Post-Traumatic Headache in Children after Minor Head Trauma: Incidence, Phenotypes, and Risk Factors
Abstract
1. Introduction
2. Materials and Methods
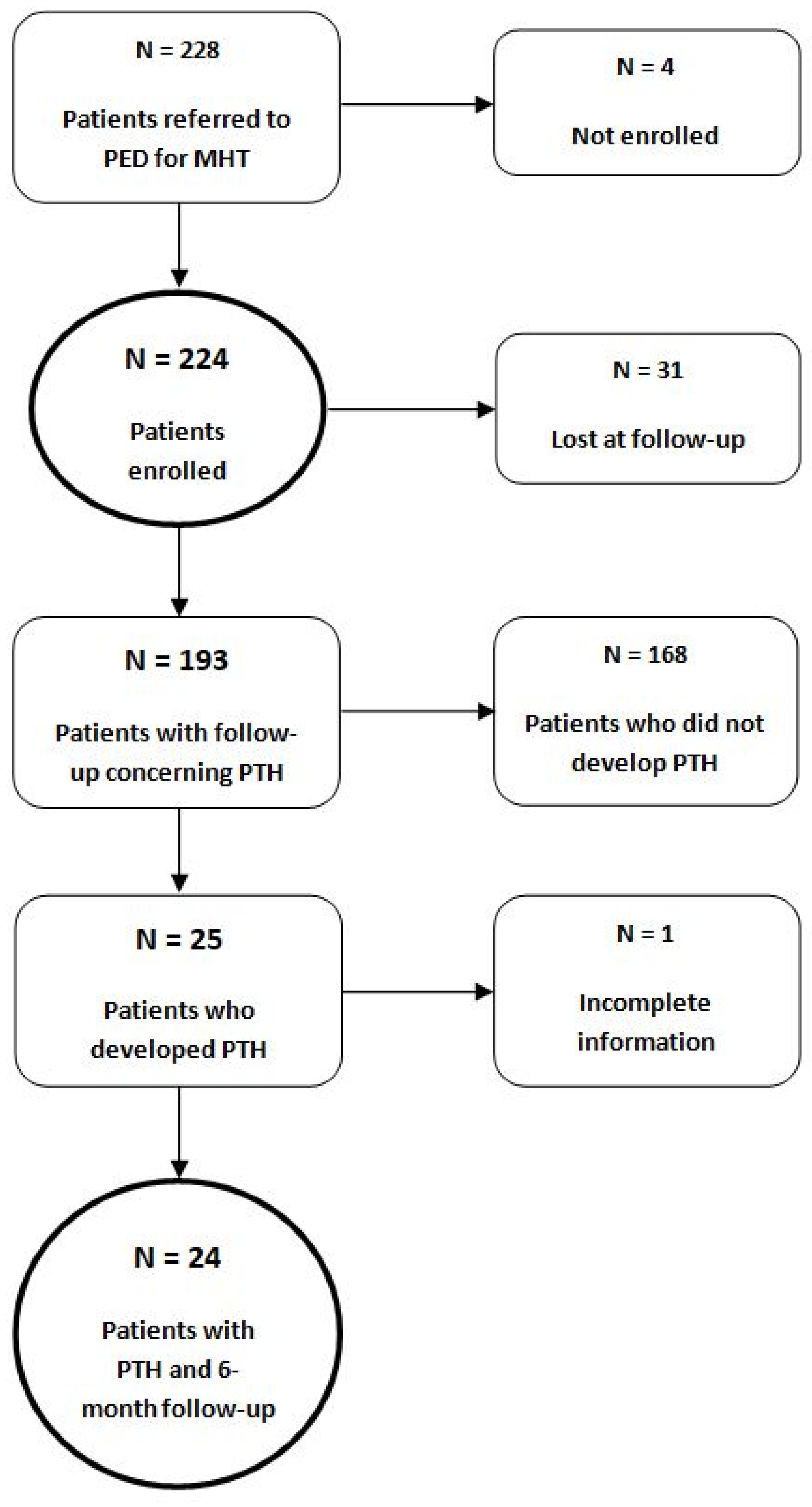
2.1. Recruitment to the Study
2.2. Inclusion and Exclusion Criteria
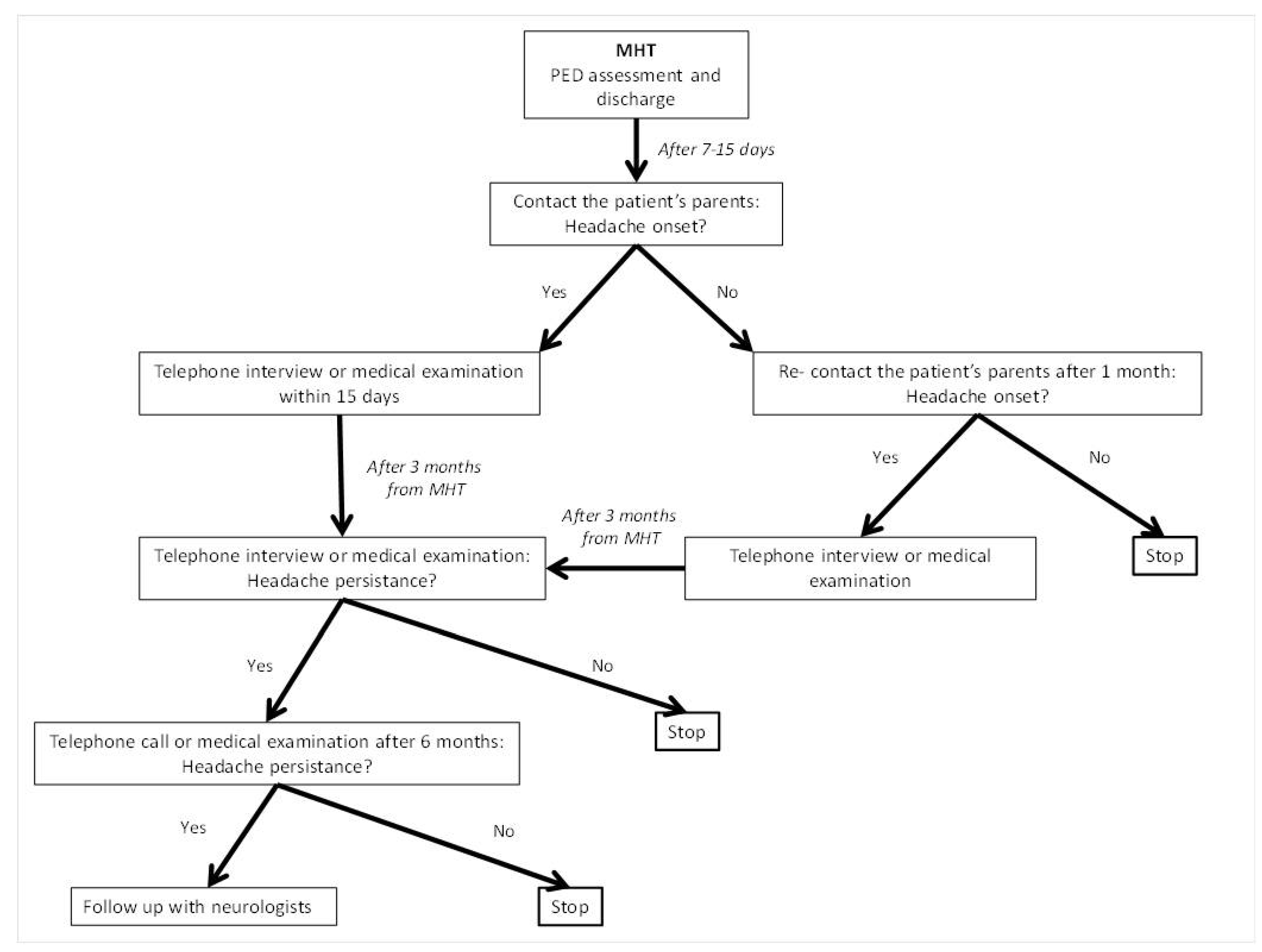
2.3. Study Design
2.4. Statistical Analysis
2.5. Ethical Issues
3. Results
3.1. Population and Characteristics of MHT at PED Evaluation
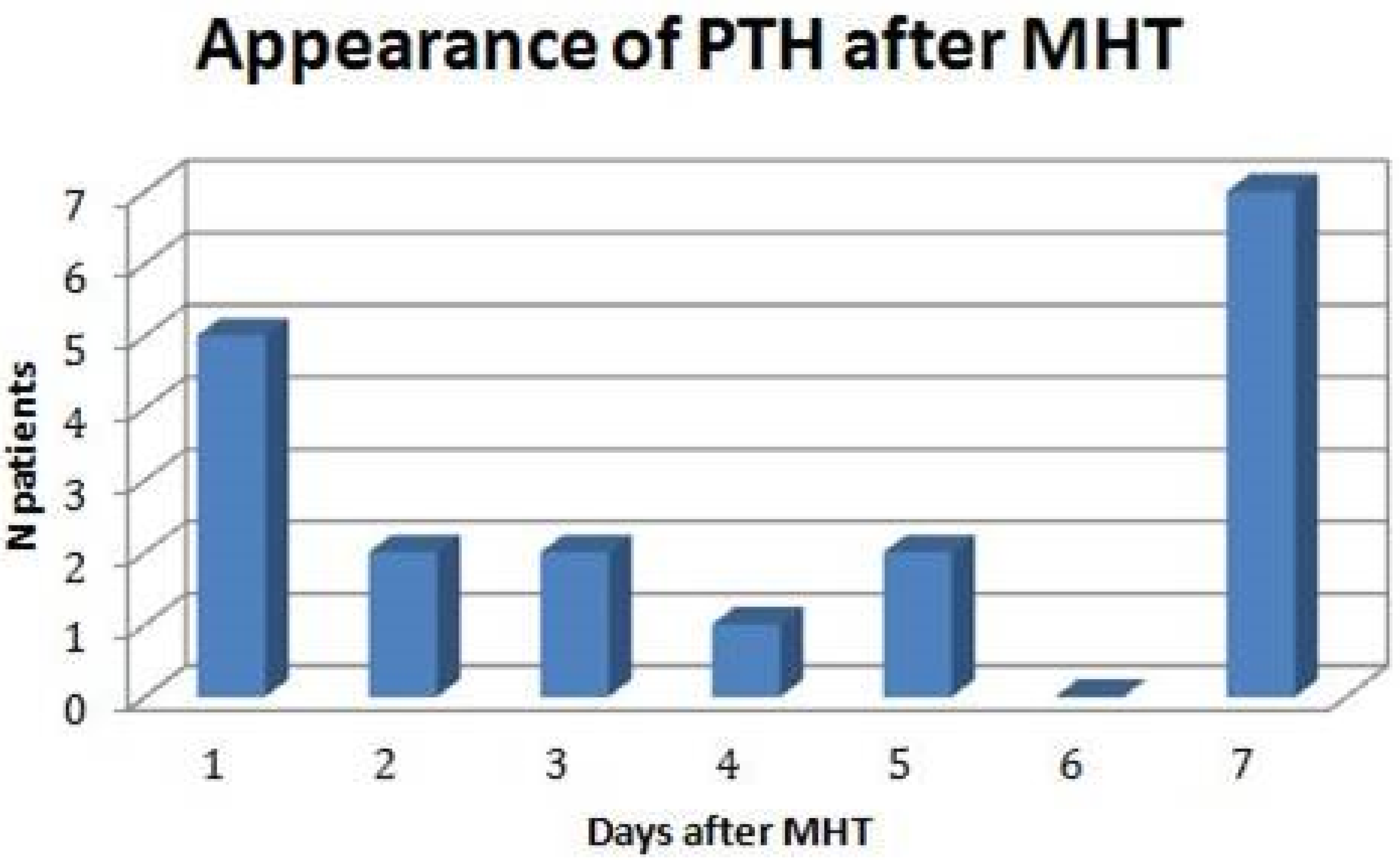
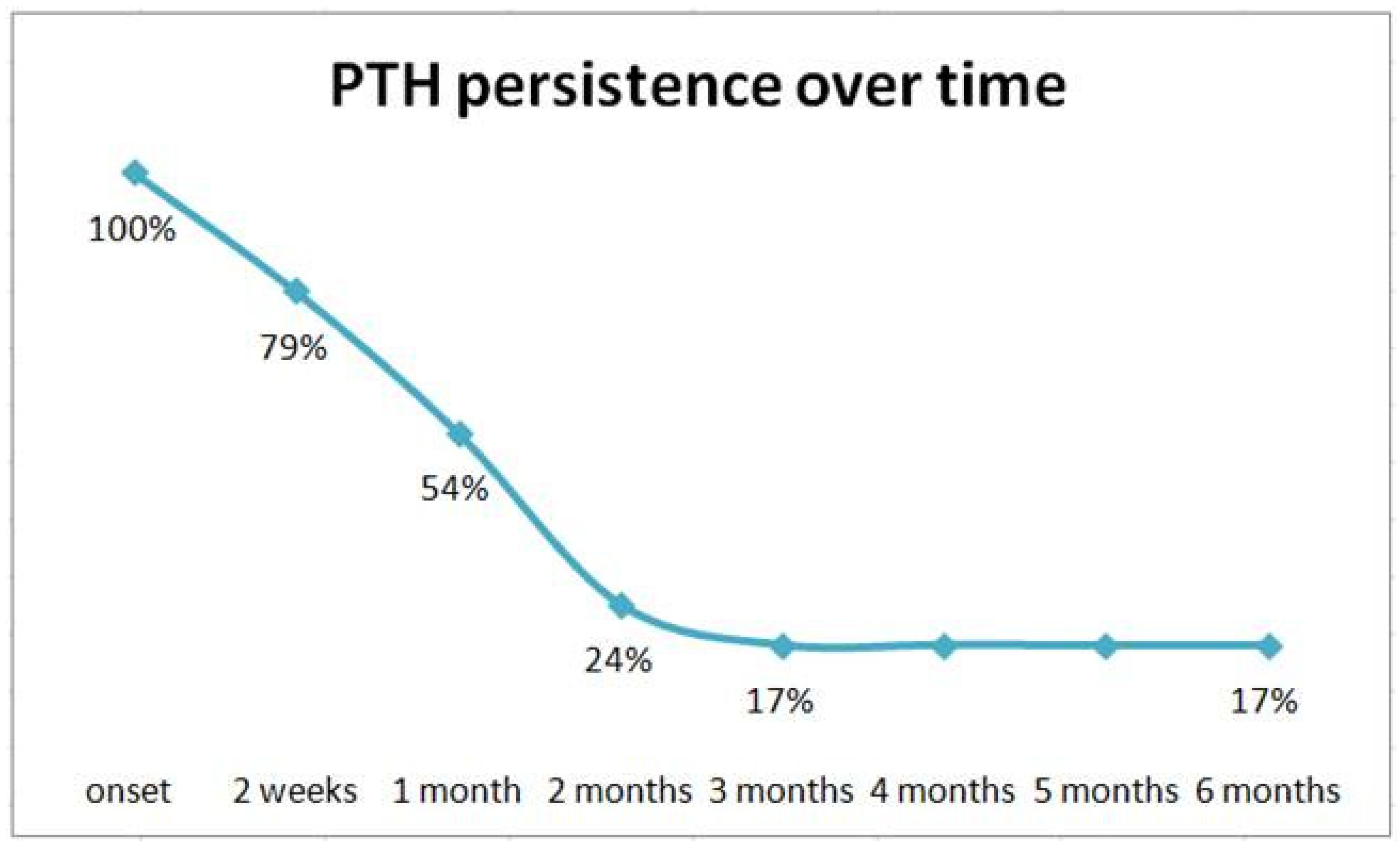
3.2. Incidence, Timing, and Characteristics of PTH
3.3. Risk Factors for PTH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quayle, K.S.; Holmes, J.F.; Kuppermann, N. Others Epidemiology of Blunt Head Trauma in Children in U.S. Emergency Departments. N. Engl. J. Med. 2014, 371, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Ohbuchi, H.; Hagiwara, S.; Hirota, K.; Koseki, H.; Kuroi, Y.; Arai, N.; Kasuya, H. Clinical Predictors of Intracranial Injuries in Infants with Minor Head Trauma. World Neurosurg. 2017, 98, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Bressan, S.; Eapen, N.; Phillips, N.; Gilhotra, Y.; Kochar, A.; Dalton, S.; Cheek, J.A.; Furyk, J.; Neutze, J.; Williams, A.; et al. PECARN algorithms for minor head trauma: Risk strat-ification estimates from a prospective PREDICT cohort study. Acad. Emerg. Med. 2021, 28, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Gagner, C.; Landry-Roy, C.; Bernier, A.; Gravel, J.; Beauchamp, M.H. Behavioral consequences of mild traumatic brain injury in preschoolers. Psychol. Med. 2018, 48, 1551–1559. [Google Scholar] [CrossRef]
- Van Gils, A.; Stone, J.; Welch, K.; Davidson, L.R.; Kerslake, D.; Caesar, D.; McWhirter, L.; Carson, A. Management of mild traumatic brain injury. Pract. Neurol. 2020, 20, 213–221. [Google Scholar] [CrossRef]
- Emery, C.A.; Barlow, K.M.; Brooks, B.L.; Max, J.E.; Villavicencio-Requis, A.; Gnanakumar, V.; Robertson, H.L.; Schneider, K.; Yeates, K.O. A Systematic Review of Psychiatric, Psychological, and Behavioural Outcomes following Mild Traumatic Brain Injury in Children and Adolescents. Can. J. Psychiatry 2016, 61, 259–269. [Google Scholar] [CrossRef]
