Quality of Life Outcomes for Patients Who Underwent Conventional Resection and Liver Transplantation for Locally Advanced Hepatoblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Survey Measures
3. Results
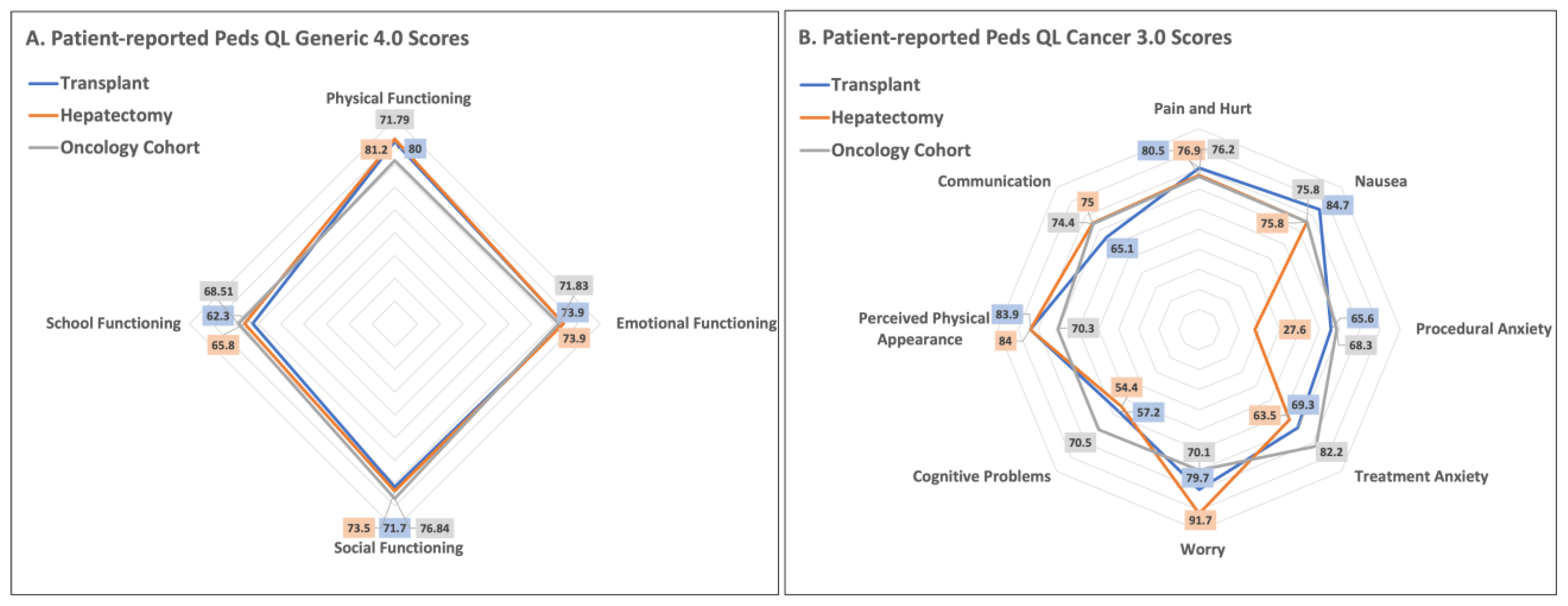
3.1. Peds QL 4.0 Generic Core Scales
3.2. Peds QL 3.0 Cancer Module
4. Discussion
4.1. Practical Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trobaugh-Lotrario, A.D.; Meyers, R.L.; Tiao, G.M.; Feusner, J.H. Pediatric liver transplantation for hepatoblastoma. Transl. Gastroenterol. Hepatol. 2016, 1, 44. [Google Scholar] [CrossRef]
- Darbari, A.; Sabin, K.M.; Shapiro, C.N.; Schwarz, K.B. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003, 38, 560–566. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Nasca, P.C.; Zdeb, M.S. Maternal and infant birth characteristics and hepatoblastoma. Am. J. Epidemiol. 2006, 163, 818–828. [Google Scholar] [CrossRef]
- Linabery, A.M.; Ross, J.A. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer 2008, 112, 416–432. [Google Scholar] [CrossRef]
- Cruz, R.J.; Ranganathan, S.; Mazariegos, G.; Soltys, K.; Nayyar, N.; Sun, Q.; Bond, G.; Shaw, P.H.; Haberman, K.; Krishnamurti, L.; et al. Analysis of national and single-center incidence and survival after liver transplantation for hepatoblastoma: New trends and future opportunities. Surgery 2013, 153, 150–159. [Google Scholar] [CrossRef]
- Küpesiz, F.T.; Akınel, A.N.; Akbaş, H.; Sivrice, Ç.; Kintrup, G.T.; Karagüzel, G.; Melikoğlu, M.; Gelen, M.T.; Aydınlı, B.; Küpesiz, A. Multidisciplinary Management of Pediatric Hepatoblastoma: A 20-Year Single-Center Experience. Turk. J. Gastroenterol. 2022, 33, 1069. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.L.; Tiao, G.; de Ville de Goyet, J.; Superina, R.; Aronson, D.C. Hepatoblastoma state of the art: Pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr. Opin. Pediatr. 2014, 26, 29–36. [Google Scholar] [CrossRef]
- Pham, T.H.; Iqbal, C.W.; Grams, J.M.; Zarroug, A.E.; Wall, J.C.; Ishitani, M.B.; Nagorney, D.M.; Moir, C. Outcomes of primary liver cancer in children: An appraisal of experience. J. Pediatr. Surg. 2007, 42, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Schnater, J.M.; Aronson, D.C.; Plaschkes, J.; Perilongo, G.; Brown, J.; Otte, J.B.; Brugieres, L.; Czauderna, P.; MacKinlay, G.; Vos, A. Surgical view of the treatment of patients with hepatoblastoma: Results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 2002, 94, 1111–1120. [Google Scholar] [CrossRef]
