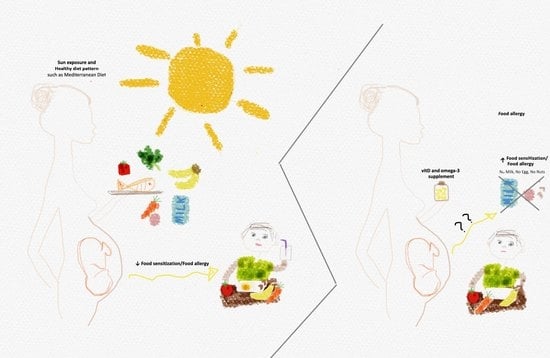
Vitamin D and Omega-3 (Fatty Acid) Supplementation in Pregnancy for the Primary Prevention of Food Allergy in Children-Literature Review
Abstract
1. Introduction
Literature Review Strategy and Methods
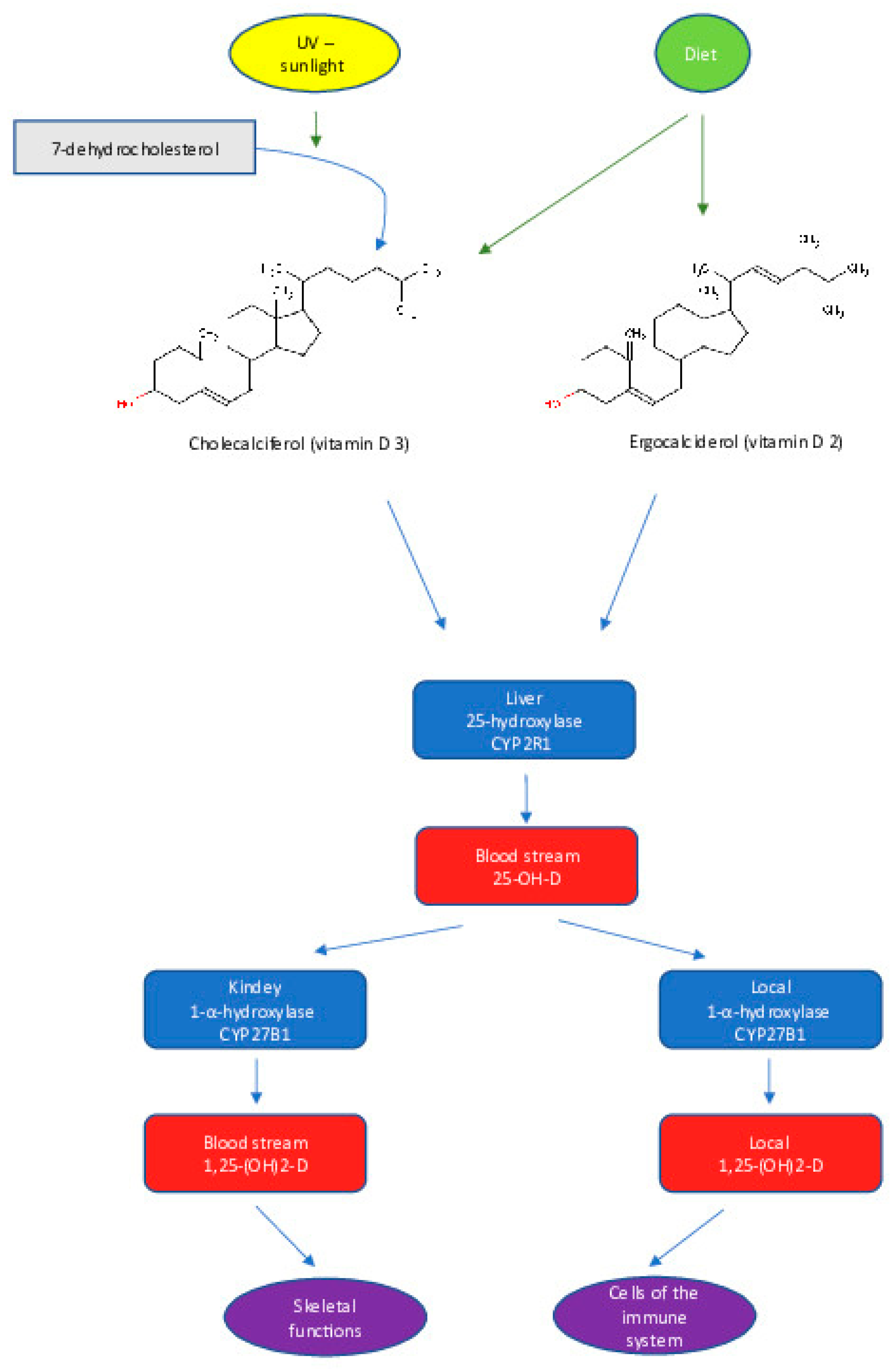
2. Vitamin D: Synthesis and Metabolism
3. Vitamin D: Biological Actions and Health Benefits
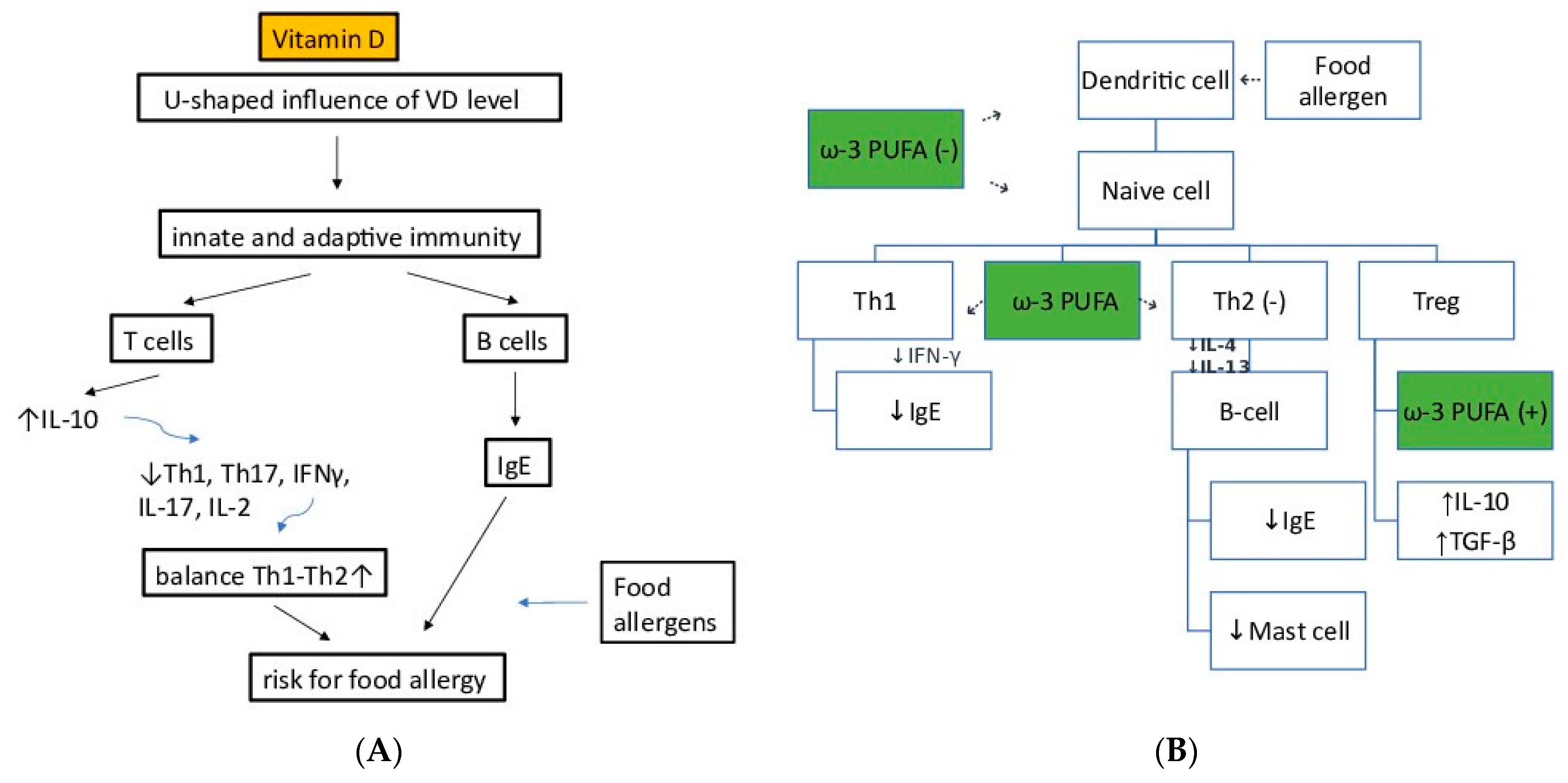
4. Vitamin D and Immune Function
5. Vitamin D: Sources
6. Vitamin D Supplementation in Pregnancy; Nutritional Benefits and/or Prevention of FA
| Authors, Country | Maternal Characteristics | Intervention (Nutrient, Concentration, etc.) | Period of Intervention | Follow-up Age | Food Allergy (FA) Outcomes in the Offspring | Other Allergy Outcomes in the Offspring |
|---|---|---|---|---|---|---|
| Vitamin D | ||||||
| Goldring et al., 2013 UK [61] | 180 pregnant women, at 27 weeks’ gestation, | Either no vitamin D, 800 IU ergocalciferol (D2) daily until delivery, or a single oral bolus of 200,000 IU cholecalciferol (D3) | April to November 2007. | 3 years | No significant difference reported in the offspring in food-specific IgE or “doctor-diagnosed FA” | No significant difference between groups of infants in ‘wheeze ever’, prevalence of eczema or atopy, baseline respiratory resistance, total IgE level, eNO or eosinophil count |
| Omega-3 | ||||||
| Dunstan et al., 2003 Australia [92] | 98 pregnant women, atopic, (i.e., offspring at high risk of allergic disease) nonsmoking, at 20 weeks’ gestation, | Either four 1-g fish oil capsules per day, comprising a total of 3.7 g of Ω-3 PUFAs, with 56.0% as docosahexaenoic acid (DHA) and 27.7% as eicosapentaenoic acid (EPA), or four 1-g capsules of olive oil per day, containing 66.6% n-9 oleic acid and < 1% Ω-3 PUFAs, as a placebo | 1999–2001 | 12 months | Infants in the fish oil group were three times less likely to be sensitized to egg allergen | Infants in the fish oil group were less likely to develop recurrent wheeze, persistent cough, diagnosed asthma, FA, angioedema, or anaphylaxis, but the differences were not statistically significant. |
| Furuhjelm et al., 2009 Sweden [93] | 145 pregnant women at 25 weeks’ gestation, with at least one family member having allergic symptoms (i.e., offspring at high risk of allergic disease) | Nine capsules a day containing Ω-3 PUFAs (35% EPA, 1.6 g/day and 25% DHA, 1.1 g/day), or soya bean oil (58% linoleic acid (LA), 2.5 g/day and 6% a-linolenic acid (LNA), 0.28 g/day) as a placebo | 2003–2005 | 12 months | FA significantly less frequent, and the risk of developing allergic sensitization to egg lower in the Ω-3 group | A lower period prevalence of IgE-associated eczema in the Ω-3 PUFAs group |
| Furuhjelm et al., 2011 Sweden [94] | 145 pregnant women at 25 weeks’ gestation, with at least one family member having allergic symptoms (i.e., offspring at high risk of allergic disease) | Nine capsules a day containing Ω-3 PUFAs (35% EPA, 1.6 g/day and 25% DHA, 1.1 g/day), or soya bean oil (58% linoleic acid (LA), 2.5 g/day and 6% a-linolenic acid (LNA), 0.28 g/day) as a placebo | 2003–2005, | 24 months | IgE-mediated food reactions, significantly less frequent and positive skin prick tests (SPTs) to food lower in the Ω-3 group | No difference between groups for “any asthma,” IgE-associated asthma, “any eczema,” “any rhino-conjunctivitis,” IgE-associated rhino-conjunctivitis Significant association between higher proportions of Ω-3 PUFAs in maternal and infant phospholipids and lower frequency and less severity of allergic diseases |