- Arbogast, K.B.; Curry, A.E.; Pfeiffer, M.R.; Zonfrillo, M.R.; Haarbauer-Krupa, J.; Breiding, M.J.; Coronado, V.G.; Master, C.L. Point of Health Care Entry for Youth with Concussion Within a Large Pediatric Care Network. JAMA Pediatr. 2016, 170, e160294. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, N.; Holmes, J.F.; Dayan, P.S.; Hoyle, J.D.; Atabaki, S.M.; Holubkov, R.; Nadel, F.M.; Monroe, D.; Stanley, R.M.; A Borgialli, D.; et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: A prospective cohort study. Lancet 2009, 374, 1160–1170. [Google Scholar] [CrossRef]
- Gambacorta, A.; Moro, M.; Curatola, A.; Brancato, F.; Covino, M.; Chiaretti, A.; Gatto, A. PECARN Rule in diagnostic process of pediatric patients with minor head trauma in emergency department. Eur. J. Pediatr. 2022, 181, 2147–2154. [Google Scholar] [CrossRef]
- Sert, E.T.; Mutlu, H.; Kokulu, K. The Use of PECARN and CATCH Rules in Children with Minor Head Trauma Presenting to Emergency Department 24 Hours after Injury. Pediatr. Emerg. Care 2022, 38, e524–e528. [Google Scholar] [CrossRef]
- Lee, L.K.; Monroe, D.; Bachman, M.C.; Glass, T.F.; Mahajan, P.V.; Cooper, A.; Stanley, R.M.; Miskin, M.; Dayan, P.S.; Holmes, J.F.; et al. Isolated Loss of Consciousness in Children with Minor Blunt Head Trauma. JAMA Pediatr. 2014, 168, 837–843. [Google Scholar] [CrossRef] [PubMed]
- De Pava, M.V.; Milani, G.P.; Zuccotti, G.V.; Tommasi, P.; Calvi, M.; Amoroso, A.; Montesano, P.; Boselli, G.; Castellazzi, M.L.; Agosti, M.; et al. Multicentre study found no increased risk of clinically important brain injuries when children presented more than 24 h after a minor head trauma. Acta Paediatr. 2022, 111, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004, 24 (Suppl. 1), 9–160. [Google Scholar]
- Lucas, S.; Hoffman, J.M.; Bell, K.; Dikmen, S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014, 34, 93–102. [Google Scholar] [CrossRef]
- Lieba-Samal, D.; Platzer, P.; Seidel, S.; Klaschterka, P.; Knopf, A.; Wöber, C. Characteristics of acute posttraumatic headache following mild head injury. Cephalalgia 2011, 31, 1618–1626. [Google Scholar] [CrossRef]
- Nampiaparampil, D.E. Prevalence of Chronic Pain After Traumatic Brain Injury: A Systematic Review. JAMA 2008, 300, 711. [Google Scholar] [CrossRef]
- Kuczynski, A.; Crawford, S.; Bodell, L.; Dewey, D.; Barlow, K.M. Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: A prospective cohort. Dev. Med. Child Neurol. 2013, 55, 636–641. [Google Scholar] [CrossRef]
