Home-Based Exercise Training in the Recovery of Multisystem Inflammatory Syndrome in Children: A Case Series Study
Abstract
1. Introduction
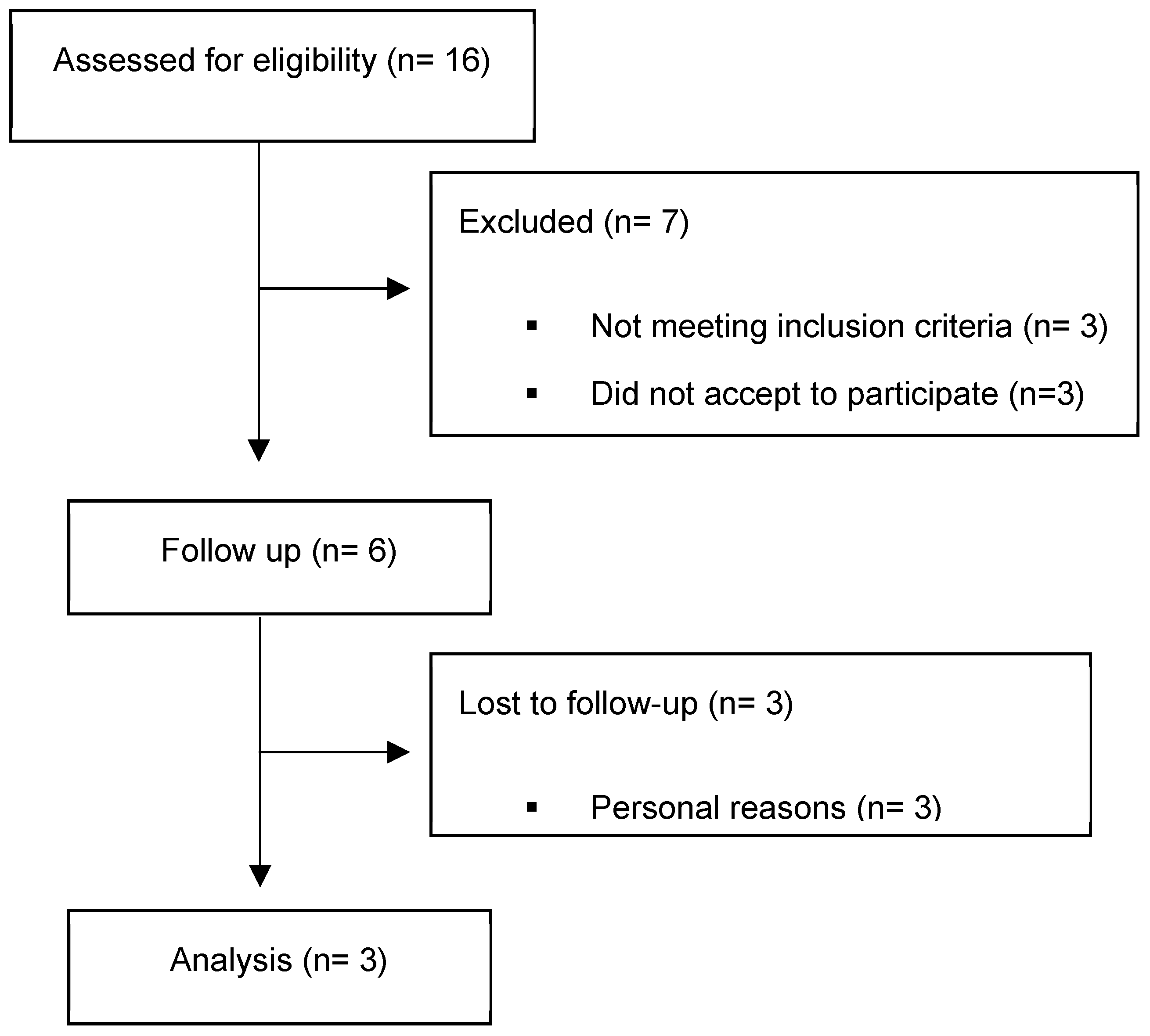
2. Material and Methods
2.1. Study Design and Patients
2.2. Ethics
2.3. Exercise Training Program
2.4. Pediatric Outcomes Data Collection Instrument (PODCI)
2.5. Coronary Flow Reserve Imaging Protocol by 13N-Ammonia PET-CT (13N PET-CT)
2.6. 2 DST Echocardiography and Speckle Tracking
2.7. Cardiopulmonary Exercise Test
2.8. Statistical Analysis
3. Results
3.1. Exercised Patients
3.2. Non-Exercised Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. United States COVID-19 Cases and Deaths by State. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/casesin-%0Dus.html (accessed on 20 March 2021).