| Palmer et al., 2012 Australia [95] | 706 pregnant women at 21 weeks’ gestation | Three 500 mg capsules of fish oil concentrate daily, providing 800 mg of DHA and 100 mg of EPA, or three 500 mg vegetable oil capsules without Ω-3 PUFAs as a placebo. | 2005–2007 | 12 months | No significant difference in IgE-mediated FA between groups. The incidence of sensitization to egg was lower in the Ω-3 PUFA group. | The incidence of IgE-associated eczema was lower in the intervention group, although not to a significant degree |
| Palmer et al., 2013 Australia [96] | 706 pregnant women at 21 weeks’ gestation | Three 500 mg capsules of fish oil concentrate daily, providing 800 mg of DHA and 100 mg of EPA, or three 500 mg vegetable oil capsules without Ω-3 PUFAs as placebo | 2005–2007 | 3 years | No significant difference between groups in IgE-mediated. No difference between groups in sensitization to at least one allergen, including egg. | A lower, but not statistically significant, incidence of eczema with sensitization in the Ω-3 PUFAs group No significant reduction in IgE-associated allergic disease. |
| Best et al., 2018 Australia [97] | 706 pregnant women at 21 weeks’, | Three 500 mg capsules of fish oil concentrate daily, providing 800 mg of DHA and 100 mg of EPA, or three 500 mg vegetable oil capsules without Ω-3 PUFAs as placebo | 2005–2007 | 6 years | No significant difference between groups in the risk of sensitization to egg, peanut, cashew | No difference between groups in the risk of ‘any’ IgE mediated allergic disease or ‘individual’ IgE mediated allergic disease symptoms (eczema, rhinitis, rhino-conjunctivitis or wheeze) |
7. Omega3 Fatty Acids: Synthesis and Metabolism
8. Omega3 Fatty Acids: Biological Actions-Health Benefits
9. Omega3 Fatty Acids and Immune Function
10. Omega3 Fatty Acids: Dietary Sources
11. Omega3 Fatty Acid Supplementation during Pregnancy, and Food Allergy Prevention
12. Future Considerations
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Prim. 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.L.; Wake, M.; Tang, M.L.; Dharmage, S.C.; et al. Prevalence of challenge-proven ige-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011, 127, 668–676.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Burks, A.W.; Tang, M.; Sicherer, S.; Muraro, A.; Eigenmann, P.A.; Ebisawa, M.; Fiocchi, A.; Chiang, W.; Beyer, K.; Wood, R.; et al. ICON: Food allergy. J. Allergy Clin. Immunol. 2012, 129, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update-2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025.e1043. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the Diagnosis and Management of food allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Howe, L.; Franxman, T.; Teich, E.; Greenhawt, M. What affects quality of life among caregivers of food-allergic children? Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2014, 113, 69–74.e62. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The Public Health impact of parent-reported childhood food allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef]
- Spolidoro, G.C.I.; Tesfaye Amera, Y.; Ali, M.M.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Frequency of food allergy in Europe: An updated systematic review and meta-analysis. Allergy 2022, 78, 351–368. [Google Scholar] [CrossRef]