- McEvoy, H.; Borsook, D.; A Holmes, S. Clinical features and sex differences in pediatric post-traumatic headache: A retrospective chart review at a Boston area concussion clinic. Cephalalgia 2020, 40, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, Y.; Mäki, K.; Marinkovic, I.; Nybo, T.; Isokuortti, H.; Huovinen, A.; Korvenoja, A.; Melkas, S.; Harno, H. Post-traumatic headache after mild traumatic brain injury in a one-year follow up study—Risk factors and return to work. J. Headache Pain 2022, 23, 27. [Google Scholar] [CrossRef]
- Maleki, N.; Finkel, A.; Cai, G.; Ross, A.; Moore, R.D.; Feng, X.; Androulakis, X.M. Post-traumatic Headache and Mild Traumatic Brain Injury: Brain Networks and Connectivity. Curr. Pain Headache Rep. 2021, 25, 20. [Google Scholar] [CrossRef]
- Yilmaz, T.; Roks, G.; De Koning, M.; Scheenen, M.; Van Der Horn, H.; Plas, G.; Hageman, G.; Schoonman, G.; Spikman, J.; Van Der Naalt, J. Risk factors and outcomes associated with post-traumatic headache after mild traumatic brain injury. Emerg. Med. J. 2017, 34, 800–805. [Google Scholar] [CrossRef]
- Doll, E.; Gong, P.; Sowell, M.; Evanczyk, L. Post-traumatic Headache in Children and Adolescents. Curr. Pain Headache Rep. 2021, 25, 51. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Porreca, F.; Anderson, T.; Amin, F.M.; Ashina, M.; Schytz, H.W.; Dodick, D.W. Post-traumatic headache: Epidemiology and pathophysiological insights. Nat. Rev. Neurol. 2019, 15, 607–617. [Google Scholar] [CrossRef]
- Blume, H.K. Posttraumatic headache in pediatrics: An update and review. Curr. Opin. Pediatr. 2018, 30, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.H.; Friedman, S.D.; Perez, F.A.; Ellenbogen, R.G.; Browd, S.R.; Mac Donald, C.L.; Vavilala, M.S.; Rivara, F.P. Prevalence of Abnormal Magnetic Resonance Imaging Findings in Children with Persistent Symptoms after Pediatric Sports-Related Concussion. J. Neurotrauma 2017, 34, 2706–2712. [Google Scholar] [CrossRef]
- Giza, C.C.; Choe, M.C. Diagnosis and Management of Acute Concussion. Semin. Neurol. 2015, 35, 029–041. [Google Scholar] [CrossRef]
- Abu-Arafeh, I.; Howells, R. Chronic post-traumatic headache in pediatrics. Pain Manag. 2014, 4, 303–308. [Google Scholar] [CrossRef]
- Kamins, J.; Richards, R.; Barney, B.J.; Locandro, C.; Pacchia, C.F.; Charles, A.C.; Cook, L.J.; Gioia, G.; Giza, C.C.; Blume, H.K. Evaluation of Posttraumatic Headache Phenotype and Recovery Time After Youth Concussion. JAMA Netw. Open 2021, 4, e211312. [Google Scholar] [CrossRef]
