Pediatric Pancreatic Endocrine Tumor Presenting as Acute Pancreatitis: A Case Report
Abstract
1. Introduction
2. Case Report
2.1. Patient Information
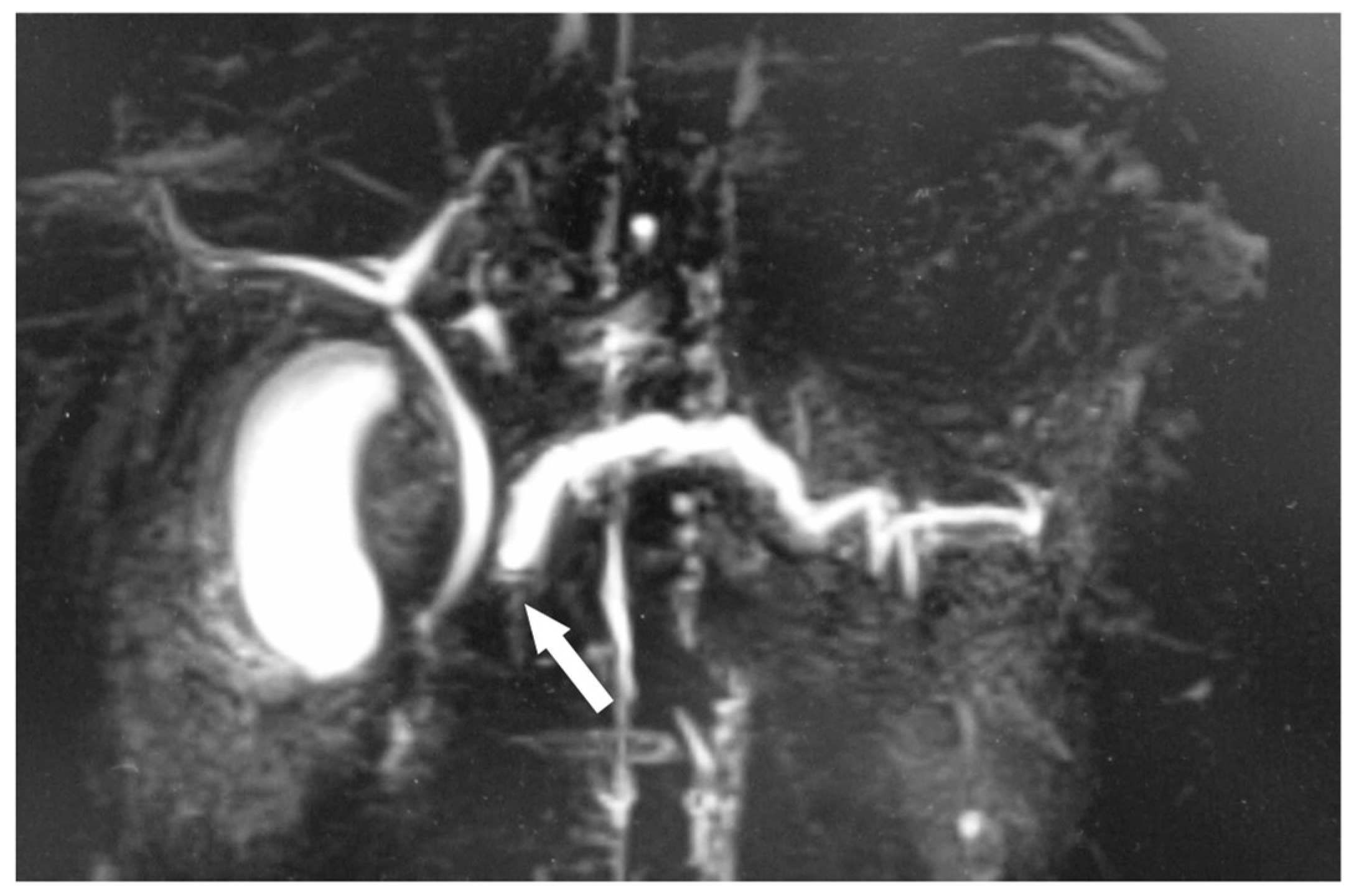
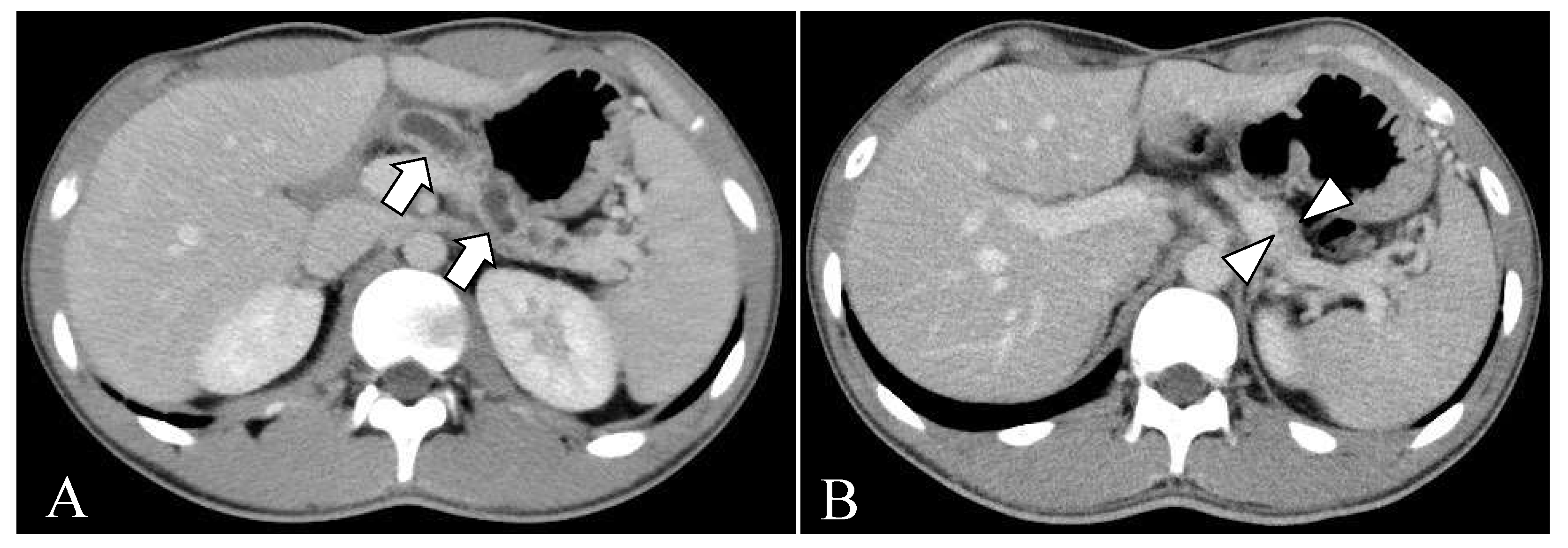
2.2. Clinical Course before Surgery
2.3. Surgical Treatment
2.4. Histological Examinations
2.5. Follow-Up and Outcome
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, M.; Sai, J.K.; Shimizu, T. Acute pancreatitis in children and adolescents. World J. Gastrointest. Pathophysiol. 2014, 5, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Igarashi, H.; Nakamura, K.; Sasano, H.; Okusaka, T.; Takano, K.; Komoto, I.; Tanaka, M.; Imamura, M.; Jensen, R.T.; et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: A nationwide survey analysis. J. Gastroenterol. 2015, 50, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gaiani, F.; de’Angelis, N.; Minelli, R.; Kayali, S.; Carra, M.C.; de’Angelis, G.L. Pediatric gastroenteropancreatic neuroendocrine tumor: A case report and review of the literature. Medicine 2019, 98, e17154. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, S.; Shi, C.; Hruban, R.H.; Choti, M.A.; Schulick, R.D.; Fishman, E.K.; Siegelman, S.S. Small serotonin-producing neuroendocrine tumor of the pancreas associated with pancreatic duct obstruction. Am. J. Roentgenol. 2011, 197, W482–W488. [Google Scholar] [CrossRef]
- Tejedor Bravo, M.; Justo, L.M.; Lasala, J.P.; Moreira Vicente, V.F.; Ruiz, A.C.; Scapa Mde, L. Acute pancreatitis secondary to neuroendocrine pancreatic tumors: Report of 3 cases and literature review. Pancreas 2012, 41, 485–489. [Google Scholar] [CrossRef]
- Kasai, T.; Kawabe, K.; Eto, H.; Ogino, T.; Muramatsu, S.; Nakamura, M.; Miyahara, Y.; Ishikawa, F.; Nitta, H.; Fujita, Y.; et al. A patient with pancreatic neuroedocrine tumor and severe acute pancreatitis: Case report. Suizo 2021, 36, 104–111. [Google Scholar] [CrossRef]