- Warmann, S.W.; Fuchs, J. Drug resistance in hepatoblastoma. Curr. Pharm. Biotechnol. 2007, 8, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.B. Progress in the surgical treatment of malignant liver tumors in children. Cancer Treat. Rev. 2010, 36, 360–371. [Google Scholar] [CrossRef] [PubMed]
- McAteer, J.P.; Goldin, A.B.; Healey, P.J.; Gow, K.W. Surgical treatment of primary liver tumors in children: Outcomes analysis of resection and transplantation in the SEER database. Pediatr. Transplant. 2013, 17, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Gallo, A.M.; Concepcion, W.; Esquivel, C.O.; Bonham, C.A. Effect of Liver Transplant on Long-term Disease-Free Survival in Children With Hepatoblastoma and Hepatocellular Cancer. JAMA Surg. 2015, 150, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh-Lotrario, A.D.; Meyers, R.L.; O’Neill, A.F.; Feusner, J.H. Unresectable hepatoblastoma: Current perspectives. Hepat. Med. 2017, 9, 1–6. [Google Scholar] [CrossRef]
- Grewal, S.; Merchant, T.; Reymond, R.; McInerney, M.; Hodge, C.; Shearer, P. Auditory late effects of childhood cancer therapy: A report from the Children’s Oncology Group. Pediatrics 2010, 125, e938–e950. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Katz, E.R.; Meeske, K.; Dickinson, P. The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002, 94, 2090–2106. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med. Care 2001, 39, 800–812. [Google Scholar] [CrossRef]
- Parmar, A.; Vandriel, S.M.; Ng, V.L. Health-related quality of life after pediatric liver transplantation: A systematic review. Liver Transpl. 2017, 23, 361–374. [Google Scholar] [CrossRef]
- Ng, V.L.; Alonso, E.M.; Bucuvalas, J.C.; Cohen, G.; Limbers, C.A.; Varni, J.W.; Mazariegos, G.; Magee, J.; McDiarmid, S.V.; Anand, R.; et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: Report of the studies of pediatric liver transplantation experience. J. Pediatr. 2012, 160, 820–826.e823. [Google Scholar] [CrossRef]