- Worm, M.; Reese, I.; Ballmer-Weber, B.; Beyer, K.; Bischoff, S.C.; Classen, M.; Fischer, P.J.; Fuchs, T.; Huttegger, I.; Jappe, U.; et al. Guidelines on the management of IgE-mediated food allergies: S2k-Guidelines of the German Society for Allergology and Clinical Immunology (DGAKI) in collaboration with the German Medical Association of Allergologists (AeDA), the German Professional Association of Pediatricians (BVKJ), the German Allergy and Asthma Association (DAAB), German Dermatological Society (DDG), the German Society for Nutrition (DGE), the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS), the German Society for Oto-Rhino-Laryngology, Head and Neck Surgery, the German Society for Pediatric and Adolescent Medicine (DGKJ), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Society for Pneumology (DGP), the German Society for Pediatric Gastroenterology and Nutrition (GPGE), German Contact Allergy Group (DKG), the Austrian Society for Allergology and Immunology (Æ-GAI), German Professional Association of Nutritional Sciences (VDOE) and the Association of the Scientific Medical Societies Germany (AWMF). Allergo J. Int. 2015, 24, 256–293. [Google Scholar] [CrossRef]
- Peters, R.L.; Mavoa, S. An overview of environmental risk factors for food allergy. Int. J. Environ. Res. Public Health 2022, 19, 722. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Furlong, T.J.; Maes, H.H.; Desnick, R.J.; Sampson, H.A.; Gelb, B.D. Genetics of peanut allergy: A twin study. J. Allergy Clin. Immunol. 2000, 106, 53–56. [Google Scholar] [CrossRef]
- Kusunoki, T.; Okafuji, I.; Yoshioka, T.; Saito, M.; Nishikomori, R.; Heike, T.; Sugai, M.; Shimizu, A.; Nakahata, T. SPINK5 polymorphism is associated with disease severity and food allergy in children with atopic dermatitis. J. Allergy Clin. Immunol. 2005, 115, 636–638. [Google Scholar] [CrossRef]
- Amoli, M.M.; Hand, S.; Hajeer, A.H.; Jones, K.P.; Rolf, S.; Sting, C.; Davies, B.H.; Ollier, W.E. Polymorphism in the STAT6 gene encodes risk for nut allergy. Genes Immun. 2002, 3, 220–224. [Google Scholar] [CrossRef]
- Hand, S.; Darke, C.; Thompson, J.; Stingl, C.; Rolf, S.; Jones, K.P.; Davies, B.H. Human leucocyte antigen polymorphisms in nut-allergic patients in South Wales. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2004, 34, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.H.; Ellis, J.A.; Saffery, R.; Allen, K.J. The role of genetics and environment in the rise of childhood food allergy. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2012, 42, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Palumbo, M.P. The maternal diet index in pregnancy is associated with offspring allergic diseases: The Healthy Start study. Allergy 2022, 77, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Sharief, S.; Jariwala, S.; Kumar, J.; Muntner, P.; Melamed, M.L. Vitamin D levels and food and environmental allergies in the United States: Results from the National Health and Nutrition Examination Survey 2005-2006. J. Allergy Clin. Immunol. 2011, 127, 1195–1202. [Google Scholar] [CrossRef]
- Pichler, J.; Gerstmayr, M.; Szépfalusi, Z.; Urbanek, R.; Peterlik, M.; Willheim, M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr. Res. 2002, 52, 12–18. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.Y.; Koenen, H.J.; Sweep, F.C.; Joosten, I.; Netea, M.G.; van der Ven, A.J. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin. Exp. Immunol. 2011, 164, 72–79. [Google Scholar] [CrossRef]