- Blume, H.K. Headaches after Concussion in Pediatrics: A Review. Curr. Pain Headache Rep. 2015, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Markus, T.E.; Zeharia, A.; Cohen, Y.H.; Konen, O. Persistent Headache and Cephalic Allodynia Attributed to Head Trauma in Children and Adolescents. J. Child Neurol. 2016, 31, 1213–1219. [Google Scholar] [CrossRef]
- Bonfert, M.; Ebinger, F.; Blankenburg, M.; Ertl-Wagner, B.; Heinen, F.; Roser, T. Primary versus Secondary Headache in Children: A Frequent Diagnostic Challenge in Clinical Routine. Neuropediatrics 2013, 44, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Blume, H.K.; Vavilala, M.S.; Jaffe, K.M.; Koepsell, T.D.; Wang, J.; Temkin, N.; Durbin, D.; Dorsch, A.; Rivara, F.P. Headache after Pediatric Traumatic Brain Injury: A Cohort Study. Pediatrics 2012, 129, e31–e39. [Google Scholar] [CrossRef]
- Moscato, D.; Peracchi, M.I.; Mazzotta, G.; Savi, L.; Battistella, P.A. Post–traumatic headache from moderate head injury. J. Headache Pain 2005, 6, 284–286. [Google Scholar] [CrossRef]
- Kirk, C.; Nagiub, G.; Abu-Arafeh, I. Chronic post-traumatic headache after head injury in children and adolescents. Dev. Med. Child Neurol. 2008, 50, 422–425. [Google Scholar] [CrossRef]
- Packard, R.C.; Ham, L.P. Pathogenesis of Posttraumatic Headache and Migraine: A Common Headache Pathway? Headache 1997, 37, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Babcock, L.; Byczkowski, T.; Wade, S.L.; Ho, M.; Mookerjee, S.; Bazarian, J.J. Predicting Postconcussion Syndrome After Mild Traumatic Brain Injury in Children and Adolescents Who Present to the Emergency Department. JAMA Pediatr. 2013, 167, 156–161. [Google Scholar] [CrossRef]
- Barlow, K.M.; Crawford, S.; Stevenson, A.; Sandhu, S.S.; Belanger, F.; Dewey, D. Epidemiology of postconcussion syndrome in pedi-atric mild traumatic brain injury. Pediatrics 2010, 126, e374–e381. [Google Scholar] [CrossRef]
- Raieli, V.; Eliseo, M.; Pandolfi, E.; La Vecchia, M.; La Franca, G.; Puma, D.; Ragusa, D. Recurrent and chronic headaches in children below 6 years of age. J. Headache Pain 2005, 6, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Lucas, S.; Dikmen, S.; Braden, C.A.; Brown, A.W.; Brunner, R.; Diaz-Arrastia, R.; Walker, W.C.; Watanabe, T.K.; Bell, K.R. Natural History of Headache after Traumatic Brain Injury. J. Neurotrauma 2011, 28, 1719–1725. [Google Scholar] [CrossRef]