- Weissberg-Benchell, J.; Zielinski, T.E.; Rodgers, S.; Greenley, R.N.; Askenazi, D.; Goldstein, S.L.; Fredericks, E.M.; McDiarmid, S.; Williams, L.; Limbers, C.A.; et al. Pediatric health-related quality of life: Feasibility, reliability and validity of the PedsQL transplant module. Am. J. Transplant. 2010, 10, 1677–1685. [Google Scholar] [CrossRef]
- Sorensen, L.G.; Neighbors, K.; Martz, K.; Zelko, F.; Bucuvalas, J.C.; Alonso, E.M.; (FOG), S.o.P.L.T.S.a.F.O.G. Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am. J. Transplant. 2011, 11, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Radosevich, D.M.; Lake, J.R. Health-related quality of life: Two decades after liver transplantation. Liver Transpl. 2014, 20, 649–654. [Google Scholar] [CrossRef]
- Otte, J.B. Paediatric liver transplantation--a review based on 20 years of personal experience. Transpl. Int. 2004, 17, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Fardell, J.E.; Wakefield, C.E.; De Abreu Lourenco, R.; Signorelli, C.; McCarthy, M.; McLoone, J.; Osborn, M.; Gabriel, M.; Anazodo, A.; Alvaro, F.; et al. Long-term health-related quality of life in young childhood cancer survivors and their parents. Pediatr. Blood Cancer 2021, 68, e29398. [Google Scholar] [CrossRef]
- Wengenroth, L.; Gianinazzi, M.E.; Rueegg, C.S.; Lüer, S.; Bergstraesser, E.; Kuehni, C.E.; Michel, G. Health-related quality of life in young survivors of childhood cancer. Qual. Life Res. 2015, 24, 2151–2161. [Google Scholar] [CrossRef]
- Alpert, O.; Sharma, V.; Cama, S.; Spencer, S.; Huang, H. Liver transplant and quality of life in the pediatric population: A review update (2013-2014). Curr. Opin. Organ. Transplant. 2015, 20, 216–221. [Google Scholar] [CrossRef]
- Taylor, R.; Franck, L.S.; Gibson, F.; Dhawan, A. A critical review of the health-related quality of life of children and adolescents after liver transplantation. Liver Transpl. 2005, 11, 51–60; discussion 57–59. [Google Scholar] [CrossRef] [PubMed]
- Bucuvalas, J.C.; Britto, M.; Krug, S.; Ryckman, F.C.; Atherton, H.; Alonso, M.P.; Balistreri, W.F.; Kotagal, U. Health-related quality of life in pediatric liver transplant recipients: A single-center study. Liver Transpl. 2003, 9, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Dembowska-Bagińska, B.; Więckowska, J.; Brożyna, A.; Święszkowska, E.; Ismail, H.; Broniszczak-Czyszek, D.; Stefanowicz, M.; Grajkowska, W.; Kaliciński, P. Health Status in Long-Term Survivors of Hepatoblastoma. Cancers 2019, 11, 1777. [Google Scholar] [CrossRef]