- Muthian, G.; Raikwar, H.P.; Rajasingh, J.; Bright, J.J. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res 2006, 83, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Chehade, M.; Mayer, L. Oral tolerance and its relation to food hypersensitivities. J. Allergy Clin. Immunol. 2005, 115, 3–12; quiz 13. [Google Scholar] [CrossRef] [PubMed]
- Dimeloe, S.; Nanzer, A.; Ryanna, K.; Hawrylowicz, C. Regulatory T cells, inflammation and the allergic response—The role of glucocorticoids and Vitamin D. J. Steroid Biochem. Mol. Biol. 2010, 120, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Burton, O.T.; Oettgen, H.C.; Chatila, T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J. Allergy Clin. Immunol. 2016, 138, 801–811.e809. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, Y.; O’Sullivan, M.; Pereira, G.; Loh, R.; Zhang, G.B. The Implications of DNA Methylation on Food Allergy. Int. Arch. Allergy Immunol. 2017, 173, 183–192. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Ahonen, S.; Kaila, M.; Erkkola, M.; Haapala, A.M.; Kronberg-Kippilä, C.; Veijola, R.; Ilonen, J.; Simell, O.; Knip, M.; et al. Maternal diet during pregnancy and allergic sensitization in the offspring by 5 yrs of age: A prospective cohort study. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2010, 21, 29–37. [Google Scholar] [CrossRef]
- Kostara, M.; Giapros, V.; Serbis, A.; Siomou, E.; Cholevas, V.; Rallis, D. Food allergy in children is associated with Vitamin D deficiency: A case-control study. Acta Paediatr. 2022, 111, 644–645. [Google Scholar] [CrossRef]
- Feketea, G.; Vlacha, V.; Tsiros, G.; Voila, P.; Pop, R.M.; Bocsan, I.C.; Stanciu, L.A.; Zdrenghea, M. Vitamin D levels in asymptomatic children and adolescents with atopy during the COVID-19 Era. J. Pers. Med. 2021, 11, 712. [Google Scholar] [CrossRef]
- van den Elsen, L.; Garssen, J.; Willemsen, L. Long chain N-3 polyunsaturated fatty acids in the prevention of allergic and cardiovascular disease. Curr. Pharm. Des. 2012, 18, 2375–2392. [Google Scholar] [CrossRef]
- Calvani, M.; Alessandri, C.; Sopo, S.M.; Panetta, V.; Pingitore, G.; Tripodi, S.; Zappalà, D.; Zicari, A.M. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: Role of maternal atopy. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2006, 17, 94–102. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zappulo, F.; Cappuccilli, M.; Cingolani, A.; Scrivo, A.; Chiocchini, A.L.C.; Nunzio, M.D.; Donadei, C.; Napoli, M.; Tondolo, F.; Cianciolo, G.; et al. Vitamin D and the kidney: Two players, one console. Int. J. Mol. Sci. 2022, 23, 9135. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Li, W.; Szczesniewski, A.; Tuckey, R.C. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005, 272, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 132–137. [Google Scholar] [CrossRef]
- Khammissa, R.A.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The biological activities of vitamin d and its receptor in relation to calcium and bone homeostasis, cancer, immune and cardiovascular systems, skin biology, and oral health. BioMed. Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. The function of vitamin D receptor in vitamin D action. J. Biochem. 2000, 127, 717–722. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; Vaes, A.M.M.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Swart, K.M.A.; Ham, A.C.; van Dijk, S.C.; Enneman, A.W.; Sohl, E.; van Schoor, N.M.; et al. Relative importance of summer sun exposure, vitamin D intake, and genes to vitamin D status in Dutch older adults: The B-PROOF study. J. Steroid Biochem. Mol. Biol. 2016, 164, 168–176. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brożyna, A.A.; Kim, T.-K.; Elsayed, M.M.; Janjetovic, Z.; Qayyum, S.; Slominski, R.M.; Oak, A.S.W.; Li, C.; Podgorska, E.; et al. CYP11A1-derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas. Int. J. Oncol. 2022, 61, 96. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Tuckey, R.C.; Li, W.; Raman, C.; Panich, U.; Slominski, A.T. CYP11A1-derived vitamin D(3) products protect against UVB-induced inflammation and promote keratinocytes differentiation. Free Radic. Biol. Med. 2020, 155, 87–98. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Rolf, L.; Muris, A.-H.; Hupperts, R.; Damoiseaux, J. Vitamin D effects on B cell function in autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Silalahi, E.R.; Wibowo, N.; Prasmusinto, D.; Djuwita, R.; Rengganis, I.; Mose, J.C. Decidual dendritic cells 10 and CD4(+)CD25(+)FOXP3 regulatory T cell in preeclampsia and their correlation with nutritional factors in pathomechanism of immune rejection in pregnancy. J. Reprod. Immunol. 2022, 154, 103746. [Google Scholar] [CrossRef] [PubMed]
- Erem, A.S.; Razzaque, M.S. Vitamin D-independent benefits of safe sunlight exposure. J. Steroid Biochem. Mol. Biol. 2021, 213, 105957. [Google Scholar] [CrossRef]
- Chen, B.; Jin, L. Low serum level of 25-OH vitamin D relates to Th17 and treg changes in colorectal cancer patients. Immun. Inflamm. Dis. 2022, 10, e723. [Google Scholar] [CrossRef]
- Mousa, A.; Misso, M.; Teede, H.; Scragg, R.; de Courten, B. Effect of vitamin D supplementation on inflammation: Protocol for a systematic review. BMJ Open 2016, 6, e010804. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Mathieu, C. 1α,25-Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: A comparison dose-titration study. J. Steroid Biochem. Mol. Biol. 2013, 136, 160–165. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Feketea, G.M.; Bocsan, I.C.; Tsiros, G.; Voila, P.; Stanciu, L.A.; Zdrenghea, M. Vitamin D status in children in Greece and its relationship with sunscreen application. Children 2021, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar] [CrossRef]
- Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Grossman, Z.; Hadjipanayis, A.; Stiris, T.; Del Torso, S.; Mercier, J.C.; Valiulis, A.; Shamir, R. Vitamin D in European children-statement from the European Academy of Paediatrics (EAP). Eur. J. Pediatr. 2017, 176, 829–831. [Google Scholar] [CrossRef]
- Saunders, C.M.; Rehbinder, E.M.; Lødrup Carlsen, K.C.; Gudbrandsgard, M.; Carlsen, K.-H.; Haugen, G.; Hedlin, G.; Monceyron Jonassen, C.; Dønvold Sjøborg, K.; Landrø, L.; et al. Food and nutrient intake and adherence to dietary recommendations during pregnancy: A Nordic mother–child population-based cohort. Food Nutr. Res. 2019, 63, 3676. [Google Scholar] [CrossRef]
- Shimizu, M.; Kato, T.; Adachi, Y.; Wada, T.; Murakami, S.; Ito, Y.; Itazawa, T.; Adachi, Y.S.; Tsuchida, A.; Matsumura, K.; et al. Association between maternal Vitamin D intake and infant allergies: The Japan environment and children’s study. J. Nutr. Sci. Vitaminol. 2022, 68, 375–382. [Google Scholar] [CrossRef]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L., 3rd; Reidy, K.C.; Catalano, P.M. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Vitamin D Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland.