- Suratannon, N.; Dik, W.A.; Chatchatee, P.; van Hagen, P.M. COVID-19 in children: Heterogeneity within the disease and hypothetical pathogenesis. Asian Pac. J. Allergy Immunol. 2020, 38, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Biglari, H.N.; Sinaei, R.; Pezeshki, S.; Hasani, F.K. Acute transverse myelitis of childhood due to novel coronavirus disease 2019: The first pediatric case report and review of literature. Iran. J. Child Neurol. 2020, 15, 107–112. [Google Scholar] [CrossRef]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical Characteristics of 58 Children with a Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2. JAMA-J. Am. Med. Assoc. 2020, 324, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemi. Arch. Cardiovasc. Dis. Suppl. 2021, 13, 271. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Sperotto, F.; Friedman, K.G.; Son, M.B.F.; VanderPluym, C.J.; Newburger, J.W.; Dionne, A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur. J. Pediatr. 2021, 180, 307–322. [Google Scholar] [CrossRef]
- Çalışkan, M.; Baycan, F.; Çelik, F.B.; Güvenç, T.S.; Atıcı, A.; Çağ, Y.; Konal, O.; İrgi, T.; Bilgili, Z.; Ağırbaşlı, M.A. Coronary microvascular dysfunction is common in patients hospitalized with COVID-19 infection. Microcirculation 2022, 29, e12757. [Google Scholar] [CrossRef]
- Leal, G.N.; Astley, C.; Lima, M.S.; Diniz, M.D.F.R.; Lianza, A.C.; Sawamura, K.S.S.; Menezes, C.R.B.; da Silva, C.L.M.R.; Bain, V.; Imada, R.; et al. Segmental cardiac strain assessment by two-dimensional speckle-tracking echocardiography in surviving MIS-c patients: Correlations with myocardial flow reserve (MFR) by 13 N-ammonia PET-CT. Microcirculation 2022, 29, e12750. [Google Scholar] [CrossRef]
- Astley, C.; Pereira, M.F.B.; Lima, M.S.; Buchpiguel, C.A.; Carneiro, C.G.; Sapienza, M.T.; Leal, G.N.; Prado, D.M.L.; Peçanha, T.; Sieczkowska, S.M.; et al. In-depth cardiovascular and pulmonary assessments in children with multisystem inflammatory syndrome after SARS-CoV-2 infection: A case series study. Physiol. Rep. 2022, 10, e15201. [Google Scholar] [CrossRef]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Merz, C.N.B. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef]
- Penner, J.; Abdel-Mannan, O.; Grant, K.; Maillard, S.; Kucera, F.; Hassell, J.; Eyre, M.; Berger, Z.; Hacohen, Y.; Moshal, K.; et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: A retrospective cohort study. Lancet Child Adolesc. Health 2021, 5, 473–482. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Pence, B.D. Exercise immunology: Future directions. J. Sport Health Sci. 2020, 9, 432–445. [Google Scholar] [CrossRef]
- Sigal, R.J.; Alberga, A.S.; Goldfield, G.S.; Prud’Homme, D.; Hadjiyannakis, S.; Gougeon, R.; Phillips, P.; Tulloch, H.; Malcolm, J.; Doucette, S.; et al. Effects of Aerobic Training, Resistance Training, or Both on Percentage Body Fat and Cardiometabolic Risk Markers in Obese Adolescents: The healthy eating aerobic and resistance training in youth randomized clinical trial. JAMA Pediatr. 2014, 168, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Bonfa, E.; Pereira, R.M.R.; Silva, C.A. Physical activity for paediatric rheumatic diseases: Standing up against old paradigms. Nat. Rev. Rheumatol. 2017, 13, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Wurz, A.; McLaughlin, E.; Lategan, C.; Viña, C.C.; Grimshaw, S.L.; Hamari, L.; Götte, M.; Kesting, S.; Rossi, F.; van der Torre, P.; et al. The international Pediatric Oncology Exercise Guidelines (iPOEG). Transl. Behav. Med. 2021, 11, 1915–1922. [Google Scholar] [CrossRef]
- CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) n.d. Available online: https://emergency.cdc.gov/han/2020/han00432.asp?deliveryName=USCDC_511-DM28431 (accessed on 22 September 2021).