- Nordhaug, L.H.; Linde, M.; Follestad, T.; Skandsen, N.; Bjarkø, V.V.; Skandsen, T.; Vik, A. Change in Headache Suffering and Predictors of Headache after Mild Traumatic Brain Injury: A Population-Based, Controlled, Longitudinal Study with Twelve-Month Follow-Up. J. Neurotrauma 2019, 36, 3244–3252. [Google Scholar] [CrossRef]
- Langdon, R.; Taraman, S. Posttraumatic Headache. Pediatr. Ann. 2018, 47, e61–e68. [Google Scholar] [CrossRef] [PubMed]
- Necajauskaite, O.; Endziniene, M.; Jurieniene, K. Prevalence, clinical features and accompanying signs of post-traumatic headache in children. Medicina 2005, 41, 100-8. [Google Scholar]
- De Kruijk, J.R.; Leffers, P.; Menheere, P.P.C.A.; Meerhoff, S.; Rutten, J.; Twijnstra, A. Prediction of post-traumatic complaints after mild traumatic brain injury: Early symptoms and biochemical markers. J. Neurol. Neurosurg. Psychiatry 2002, 73, 727–732. [Google Scholar] [CrossRef]
- Chan, T.L.H.; Woldeamanuel, Y.W. Exploring naturally occurring clinical subgroups of post-traumatic headache. J. Headache Pain 2020, 21, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mishra, D.; Pandey, P.N.; Juneja, M. Clinical profile and short-term course of post-traumatic headache in children with mild traumatic brain injury: A prospective cohort study. Child’s Nerv. Syst. 2021, 37, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.C.; Blume, H.K. Pediatric Posttraumatic Headache: A Review. J. Child Neurol. 2016, 31, 76–85. [Google Scholar] [CrossRef]
- Lee, M.J.; Zhou, Y.; Greenwald, B.D. Update on Non-Pharmacological Interventions for Treatment of Post-Traumatic Headache. Brain Sci. 2022, 12, 1357. [Google Scholar] [CrossRef]
- Standiford, L.; O’Daniel, M.; Hysell, M.; Trigger, C. A randomized cohort study of the efficacy of PO magnesium in the treatment of acute concussions in adolescents. Am. J. Emerg. Med. 2020, 44, 419–422. [Google Scholar] [CrossRef]
- Kallianezos, P.; Sinopidis, X.; Petropoulos, C.; Gkentzi, D.; Plotas, P.; Fouzas, S.; Karatza, A.; Jelastopulu, E. Anxiety and depression among parents of children with mild head injuries. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1530–1535. [Google Scholar] [CrossRef]