- Goldring, S.T.; Griffiths, C.J.; Martineau, A.R.; Robinson, S.; Yu, C.; Poulton, S.; Kirkby, J.C.; Stocks, J.; Hooper, R.; Shaheen, S.O.; et al. Prenatal vitamin d supplementation and child respiratory health: A randomised controlled trial. PLoS ONE 2013, 8, e66627. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E., Jr.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of prenatal supplementation with Vitamin D on asthma or recurrent wheezing in offspring by age 3 years: The VDAART randomized clinical trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef]
- Chawes, B.L.; Bonnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadottir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdottir, S.; et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: A randomized clinical trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef]
- Anderson, L.N.; Chen, Y.; Omand, J.A.; Birken, C.S.; Parkin, P.C.; To, T.; Maguire, J.L. Vitamin D exposure during pregnancy, but not early childhood, is associated with risk of childhood wheezing. J. Dev. Orig. Health Dis. 2015, 6, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xun, P.; Pike, K.; Wills, A.K.; Chawes, B.L.; Bisgaard, H.; Cai, W.; Wan, Y.; He, K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J. Allergy Clin. Immunol. 2017, 139, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Eliasen, A.U.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H.; Chawes, B.L. High-Dose Vitamin D supplementation during pregnancy and asthma in offspring at the age of 6 years. JAMA 2019, 321, 1003–1005. [Google Scholar] [CrossRef]
- Litonjua, A.A. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 179–185. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- Best, C.M.; Xu, J.; Patchen, B.K.; Cassano, P.A. Vitamin D supplementation in pregnant or breastfeeding women or young children for preventing asthma. Cochrane Database Syst. Rev. 2019, 2019, CD013396. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo, C.A., Jr.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J. Allergy Clin. Immunol. 2016, 137, 1063–1070.e1062. [Google Scholar] [CrossRef]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Vassallo, M.F.; Banerji, A.; Rudders, S.A.; Clark, S.; Mullins, R.J.; Camargo, C.A. Season of birth and food allergy in children. Ann. Allergy Asthma Immunol. 2010, 104, 307–313. [Google Scholar] [CrossRef]
- Rudders, S.A.; Camargo, C.A., Jr. Sunlight, vitamin D and food allergy. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Feketea, G.; Konstantinou, G.N.; Zekakos Xypolias, D.; Valianatou, M.; Petrodimopoulou, M.; Vourga, V.; Tasios, I.; Papadopoulos, N.G. Food protein-induced allergic proctocolitis: The effect of maternal diet during pregnancy and breastfeeding in a Mediterranean population. Front. Nutr. 2022, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Tapsell, L.C. Food synergy: The key to a healthy diet. Proc. Nutr. Soc. 2013, 72, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.; Devereux, G.; Sheikh, A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011, 127, e721–e730. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Guibas, G.V.; Papadopoulos, N.G. Mediterranean-type diets as a protective factor for asthma and atopy. Nutrients 2022, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- Feketea, G.; Lakoumentas, J.; Konstantinou, G.N.; Douladiris, N.; Papadopoulos, N.G.; Petrodimopoulou, M.; Tasios, I.; Valianatou, M.; Vourga, V.; Vassilopoulou, E. Dietary factors may delay tolerance acquisition in food protein-induced allergic proctocolitis. Nutrients 2023, 15, 425. [Google Scholar] [CrossRef]
- CDC. Available online: https://www.cdc.gov/pregnancy/index.html (accessed on 20 December 2022).
