3390/children10061012
Chicago/Turabian StyleOreto, Lilia, Paolo Guccione, Placido Gitto, Letteria Bruno, Rosanna Zanai, Nadia Grasso, Enrico Iannace, Concetta Zito, Scipione Carerj, and Salvatore Agati. 2023. "Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography" Children 10, no. 6: 1012. https://doi.org/10.3390/children10061012
APA StyleOreto, L., Guccione, P., Gitto, P., Bruno, L., Zanai, R., Grasso, N., Iannace, E., Zito, C., Carerj, S., & Agati, S. (2023). Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography. Children, 10(6), 1012. https://doi.org/10.3390/children10061012