- Shen, B.; Zheng, Y.; Zhang, X.; Zhang, W.; Wang, D.; Jin, J.; Lin, R.; Zhang, Y.; Zhu, G.; Zhu, H.; et al. Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG. Am. J. Transl. Res. 2020, 12, 1348–1354. [Google Scholar]
- Astley, C.; Clemente, G.; Terreri, M.T.; Carneiro, C.G.; Lima, M.S.; Buchpiguel, C.A.; Filho, H.L.; Pinto, A.L.D.S.; Silva, C.A.; Campos, L.M.A.; et al. Home-Based Exercise Training in Childhood-Onset Takayasu Arteritis: A Multicenter, Randomized, Controlled Trial. Front. Immunol. 2021, 12, 705250. [Google Scholar] [CrossRef]
- Sieczkowska, S.M.; Astley, C.; Marques, I.G.; Iraha, A.Y.; Franco, T.C.; Ihara, B.P.; Lavorato, S.S.M.; Lindoso, L.; Setoue, D.N.D.; Tanigava, N.Y.; et al. A home-based exercise program during COVID-19 pandemic: Perceptions and acceptability of juvenile systemic lupus erythematosus and juvenile idiopathic arthritis adolescents. Lupus 2022, 31, 443–456. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Boer, N.F.; Peoples, J.A.; Foreman, A.J.; Dabayebeh, I.M.; Millich, N.B.; Balasekaran, G.; Riechman, S.E.; Gallagher, J.D.; et al. Children’s OMNI Scale of Perceived Exertion: Mixed gender and race validation. Med. Sci. Sport. Exerc. 2000, 32, 452–458. [Google Scholar] [CrossRef]
- Helito, A.C.; Lindoso, L.; Sieczkowska, S.M.; Astley, C.; Queiroz, L.B.; Rose, N.; Santos, C.R.P.; Bolzan, T.; Peralta, R.M.I.; Franco, R.R.; et al. Poor Sleep quality and health-related quality of life impact in adolescents with and without chronic immunosuppressive conditions during COVID-19 quarantine. Clinics 2021, 76, e3501. [Google Scholar] [CrossRef]
- Waller, A.H.; Blankstein, R.; Kwong, R.Y.; Di Carli, M.F. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr. Cardiol. Rep. 2014, 16, 483. [Google Scholar] [CrossRef]
- Bateman, T.M.; Heller, G.V.; Beanlands, R.; Calnon, D.A.; Case, J.; Dekemp, R.; DePuey, E.G.; Di Carli, M.; Guler, E.C.; Murthy, V.L.; et al. Practical guide for interpreting and reporting cardiac PET measurements of myocardial blood flow: An Information Statement from the American Society of Nuclear Cardiology, and the Society of Nuclear Medicine and Molecular Imaging. J. Nucl. Cardiol. 2021, 28, 768–795. [Google Scholar] [CrossRef]
- De Vries, J.; DeJongste, M.J.; Jessurun, G.A.; Jager, P.L.; Staal, M.J.; Slart, R.H. Myocardial perfusion quantification in patients suspected of cardiac syndrome X with positive and negative exercise testing: A [13N]ammonia positron emission tomography study. Nucl. Med. Commun. 2006, 27, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for Quantification Methods during the Performance of a Pediatric Echocardiogram: A Report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef]
- Colan, S.D. Normal Echocardiographic Values for Cardiovascular Structures. Echocardiogr. Pediatr. Congenit. Heart Dis. 2016, 21, 883–901. [Google Scholar] [CrossRef]