- Chiu, C.Y.; Yao, T.C.; Chen, S.H.; Tsai, M.H.; Tu, Y.L.; Hua, M.C.; Yeh, K.W.; Huang, J.L. Low cord blood vitamin D levels are associated with increased milk sensitization in early childhood. Pediatr. Allergy Immunol. 2014, 25, 767–772. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Huang, S.Y.; Peng, Y.C.; Tsai, M.H.; Hua, M.C.; Yao, T.C.; Yeh, K.W.; Huang, J.L. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr. Allergy Immunol. 2015, 26, 337–343. [Google Scholar] [CrossRef]
- He, C.; Xiao, G.; Liu, S.; Hua, Z.; Wang, L.; Wang, N. A prospective cohort study of cord blood 25(OH)D3 and food allergies in 6-month-old Chinese infants. Asian Pac. J. Allergy Immunol. 2021, 39, 258–265. [Google Scholar] [CrossRef]
- Wang, N.R.; Liu, S.J.; Xiao, G.Y.; Zhang, H.; Huang, Y.J.; Wang, L.; He, C.Y. Cord blood 25(OH)D(3), cord blood total immunoglobulin E levels, and food allergies in infancy: A birth cohort study in Chongqing, China. World Allergy Organ J. 2022, 15, 100645. [Google Scholar] [CrossRef]
- Weisse, K.; Winkler, S.; Hirche, F.; Herberth, G.; Hinz, D.; Bauer, M.; Röder, S.; Rolle-Kampczyk, U.; von Bergen, M.; Olek, S.; et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 2013, 68, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Norizoe, C.; Akiyama, N.; Segawa, T.; Tachimoto, H.; Mezawa, H.; Ida, H.; Urashima, M. Increased food allergy and vitamin D: Randomized, double-blind, placebo-controlled trial. Pediatr. Int. 2014, 56, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Pelkonen, A.S.; Helve, O.; Hauta-Alus, H.; Holmlund-Suila, E.; Valkama, S.; Enlund-Cerullo, M.; Viljakainen, H.; Hytinantti, T.; Mäkitie, O.; et al. High-dose vitamin D supplementation does not prevent allergic sensitization of infants. J. Pediatr. 2019, 209, 139–145.e131. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.O.; Warner, J.A. The foetal origins of allergy and potential nutritional interventions to prevent disease. Nutrients 2022, 14, 1590. [Google Scholar] [CrossRef]
- Douros, K.; Loukou, I.; Tsabouri, S. More data are needed about vitamin D supplements in pregnancy and infancy including any impact on allergies. Acta Paediatr. 2020, 110, 753–754. [Google Scholar] [CrossRef]
- Tuokkola, J.; Luukkainen, P.; Kaila, M.; Takkinen, H.M.; Niinistö, S.; Veijola, R.; Virta, L.J.; Knip, M.; Simell, O.; Ilonen, J.; et al. Maternal dietary folate, folic acid and vitamin D intakes during pregnancy and lactation and the risk of cows’ milk allergy in the offspring. Br. J. Nutr. 2016, 116, 710–718. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Maternal consumption of dairy products, calcium, and vitamin D during pregnancy and infantile allergic disorders. Ann. Allergy Asthma Immunol. 2014, 113, 82–87. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef]
- Furuhjelm, C.; Warstedt, K.; Larsson, J.; Fredriksson, M.; Böttcher, M.F.; Fälth-Magnusson, K.; Duchén, K. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. 2009, 98, 1461–1467. [Google Scholar] [CrossRef]
- Furuhjelm, C.; Warstedt, K.; Fagerås, M.; Fälth-Magnusson, K.; Larsson, J.; Fredriksson, M.; Duchén, K. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2011, 22, 505–514. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: Randomised controlled trial. BMJ 2012, 344, e184. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013, 68, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Best, K.P.; Sullivan, T.R.; Palmer, D.J.; Gold, M.; Martin, J.; Kennedy, D.; Makrides, M. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—A longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ. J. 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Arguelles, L.; Zhou, Y.; Wang, G.; Chen, Q.; Tsai, H.J.; Hong, X.; Liu, R.; Price, H.E.; Pearson, C.; et al. Longitudinal trajectory of vitamin D status from birth to early childhood in the development of food sensitization. Pediatr. Res. 2013, 74, 321–326. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Understanding omega-3 polyunsaturated fatty acids. Postgrad. Med. 2009, 121, 148–157. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592s–599s. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Dietary alpha-linolenic acid and health-related outcomes: A metabolic perspective. Nutr. Res. Rev. 2006, 19, 26–52. [Google Scholar] [CrossRef]
- Birch, E.E.; Castañeda, Y.S.; Wheaton, D.H.; Birch, D.G.; Uauy, R.D.; Hoffman, D.R. Visual maturation of term infants fed long-chain polyunsaturated fatty acid–supplemented or control formula for 12 mo. Am. J. Clin. Nutr. 2005, 81, 871–879. [Google Scholar] [CrossRef]
- Farquharson, J.; Jamieson, E.C.; Abbasi, K.A.; Patrick, W.J.; Logan, R.W.; Cockburn, F. Effect of diet on the fatty acid composition of the major phospholipids of infant cerebral cortex. Arch. Dis. Child. 1995, 72, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Torrejon, C.; Tighe, A.P.; Deckelbaum, R.J. n−3 Fatty acids and cardiovascular disease: Mechanisms underlying beneficial effects. Am. J. Clin. Nutr. 2008, 87, 2003S–2009S. [Google Scholar] [CrossRef]