- Langer, L.K.; Bayley, M.T.; Lawrence, D.W.; Comper, P.; Kam, A.; Tam, A.; Saverino, C.; Wiseman-Hakes, C.; Ruttan, L.; Chandra, T.; et al. Revisiting the ICHD-3 criteria for headache attributed to mild traumatic injury to the head: Insights from the Toronto Concussion Study Analysis of Acute Headaches Following Concussion. Cephalalgia 2022, 42, 1172–1183. [Google Scholar] [CrossRef]
- Ledoux, A.-A.; Barrowman, N.; Bijelić, V.; Borghese, M.M.; Davis, A.; Reid, S.; Sangha, G.; Yeates, K.O.; Tremblay, M.S.; McGahern, C.; et al. Is early activity resumption after paediatric concussion safe and does it reduce symptom burden at 2 weeks post injury? The Pediatric Concussion Assessment of Rest and Exertion (PedCARE) multicentre randomised clinical trial. Br. J. Sports Med. 2021, 56, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Irwin, S.L.; Kacperski, J.; Rastogi, R.G. Pediatric Post-Traumatic Headache and Implications for Return to Sport: A Narrative Review. Headache 2020, 60, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
| Patients | n | 224 |
| Age | Mean ± SD | 6.7 ± 3.2 |
| Median | 6 | |
| Sex | M/F | 149/75 |
| HT characteristics, n (%) | ||
| mechanism | Car accident | 13 (5.8%) |
| High fall * | 13 (5.8%) | |
| Low fall ** | 122 (54.5%) | |
| Play/sports strike | 19 (8.5%) | |
| Head struck by object | 57 (25.5%) | |
| Location | Frontal | 95 (42.4%) |
| Non-frontal | 129 (57.6%) | |
| Symptoms and physical findings at PED, n (%) | ||
| Headache alone | 43 (19.2%) | |
| Headache associated with other symptoms and findings ° | 25 (11.2%) | |
| Visual disturbances | 8 (3.6%) | |
| Hematoma | 26 (11.6%) | |
| Swelling | 44 (19.6%) | |
| Soft tissue localized pain in the trauma site | 97 (43.3%) | |
| Altered mental status | 18 (8.0%) | |
| Amnesia | 6 (2.7%) | |
| Nausea and/or vomiting | 29 (12.9%) | |
| Instability or dizziness | 13 (5.8%) | |
| Glasgow Coma Scale ≥ 14 | 100% | |
| Head CT scan in PED, n (%) | 5 (2.2%) | |
| Time spent in PED, median (SD) | 2:26 h (2:28) | |
| Time between MHT and PED consultation, median (SD) | 22:57 h (7:27) | |
| Triage system priority, n (%) | High risk | 23 (10.3%) |
| Low risk | 201 (89.7%) |
| Characteristics | N (%) | |
|---|---|---|
| Risk assessment in MHT according to PECARN score | High risk | 3 (12.0%) |
| Low risk | 22 (88.0%) | |
| Mechanism in low risk MHT | Fall | 13 (59.1%) |
| Head struck | 7 (31.8%) | |
| Play/sports strike | 2 (9.1%) | |
| Location of MHT | Frontal | 14 (56.0%) |
| Others | 11 (44.0%) | |
| Physical findings * | Localized pain | 15 (60.0%) |