- Funamizu, N.; Mineta, S.; Ozaki, T.; Igarashi, K.; Omura, K.; Wakabayashi, G. A case of pancreatic neuroendocrine tumor with acute pancreatitis in a young man. J. Jpn. Coll. Surg. 2020, 45, 270–274. [Google Scholar] [CrossRef]
- Puri, R.; Choudhary, N.S.; Sud, R. Recurrent acute pancreatitis secondary to pancreatic neuroendocrine tumor: Role of endoscopic ultrasound. Trop. Gastroenterol. 2014, 35, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.M.; Lee, S.J.; Kang, C.D.; Kang, M.; Kim, J.H.; Lee, S.; Cho, S.W. Pancreatic neuroendocrine tumor presenting as acute pancreatitis. Korean J. Gastroenterol. 2018, 71, 98–102. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, A.; Di Filippo, A.R.; Caruso, G.; Spaziani, M.; Baldelli, R.; Picchio, M.; Spaziani, E. Acute pancreatitis secondary to non-functioning pancreatic neuroendocrine tumor: Uncommon clinical presentation. Clinical case and review of literature. Ann. Ital. Chir. 2021, 10, S2239253X21034939. [Google Scholar] [PubMed]
- Mujica, V.R.; Barkin, J.S.; Go, V.L. Acute pancreatitis secondary to pancreatic carcinoma. Study Group Participants. Pancreas 2000, 21, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Druce, M.; Rockall, A.; Grossman, A.B. Fibrosis and carcinoid syndrome: From causation to future therapy. Nat. Rev. Endocrinol. 2009, 5, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Deandreis, D.; Scoazec, J.Y.; Goere, D.; Ducreux, M.; Baudin, E.; Tselikas, L. Imaging of neuroendocrine tumors of the pancreas. Diagn. Interv. Imaging 2016, 97, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Kloppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Sadot, E.; Reidy-Lagunes, D.L.; Tang, L.H.; Do, R.K.; Gonen, M.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Groot Koerkamp, B.; Untch, B.R.; et al. Observation versus resection for small asymptomatic pancreatic neuroendocrine tumors: A matched case-control study. Ann. Surg. Oncol. 2016, 23, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, V.; Haglund, C.; Seppanen, H. Outcomes of resected nonfunctional pancreatic neuroendocrine tumors: Do size and symptoms matter? Surgery 2015, 158, 1556–1563. [Google Scholar] [CrossRef] [PubMed]