- Khoury, P.R.; Mitsnefes, M.; Daniels, S.R.; Kimball, T.R. Age-Specific Reference Intervals for Indexed Left Ventricular Mass in Children. J. Am. Soc. Echocardiogr. 2009, 22, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Koestenberger, M.; Ravekes, W.; Everett, A.D.; Stueger, H.P.; Heinzl, B.; Gamillscheg, A.; Cvirn, G.; Boysen, A.; Fandl, A.; Nagel, B. Right Ventricular Function in Infants, Children and Adolescents: Reference Values of the Tricuspid Annular Plane Systolic Excursion (TAPSE) in 640 Healthy Patients and Calculation of z Score Values. J. Am. Soc. Echocardiogr. 2009, 22, 715–719. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef]
- Levy, P.T.; Machefsky, A.; Sanchez, A.A.; Patel, M.D.; Rogal, S.; Fowler, S.; Yaeger, L.; Hardi, A.; Holland, M.R.; Hamvas, A.; et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2016, 29, 209–225.e6. [Google Scholar] [CrossRef]
- American Thoracic Society. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef] [PubMed]
- Bongers, B.; Hulzebos, H.; Blank, A.; van Brussel, M.; Takken, T. The oxygen uptake efficiency slope in children with congenital heart disease: Construct and group validity. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Hossri, C.A.; De Souza, I.P.A.; De Oliveira, J.S.; Mastrocola, L.E. Assessment of oxygen-uptake efficiency slope in healthy children and children with heart disease: Generation of appropriate reference values for the OUES variable. Eur. J. Prev. Cardiol. 2019, 26, 177–184. [Google Scholar] [CrossRef]
- Fink, T.T.; Marques, H.H.; Gualano, B.; Lindoso, L.; Bain, V.; Astley, C.; Martins, F.; Matheus, D.; Matsuo, O.M.; Suguita, P.; et al. Persistent symptoms and decreased health-related quality of life after symptomatic pediatric COVID-19: A prospective study in a Latin American tertiary hospital. Clinics 2021, 76, e3511. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.-N.; John, A.; Oster, M.E.; Allen, K.; Tremoulet, A.H.; Saarel, E.V.; Lambert, L.M.; Miyamoto, S.D.; de Ferranti, S.D. SARS-CoV-2 Infection and Associated Cardiovascular Manifestations and Complications in Children and Young Adults: A Scientific Statement from the American Heart Association. Circulation 2022, 145, e1037–e1052. [Google Scholar] [CrossRef]
- Mazzolani, B.C.; Smaira, F.I.; Astley, C.; Iraha, A.Y.; Pinto, A.J.; Marques, I.G.; Amarante, M.C.; Rezende, N.S.; Sieczkowska, S.M.; Franco, T.C.; et al. Changes in Eating Habits and Sedentary Behavior during the COVID-19 Pandemic in Adolescents with Chronic Conditions. Front. Pediatr. 2021, 9, 714120. [Google Scholar] [CrossRef]
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 1003–1008. [Google Scholar] [CrossRef]
- Joshi, K.; Kaplan, D.; Bakar, A.; Jennings, J.F.; Hayes, D.A.; Mahajan, S.; Misra, N.; Mitchell, E.; Sweberg, T.M.; Taylor, M.D.; et al. Cardiac Dysfunction and Shock in Pediatric Patients with COVID-19. JACC Case Rep. 2020, 2, 1267–1270. [Google Scholar] [CrossRef]