| Altered mental status | 1 (4.0%) | |
| Amnesia | 3 (12.0%) | |
| Nausea and/or vomiting | 5 (20.0%) | |
| Instability or dizziness | 2 (8.0%) | |
| PTH episodes occurrence ° | >1 per week | 15 (62.5%) |
| 1 per week | 8 (33.3%) | |
| <1 per week | 1 (4.2%) | |
| Duration of episodes ° | <3 h | 16 (66.7%) |
| >3 h | 3 (12.5%) | |
| Variable | 5 (20.8%) | |
| Time of the day ° | Afternoon | 9 (37.5%) |
| Morning | 2 (8.3%) | |
| Awakening | 5 (20.8%) | |
| Evening | 3 (12.5%) | |
| Variable | 5 (20.8%) | |
| Trigger °# | Physical exercise | 6 (25.0%) |
| Food ingestion | 1 (4.2%) | |
| None | 18 (75.0%) | |
| Pain medication ° | Ibuprofen or paracetamol | 15 (62.5%) |
| Not needed | 9 (37.5%) | |
| Characteristic | No PTH (n = 168) | PTH (n = 25) | p Value | RR | CI- 95% | |
|---|---|---|---|---|---|---|
| Sex, patients | Male (%) Female (%) | 117 (90.6) 51 (79.7) | 12 (9.4) 13 (20.3) | 0.055 * | ||
| Age | Schooler Preschooler | 89 (81) 79 (95.2) | 21 (19) 4 (4.8) | 0.0042 § | 4 | 1.4–11.4 |
| Female Schooler Female Preschooler | 21 (61.7) 30 (100) | 13 (38.2) 0 | <0.001 § | |||
| Location of MHT | Frontal Others | 96 (87.3) 72 (86.7) | 14 (12.7) 11 (13.2) | 0.914 § | ||
| Scalp edema in PED | Present Absent | 34 (86.8) 134 (87) | 5 (13.1) 20 (13) | 0.978 § | ||
| Hematoma | Present Absent | 21 (91) 147 (86.5) | 2 (9) 23 (13.5) | 0.744 § | ||
| Mechanism of MHT | High risk Low risk | 23 (88.5) 145 (86,8) | 3 (11.5) 22 (13.2) | 0.817 § | ||
| Head CT scan | Yes No | 4 (66) 164 (87.7) | 2 (33) 23 (12.3) | 0.131 § | ||
| Any headache in PED | Yes No | 28 (70) 140 (91.5) | 12 (30) 13 (8.5) | 0.0008 * | 3.5 | 1.7–7.1 |
| Headache in PED with other symptoms | Yes No | 41 (70) 127 (94) | 17 (30) 8 (6) | <0.00001 * | 4.9 | 2.7–10.8 |
| Headache and/or amnesia in PED | Yes No | 29 (67.4) 139 (92.6) | 14 (32.6) 11 (7.3) | 0.000044 * | 4.4 | 2.2–9.1 |
| Headache and/or nausea/vomiting in PED | Yes No | 34 (69.4) 134 (93) | 15 (30.6) 10 (7) | 0.000059 * | 4.4 | 2.1–9.2 |
| Headache and/or gait instability in PED | Yes No | 31 (68.8) 137 (92.6) | 14 (31.2) 11 (7.4) | 0.000101 * | 4.19 | 2–8.6 |
| Headache and/or LOC in PED | Yes No | 38 (73) 130 (92.2) | 14 (27) 11 (7.8) | 0.001082 * | 3.5 | 1.7–7.1 |
| LOC and/or amnesia and/or instability and/or nausea/vomiting in PED | Yes No | 23 (79.3) 145 (88.4) | 6 (20.7) 19 (11.6) | 0.296 § |
| Characteristic | Migraine | Tension-Type | |