| Adults (n = 44) | Children (n = 3) | ||
|---|---|---|---|
| Median | Range | ||
| Age (years) | 48 | 20–76 | 11, 13, 16 |
| n | % | n | |
| Female | 20 * | 45.4 | 1 |
| Location of tumor | |||
| Head | 13 | 29.5 | 2 |
| Body | 9 | 20.5 | 1 |
| Tail | 12 | 27.3 | 0 |
| Others | 4 | 9.1 | 0 |
| Not available | 6 | 13.6 | 0 |
| Pathological diagnosis | |||
| Non-functioning | 34 | 77.3 | 3 |
| Gastrinoma | 1 | 2.3 | 0 |
| Glucagonoma | 1 | 2.3 | 0 |
| Somatostatinoma | 1 | 2.3 | 0 |
| Not available | 7 | 15.9 | 0 |
| Pancreatitis severity | |||
| Severe | 6 | 13.6 | 1 |
| Not severe | 35 | 79.5 | 2 |
| Not available | 3 | 6.8 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, S.; Suzuki, M.; Minowa, K.; Koga, H.; Yamataka, A.; Shimizu, T. Pediatric Pancreatic Endocrine Tumor Presenting as Acute Pancreatitis: A Case Report. Children 2023, 10, 900. https://doi.org/10.3390/children10050900
Fukuda S, Suzuki M, Minowa K, Koga H, Yamataka A, Shimizu T. Pediatric Pancreatic Endocrine Tumor Presenting as Acute Pancreatitis: A Case Report. Children. 2023; 10(5):900. https://doi.org/10.3390/children10050900
Chicago/Turabian StyleFukuda, Shigetaka, Mitsuyoshi Suzuki, Kei Minowa, Hiroyuki Koga, Atsuyuki Yamataka, and Toshiaki Shimizu. 2023. "Pediatric Pancreatic Endocrine Tumor Presenting as Acute Pancreatitis: A Case Report" Children 10, no. 5: 900. https://doi.org/10.3390/children10050900
APA StyleFukuda, S., Suzuki, M., Minowa, K., Koga, H., Yamataka, A., & Shimizu, T. (2023). Pediatric Pancreatic Endocrine Tumor Presenting as Acute Pancreatitis: A Case Report. Children, 10(5), 900. https://doi.org/10.3390/children10050900