- Yin, J.; Wang, S.; Liu, Y.; Chen, J.; Li, D.; Xu, T. Coronary microvascular dysfunction pathophysiology in COVID-19. Microcirculation 2021, 28, e12718. [Google Scholar] [CrossRef]
- Kiko, T.; Yokokawa, T.; Masuda, A.; Misaka, T.; Yamada, S.; Kaneshiro, T.; Oikawa, M.; Yoshihisa, A.; Nakazato, K.; Takeishi, Y. Simultaneous assessment of coronary flow reserve and left ventricular function during vasodilator stress evaluated by 13N-ammonia hybrid PET/MRI. Clin. Radiol. 2021, 76, 472.e1–472.e9. [Google Scholar] [CrossRef] [PubMed]
- Blomster, J.I.; Svedlund, S.; Westergren, H.U.; Gan, L.-M. Coronary flow reserve as a link between exercise capacity, cardiac systolic and diastolic function. Int. J. Cardiol. 2016, 217, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ziadi, M.C. Myocardial flow reserve (MFR) with positron emission tomography (PET)/computed tomography (CT): Clinical impact in diagnosis and prognosis. Cardiovasc. Diagn. Ther. 2017, 7, 206–218. [Google Scholar] [CrossRef] [PubMed]


| Exercised Patients | Non-Exercised Patients | |||||
|---|---|---|---|---|---|---|
| Patient’s Characteristics | PI | PII | PIII | PIV | PV | PVI |
| Sex | Female | Male | Female | Female | Male | Male |
| Age (years) | 16 | 7 | 9 | 8 | 11 | 11 |
| Previously medical history | No | No | No | No | Yes (T1D) | No |
| Height (cm) | 156 | 126 | 145 | 135 | 139 | 138 |
| Weight (kg) | 60.3 | 33.7 | 38.6 | 38.4 | 31.5 | 29.3 |
| BMI (kg·m−2) | 24.7 | 21.2 | 18.3 | 21.1 | 16.3 | 15.3 |
| Adherence to 12-wk home-based exercise program (supervised/online) | 83.3/62.5 | 100/100 | 100/37.5 | * | * | * |
| Signs and symptoms at admission | ||||||
| Fever (days) | Yes (12) | Yes (8) | Yes (12) | Yes (1) | Yes (3) | Yes (5) |
| Conjunctivitis | Yes | Yes | No | No | Yes | Yes |
| Arterial hypotension | Yes | Yes | Yes | No | Yes | Yes |
| Shock | No | Yes | Yes | No | Yes | Yes |
| Abdominal pain | Yes | Yes | Yes | Yes | Yes | Yes |
| Diarrhea | Yes | No | No | Yes | No | Yes |
| Echocardiogram abnormalities | Yes * | Yes # | Yes * | No | Yes $ | No |
| Treatment | ||||||
| ICU admission | No | Yes | Yes | Yes | Yes | Yes |
| Length of stay at hospital (days) | 3 | 14 | 18 | 5 | 10 | 12 |
| Respiratory support/Oxygen therapy | No/No | Yes/Yes | No/No | No/No | Yes/No | Yes/Yes |
| Anti-inflammatory treatment | No | Yes (mPRED) | Yes (mPRED) | No | Yes (mPRED) | No |
| Immunoglobulin treatment | First dose 2 g/kg | First and second dose 2 g/kg | First dose 2 g/kg | First dose 2 g/kg | First dose 2 g/kg; second dose 1 g/kg | First dose 2 g/kg |
| Exercised Patients | ||||
|---|---|---|---|---|
| Pre | Post | Fisher’s Test | ||
| I | CFR abnormal | 12/20 | 2/20 | 0.0022 |
| CFR borderline | 5/20 | 6/20 | 1.0000 | |
| CFR normal | 3/20 | 12/20 | 0.0079 | |
| II | CFR abnormal | 0/20 | 0/20 | 1.0000 |
| CFR borderline | 1/20 | 6/20 | 0.0915 | |
| CFR normal | 19/20 | 14/20 | 0.0915 | |
| III * | CFR abnormal | 11/20 | - | - |
| CFR borderline | 9/20 | - | - | |
| CFR normal | 0/20 | - | - | |
| Non-exercised patients | ||||