- Bell, D.; Ranganathan, S.; Tao, J.; Monga, S.P. Novel Advances in Understanding of Molecular Pathogenesis of Hepatoblastoma: A Wnt/β-Catenin Perspective. Gene Expr. 2017, 17, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, A.B.; Wasilewski-Masker, K.; Border, W.L.; Goodman, M.; Meacham, L.; Austin, H.; Marchak, J.G.; Mertens, A.C. Pediatric quality of life in long-term survivors of childhood cancer treated with anthracyclines. Pediatr. Blood Cancer 2016, 63, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
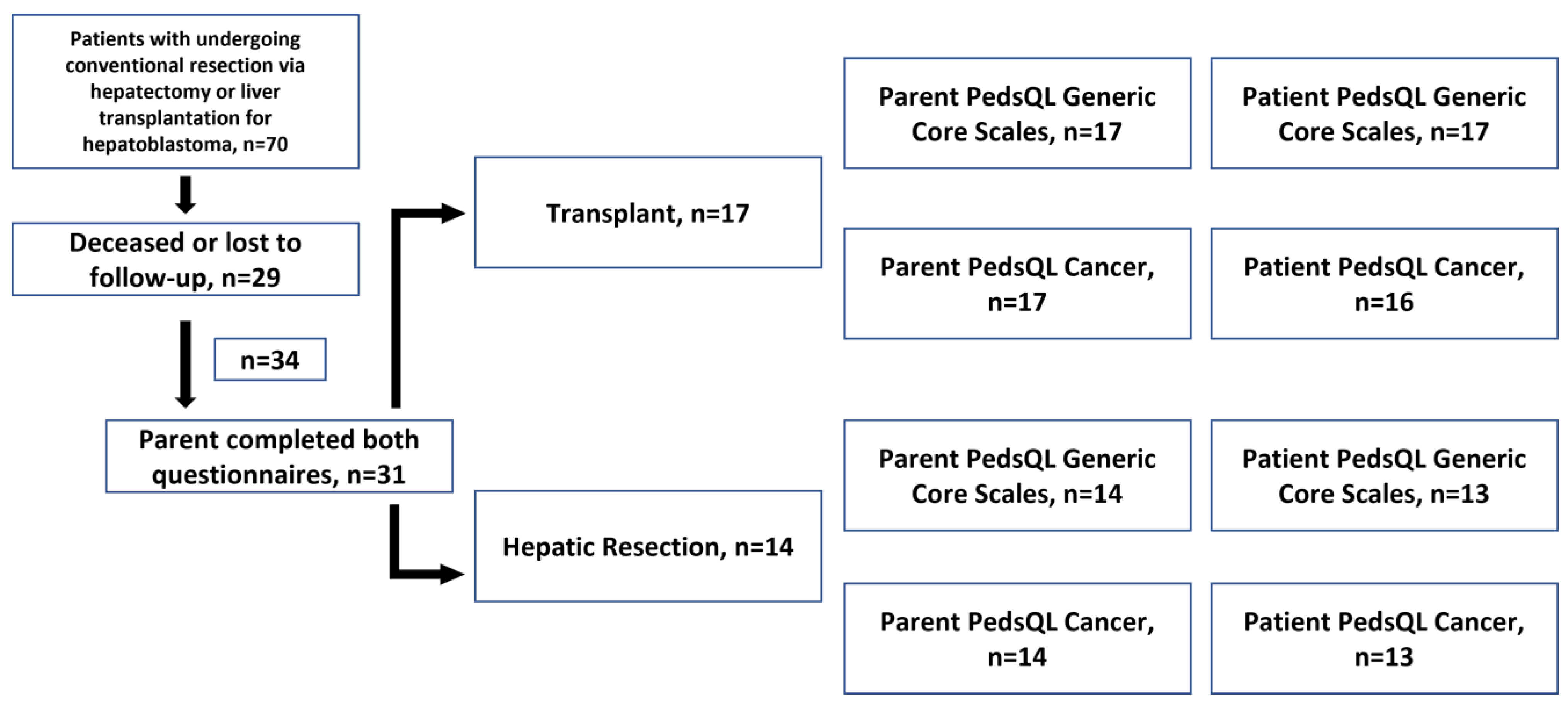
| All Patients, n = 31 | Transplant, n = 17 | Resection, n = 14 | p-Value | |
|---|---|---|---|---|
| Operation performed, n (%) | ||||
| Transplant | 17 (54.8%) | - | - | |
| Resection | 14 (45.2%) | - | - | |
| Age (years) at time of surgery, median (IQR) | 1.9 (1.4–2.7) | 1.7 (0.97–2.0) | 2.3 (1.6–3.0) | 0.08 |
| Age at time of survey by categories. n (%) | ||||
| 5 to 7 years | 5 (16.1%) | 1 (5.9%) | 4 (28.6%) | 0.06 |
| 8 to 12 years | 14 (45.2%) | 6 (35.3%) | 8 (57.1%) | |
| 13 to 18 years | 11 (35.5%) | 9 (52.9%) | 2 (14.3%) | |
| 18 to 25 years | 1 (3.2%) | 1 (5.9%) | 0 | |
| Sex, n (%) | ||||
| Male | 16 (51.6%) | 10 (52.6%) | 8 (57.1%) | 0.38 |
| Female | 15 (48.4%) | 7 (36.8%) | 6 (42.9%) | |
| Ethnicity | ||||
| White | 29 (93.5%) | 17 (100%) | 12 (85.7%) | 0.27 |
| Black | 1 (3.2%) | 0 | 1 (7.1%) | |
| Missing | 1 (3.2%) | 0 | 1 (7.1%) | |
| PRETEXT | ||||
| I | 1 (3.2%) | 1 (5.9%) | 0 (0%) | 0.12 |
| II | 11 (35.5%) | 3 (17.7%) | 8 (57.1%) | |
| III | 10 (32.3%) | 7 (41.2%) | 3 (21.4%) | |
| IV | 1 (3.2%) | 1 (5.9%) | 0 (0%) | |
| Missing | 8 (25.8%) | 5 (29.4%) | 3 (21.4%) | |
| Neoadjuvant chemotherapy given | ||||
| Yes | 19 (61.3%) | 9 (52.9%) | 10 (71.4%) | 0.77 |
| No | 5 (16.1%) | 2 (11.8%) | 3 (21.4%) | |
| Missing | 7 (22.6%) | 6 (35.3%) | 1 (7.1%) |
| Patient Survey (n = 30) | Parent Survey (n = 30) | ||
|---|---|---|---|
| Mean (SD) | p-value | ||
| Total Scale Score | 73.7 (18.8) | 73.9 (18.1) | 0.89 |
| Subscale | |||