|---|---|---|---|
| Sex, patients | Male Female | 1 5 | 9 9 |
| Age | Mean ± SD | 6.4 ± 3 | 7.9 ± 2.5 |
| Family history of headache | Yes No | 3 3 | 11 7 |
| Intensity | Mild Moderate Severe | 0 6 0 | 11 7 0 |
| Pain quality | Pulsating Continuous Gravative/burdensome Not defined | 3 3 0 0 | 1 10 6 1 |
| Location | Unilateral Bilateral Global Variable | 1 3 2 0 | 4 6 7 1 |
| Associated or prodromal symptoms * | Photophobia Phonophobia Photo- and Phonophobia Nausea or vomiting None | 5 4 0 0 1 | 3 4 0 1 11 |
| Coefficients | ODDs Ratio | Sign. | |
|---|---|---|---|
| Sex | −1.148 | 0.317 | 0.015 |
| Age | 0.089 | 1.093 | 0.235 |
| Location of MHT | −0.487 | 0.614 | 0.343 |
| Scalp edema in PED | −0.180 | 0.835 | 0.764 |
| Hematoma | −0.824 | 0.439 | 0.346 |
| Mechanism | 0.309 | 0.734 | 0.683 |
| Head CT scan | 0.337 | 1.400 | 0.804 |
| Any headache in PED | 1.956 | 7.073 | 0.001 |
| Loss of consciousness | −1.925 | 0.146 | 0.113 |
| Amnesia in PED | 1.844 | 6.320 | 0.136 |
| Nausea/vomiting in PED | −0.777 | 0.460 | 0.274 |
| Gait instability in PED | 0.286 | 1.331 | 0.774 |
| Constant | −0.370 | 0.691 | 0.887 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dondi, A.; Biserni, G.B.; Scarpini, S.; Fetta, A.; Moscano, F.; Corsini, I.; Borelli, G.; Cordelli, D.M.; Lanari, M. Post-Traumatic Headache in Children after Minor Head Trauma: Incidence, Phenotypes, and Risk Factors. Children 2023, 10, 534. https://doi.org/10.3390/children10030534
Dondi A, Biserni GB, Scarpini S, Fetta A, Moscano F, Corsini I, Borelli G, Cordelli DM, Lanari M. Post-Traumatic Headache in Children after Minor Head Trauma: Incidence, Phenotypes, and Risk Factors. Children. 2023; 10(3):534. https://doi.org/10.3390/children10030534
Chicago/Turabian StyleDondi, Arianna, Giovanni Battista Biserni, Sara Scarpini, Anna Fetta, Filomena Moscano, Ilaria Corsini, Greta Borelli, Duccio Maria Cordelli, and Marcello Lanari. 2023. "Post-Traumatic Headache in Children after Minor Head Trauma: Incidence, Phenotypes, and Risk Factors" Children 10, no. 3: 534. https://doi.org/10.3390/children10030534
APA StyleDondi, A., Biserni, G. B., Scarpini, S., Fetta, A., Moscano, F., Corsini, I., Borelli, G., Cordelli, D. M., & Lanari, M. (2023). Post-Traumatic Headache in Children after Minor Head Trauma: Incidence, Phenotypes, and Risk Factors. Children, 10(3), 534. https://doi.org/10.3390/children10030534