| IV | CFR abnormal | 0/20 | 6/20 | 0.0202 |
| CFR borderline | 0/20 | 6/20 | 0.0202 | |
| CFR normal | 20/20 | 8/20 | 0.0001 | |
| V | CFR abnormal | 1/20 | 1/20 | 1.0000 |
| CFR borderline | 1/20 | 6/20 | 0.0915 | |
| CFR normal | 18/20 | 13/20 | 0.1274 | |
| VI | CFR abnormal | 0/20 | 8/20 | 0.0033 |
| CFR borderline | 9/20 | 12/20 | 0.0012 | |
| CFR normal | 11/20 | 0/20 | 0.0001 | |
| Exercised Patients | Non-Exercised Patients | |||||
|---|---|---|---|---|---|---|
| PI | PII | PIII | PIV | PV | PVI | |
| Cardiopulmonary exercise test | ||||||
| Time to exhaustion (min) | ||||||
| Pre | 9.0 | 7 | 9.2 | 11.3 | 11.4 | 10 |
| Post | 11.0 | 10.5 | 8.5 | 9.3 | 12.3 | 10 |
| Δ, % | 22.2 | 50.0 | −7.61 | −17.7 | 7.89 | 0 |
| VO2peak (mL·kg−1·min−1) | ||||||
| Pre | 22.5 | 18.3 | 30.8 | 35.2 | 42.5 | 37.1 |
| Post | 25.6 | 20.7 | 29.3 | 28.4 | 48.9 | 38.7 |
| Δ, % | 13.8 | 13.4 | −4.87 | −19.2 | 15 | 4.39 |
| VO2VAT (mL·kg−1·min−1) | ||||||
| Pre | 9.66 | 12.9 | 11.1 | 14.6 | 16.2 | 19.2 |
| Post | 9.73 | 15.6 | 15.8 | 12.9 | 24.8 | 16.7 |
| Δ, % | 0.72 | 20.9 | −29.6 | −11.8 | 52.8 | −13.1 |
| % Predicted VO2peak (<80% abnormal) | ||||||
| Pre | 48.7 | 37.1 | 70.1 | 64.5 | 78.7 | 69.4 |
| Post | 55.5 | 42.1 | 66.7 | 62.8 | 90.5 | 72.5 |
| Δ, % | 13.8 | 13.4 | −4.87 | −2.54 | 14.9 | 4.39 |
| VE/VCO2 slope (units) (>31 abnormal) | ||||||
| Pre | 38.8 | 31.6 | 33.6 | 33.0 | 39.5 | 31.3 |
| Post | 42.2 | 32.8 | 36.9 | 36.1 | 39.6 | 37.8 |
| Δ, % | 8.76 | 3.80 | 9.82 | 9.39 | 0.25 | 20.7 |
| PetCO2 rest (mmHg) (<35 abnormal) | ||||||
| Pre | 24 | 32 | 31 | 35 | 37 | 35 |
| Post | 27 | 29 | 30 | 35 | 36 | 30 |
| Δ, % | 12.5 | −9.38 | −5.88 | 0 | −2.7 | −14.3 |
| O2 pulse peak (mL/beat) (<14 abnormal) | ||||||
| Pre | 8 | 4 | 7 | 10 | 7 | 7 |
| Post | 10 | 5 | 7 | 8 | 9 | 7 |
| Δ, % | 25 | 25 | 0 | −20 | 28.5 | 0 |
| Resting heart rate (beats/min) | ||||||
| Pre | 91 | 107 | 120 | 93 | 100 | 115 |
| Post | 94 | 99 | 114 | 94 | 91 | 90 |
| Δ, % | 9.5 | −7.4 | −5 | 1.1 | −9.0 | −21.7 |
| Heart rate peak (beats/min) | ||||||
| Pre | 178 | 134 | 195 | 188 | 195 | 170 |
| Post | 195 | 139 | 183 | 208 | 195 | 171 |
| Δ, % | 9.5 | 3.7 | −6.1 | 10.6 | 0 | 0.6 |
| Chronotropic reserve (%) (abnormal < 80%) | ||||||
| Pre | 87.9 | 35.5 | 112 | 94.6 | 106.7 | 75.3 |
| Post | 106.3 | 47.6 | 94.5 | 118.8 | 106.1 | 82.7 |
| Δ, % | 20.9 | 34.0 | −15.5 | 25.5 | −0.58 | 9.70 |
| Laboratory data (normal range) | ||||||
| C-reactive protein (0.3–10 mg/L) | ||||||
| Pre | 0.30 | 0.42 | 4.85 | 0.30 | 0.30 | 0.57 |
| Post | 0.71 | 1.06 | 2.90 | 0.32 | 0.60 | 0.30 |
| Δ, % | 136.6 | 152.3 | −40.2 | 6.67 | 100 | −47.3 |
| D-dimers (≤500 ng/mL) | ||||||
| Pre | 794 | 97572 | 1691 | * | 271 | 349 |
| Post | 665 | 215 | 640 | * | 326 | 337 |
| Δ, % | −16.2 | −9978 | −62.1 | * | 20.3 | −3.44 |
| Fibrinogen (200–400 mg/dL) | ||||||
| Pre | 311 | 190 | 465 | * | 304 | 263 |
| Post | 349 | 315 | 340 | 239 | 257 | 252 |