| Physical Functioning | 80.5 (23.4) | 79.9 (20.9) | 0.50 |
| Emotional Functioning | 73.9 (22.1) | 71.0 (22.7) | 0.14 |
| Social Functioning | 72.5 (23.9) | 74.6 (23.4) | 0.38 |
| School Functioning | 63.8 (20.3) | 66.3 (19.2) | 0.46 |
| Psychosocial Summary Score | 70.1 (18.7) | 70.6 (19.3) | 0.60 |
| Patient Responses | Parent Responses | |||||
|---|---|---|---|---|---|---|
| Transplant n = 17 | Resection n = 13 | Transplant n = 17 | Resection n = 14 | |||
| Mean (SD) | p-value | Mean (SD) | p-value | |||
| Total Scale Score | 73.0 (18.2) | 74.6 (20.2) | 0.83 | 72.4 (18.9) | 75.6 (17.6) | 0.63 |
| Subscale | ||||||
| Physical Functioning | 80.0 (20.7) | 81.2 (27.3) | 0.89 | 78.6 (17.8) | 81.5 (24.8) | 0.72 |
| Emotional Functioning | 73.9 (22.7) | 73.9 (22.0) | 0.99 | 70.6 (23.0) | 71.4 (23.2) | 0.92 |
| Social Functioning | 71.7 (23.1) | 73.5 (25.8) | 0.84 | 70.3 (24.5) | 79.7 (21.9) | 0.27 |
| School Functioning | 62.3 (20.0) | 65.8 (21.4) | 0.65 | 66.7 (23.7) | 65.7 (12.5) | 0.88 |
| Psychosocial Summary Score | 69.3 (18.7) | 71.1 (19.4) | 0.8 | 69.2 (21.0) | 72.3 (17.6) | 0.66 |
| Patient Responses | Parent Responses | |||||
|---|---|---|---|---|---|---|
| Transplant, n = 16 | Resection, n = 13 | Transplant, n = 17 | Resection, n = 14 | |||
| Mean (SD) | p-value | Mean (SD) | p-value | |||
| Total | 73.1 (16.1) | 68.0 (17.3) | 0.41 | 69.1 (19.5) | 69.5 (13.9) | 0.94 |
| Dimension Scores | ||||||
| Pain and Hurt | 80.5 (21.4) | 76.9 (25.4) | 0.69 | 79.4 (24.2) | 75 (24.5) | 0.62 |
| Nausea | 84.7 (16.7) | 75.8 (24.2) | 0.25 | 84.4 (19.9) | 84.3 (18.0) | 0.99 |
| Procedural Anxiety | 65.6 (30.6) | 27.6 (34.1) | 0.004 * | 60.3 (29.8) | 33.3 (37.3) | 0.03 * |
| Treatment Anxiety | 69.3 (24.3) | 63.5 (31.6) | 0.58 | 71.6 (28.6) | 50 (32.7) | 0.06 |
| Worry | 79.7 (20.4) | 91.7 (10.2) | 0.06 | 76.5 (25.0) | 88.1 (13.8) | 0.13 |
| Cognitive Problems | 57.2 (26.7) | 54.4 (27.4) | 0.79 | 48.8 (29.6) | 60.7 (19.8) | 0.21 |
| Perceived Physical Appearance | 83.9 (20.7) | 84.0 (20.0) | 0.99 | 75.0 (24.3) | 83.3 (19.9) | 0.31 |
| Communication | 65.1 (26.7) | 75 (30.6) | 0.36 | 62.7 (29.2) | 79.2 (30.4) | 0.14 |
| 𝛽surgery * | SE | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| PedsQL General Survey | ||||