- Das, U.N. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: But, why and how? Prostaglandins Leukot Essent Fat. Acids 2000, 63, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.C. A study of the differential effects of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) on gene expression profiles of stimulated Thp-1 macrophages. Nutrients 2017, 9, 424. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Farjadian, S.; Moghtaderi, M.; Kalani, M.; Gholami, T.; Hosseini Teshnizi, S. Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine 2016, 85, 61–66. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, K.; Kim, K.H.; Kim, J.H.; Choi, J.S.; Shim, S.C. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PLoS ONE 2018, 13, e0194331. [Google Scholar] [CrossRef]
- Han, S.C.; Koo, D.H.; Kang, N.J.; Yoon, W.J.; Kang, G.J.; Kang, H.K.; Yoo, E.S. Docosahexaenoic acid alleviates atopic dermatitis by generating tregs and IL-10/TGF-β-modified macrophages via a TGF-β-dependent mechanism. J. Investig. Dermatol. 2015, 135, 1556–1564. [Google Scholar] [CrossRef]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. Nutr. 2014, 100, 443S–448S. [Google Scholar] [CrossRef]
- Takic, M.; Pokimica, B.; Petrovic-Oggiano, G.; Popovic, T. Effects of dietary α-linolenic acid treatment and the efficiency of its conversion to eicosapentaenoic and docosahexaenoic acids in obesity and related diseases. Molecules 2022, 27, 4471. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Fountoulaki, E.; Vasilaki, A.; Hurtado, R.; Grigorakis, K.; Karacostas, I.; Nengas, I.; Rigos, G.; Kotzamanis, Y.; Venou, B.; Alexis, M.N. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile: Recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures. Aquaculture 2009, 289, 317–326. [Google Scholar] [CrossRef]
- Ruiz-León, A.M.; Lapuente, M.; Estruch, R.; Casas, R. Clinical Advances in Immunonutrition and Atherosclerosis: A Review. Front. Immunol. 2019, 10, 837. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Molendi-Coste, O.; Legry, V.; Leclercq, I.A. Why and how meet n-3 PUFA dietary recommendations? Gastroenterol Res. Pract. 2011, 2011, 364040. [Google Scholar] [CrossRef]
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic Review on N-3 and N-6 polyunsaturated fatty acid intake in European Countries in light of the current recommendations—Focus on specific population groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, A.; Padrão, P.; Teixeira, V.H.; Carvalho, P.; Delgado, L.; Lopes, C.; Severo, M.; Moreira, P. Dietary patterns and asthma prevalence, incidence and control. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 1673–1680. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; García-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef]
- van den Elsen, L.W.; Bol-Schoenmakers, M.; van Esch, B.C.; Hofman, G.A.; van de Heijning, B.J.; Pieters, R.H.; Smit, J.J.; Garssen, J.; Willemsen, L.E. DHA-rich tuna oil effectively suppresses allergic symptoms in mice allergic to whey or peanut. J. Nutr. 2014, 144, 1970–1976. [Google Scholar] [CrossRef]
- Calder, P.C.; Krauss-Etschmann, S.; de Jong, E.C.; Dupont, C.; Frick, J.S.; Frokiaer, H.; Heinrich, J.; Garn, H.; Koletzko, S.; Lack, G.; et al. Early nutrition and immunity—Progress and perspectives. Br. J. Nutr. 2006, 96, 774–790. [Google Scholar]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef]
- Clausen, M.; Jonasson, K.; Keil, T.; Beyer, K.; Sigurdardottir, S.T. Fish oil in infancy protects against food allergy in Iceland-Results from a birth cohort study. Allergy 2018, 73, 1305–1312. [Google Scholar] [CrossRef]
- Best, K.P.; Gold, M.; Kennedy, D.; Martin, J.; Makrides, M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 128–143. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, B.; Li, J.; Luo, C.Q.; Zhang, Q.; Chen, J.L.; Sinha, A.; Li, Z.Y. Fish intake during pregnancy or infancy and allergic outcomes in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2017, 28, 152–161. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 fatty acid intake of pregnant women and women of childbearing age in the United States: Potential for deficiency? Nutrients 2017, 9, 197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feketea, G.; Kostara, M.; Bumbacea, R.S.; Vassilopoulou, E.; Tsabouri, S. Vitamin D and Omega-3 (Fatty Acid) Supplementation in Pregnancy for the Primary Prevention of Food Allergy in Children-Literature Review. Children 2023, 10, 468. https://doi.org/10.3390/children10030468
Feketea G, Kostara M, Bumbacea RS, Vassilopoulou E, Tsabouri S. Vitamin D and Omega-3 (Fatty Acid) Supplementation in Pregnancy for the Primary Prevention of Food Allergy in Children-Literature Review. Children. 2023; 10(3):468. https://doi.org/10.3390/children10030468
Chicago/Turabian StyleFeketea, Gavriela, Maria Kostara, Roxana Silvia Bumbacea, Emilia Vassilopoulou, and Sophia Tsabouri. 2023. "Vitamin D and Omega-3 (Fatty Acid) Supplementation in Pregnancy for the Primary Prevention of Food Allergy in Children-Literature Review" Children 10, no. 3: 468. https://doi.org/10.3390/children10030468
APA StyleFeketea, G., Kostara, M., Bumbacea, R. S., Vassilopoulou, E., & Tsabouri, S. (2023). Vitamin D and Omega-3 (Fatty Acid) Supplementation in Pregnancy for the Primary Prevention of Food Allergy in Children-Literature Review. Children, 10(3), 468. https://doi.org/10.3390/children10030468