| Δ, % | 12.2 | 65.8 | −26.8 | * | −15.4 | −4.18 |
| Troponin T (<0.004 ng/mL) | ||||||
| Pre | 0.005 | 0.004 | 0.004 | * | 0.003 | 0.007 |
| Post | 0.003 | 0.004 | 0.003 | * | 0.003 | 0.003 |
| Δ, % | −40.0 | 0 | −25.0 | * | 0 | −57.1 |
| Hemoglobin (11.5–15.5 g/dL) | ||||||
| Pre | 10.9 | 14.6 | 12.7 | 12.7 | 12.3 | 11.2 |
| Post | 10.8 | 13.7 | 13.4 | 12.8 | 12.2 | 12.3 |
| Δ, % | −0.92 | −6.16 | 5.51 | 0.79 | −0.81 | 9.82 |
| Lymphocyte count (1.5−7 × 109/L) | ||||||
| Pre | 3.29 | 6.29 | 3.51 | 2.90 | 1.72 | 2.10 |
| Post | 2.40 | 5.18 | 2.53 | 2.56 | 1.55 | 2.28 |
| Δ, % | −27.0 | −17.6 | −27.9 | −11.7 | −9.88 | 9.09 |
| Platelet count (150−400 × 109/L) | ||||||
| Pre | 413 | 473 | 409 | 336 | 194 | 376 |
| Post | 368 | 359 | 405 | 314 | 178 | 449 |
| Δ, % | −10.9 | −24.1 | −0.98 | −6.55 | −8.25 | 19.4 |
| Urea (7–20 mg/dL) | ||||||
| Pre | 23 | 27 | 18 | 18 | 23 | 41 |
| Post | 27 | 24 | 12 | 17 | 23 | 24 |
| Δ, % | 17.3 | −11.1 | −33.3 | −5.56 | 0 | −41.6 |
| Creatinine (0.59–1.53 mg/dL) | ||||||
| Pre | 0.61 | 0.47 | 0.46 | 0.46 | 0.48 | 0.44 |
| Post | 0.77 | 0.54 | 0.54 | 0.50 | 0.53 | 0.52 |
| Δ, % | 26.2 | 14.9 | 17.3 | 8.70 | 10.4 | 18.1 |
| Alanine transaminase (7–55 U/L) | ||||||
| Pre | 20 | 19 | 36 | 20 | 19 | 25 |
| Post | 15 | 19 | 26 | 15 | 13 | 23 |
| Δ, % | −25.0 | 0 | −27.7 | −25.0 | −31.6 | −8.0 |
| Aspartate transaminase (8–33 U/L) | ||||||
| Pre | 13 | 11 | 26 | 12 | 9 | 12 |
| Post | 9 | 12 | 19 | 11 | 7 | 10 |
| Δ, % | −30.7 | 9.09 | −26.9 | −8.33 | −22.2 | −16.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astley, C.; Leal, G.N.; Gil, S.; Suguita, P.; Fink, T.; Bain, V.; Pereira, M.F.B.; Marques, H.H.; Sieczkowska, S.; Prado, D.; et al. Home-Based Exercise Training in the Recovery of Multisystem Inflammatory Syndrome in Children: A Case Series Study. Children 2023, 10, 889. https://doi.org/10.3390/children10050889
Astley C, Leal GN, Gil S, Suguita P, Fink T, Bain V, Pereira MFB, Marques HH, Sieczkowska S, Prado D, et al. Home-Based Exercise Training in the Recovery of Multisystem Inflammatory Syndrome in Children: A Case Series Study. Children. 2023; 10(5):889. https://doi.org/10.3390/children10050889
Chicago/Turabian StyleAstley, Camilla, Gabriela Nunes Leal, Saulo Gil, Priscila Suguita, Thais Fink, Vera Bain, Maria Fernanda Badue Pereira, Heloisa Helena Marques, Sofia Sieczkowska, Danilo Prado, and et al. 2023. "Home-Based Exercise Training in the Recovery of Multisystem Inflammatory Syndrome in Children: A Case Series Study" Children 10, no. 5: 889. https://doi.org/10.3390/children10050889
APA StyleAstley, C., Leal, G. N., Gil, S., Suguita, P., Fink, T., Bain, V., Pereira, M. F. B., Marques, H. H., Sieczkowska, S., Prado, D., Lima, M. S., Carneiro, C. G., Buchpiguel, C. A., Silva, C. A., & Gualano, B. (2023). Home-Based Exercise Training in the Recovery of Multisystem Inflammatory Syndrome in Children: A Case Series Study. Children, 10(5), 889. https://doi.org/10.3390/children10050889