| Total Scale Score | 3.70 | 7.66 | (−12.19, 19.60) | 0.63 |
| Subscale | ||||
| Physical Functioning | 0.11 | 9.50 | (−19.60, 19.82) | 0.99 |
| Emotional Functioning | 4.73 | 9.51 | (−15.00, 24.45) | 0.62 |
| Social Functioning | 2.69 | 9.73 | (−17.49, 22.88) | 0.78 |
| School Functioning | 9.60 | 8.70 | (−8.44, 27.63) | 0.28 |
| Peds QL Cancer 3.0 Dimension | ||||
| Pain and Hurt | −2.79 | 8.50 | (−20.48, 14.89) | 0.75 |
| Nausea | −6.91 | 8.33 | (−24.24, 10.41) | 0.42 |
| Procedural Anxiety | −33.47 | 13.00 | (−60.41, −6.53) | 0.017 * |
| Treatment Anxiety | −3.86 | 11.44 | (−27.64, 19.93) | 0.74 |
| Worry | 10.11 | 7.31 | (−5.09, 25.31) | 0.18 |
| Cognitive Problems | 5.31 | 11.76 | (−19.16, 29.77) | 0.66 |
| Perceived Physical Appearance | −3.50 | 9.28 | (−22.80, 15.80) | 0.71 |
| Communication | 4.92 | 13.52 | (−23.19, 33.03) | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqui, Z.; Johnston, M.; Schepers, E.; Brewer, N.; Hartman, S.; Jenkins, T.; Bondoc, A.; Pai, A.; Geller, J.; Tiao, G.M. Quality of Life Outcomes for Patients Who Underwent Conventional Resection and Liver Transplantation for Locally Advanced Hepatoblastoma. Children 2023, 10, 890. https://doi.org/10.3390/children10050890
Farooqui Z, Johnston M, Schepers E, Brewer N, Hartman S, Jenkins T, Bondoc A, Pai A, Geller J, Tiao GM. Quality of Life Outcomes for Patients Who Underwent Conventional Resection and Liver Transplantation for Locally Advanced Hepatoblastoma. Children. 2023; 10(5):890. https://doi.org/10.3390/children10050890
Chicago/Turabian StyleFarooqui, Zishaan, Michael Johnston, Emily Schepers, Nathalie Brewer, Stephen Hartman, Todd Jenkins, Alexander Bondoc, Ahna Pai, James Geller, and Gregory M. Tiao. 2023. "Quality of Life Outcomes for Patients Who Underwent Conventional Resection and Liver Transplantation for Locally Advanced Hepatoblastoma" Children 10, no. 5: 890. https://doi.org/10.3390/children10050890
APA StyleFarooqui, Z., Johnston, M., Schepers, E., Brewer, N., Hartman, S., Jenkins, T., Bondoc, A., Pai, A., Geller, J., & Tiao, G. M. (2023). Quality of Life Outcomes for Patients Who Underwent Conventional Resection and Liver Transplantation for Locally Advanced Hepatoblastoma. Children, 10(5), 890. https://doi.org/10.3390/children10050890







