Predictive Value of MRI in Hypoxic-Ischemic Encephalopathy Treated with Therapeutic Hypothermia
Abstract
1. Introduction
2. Materials and Methods
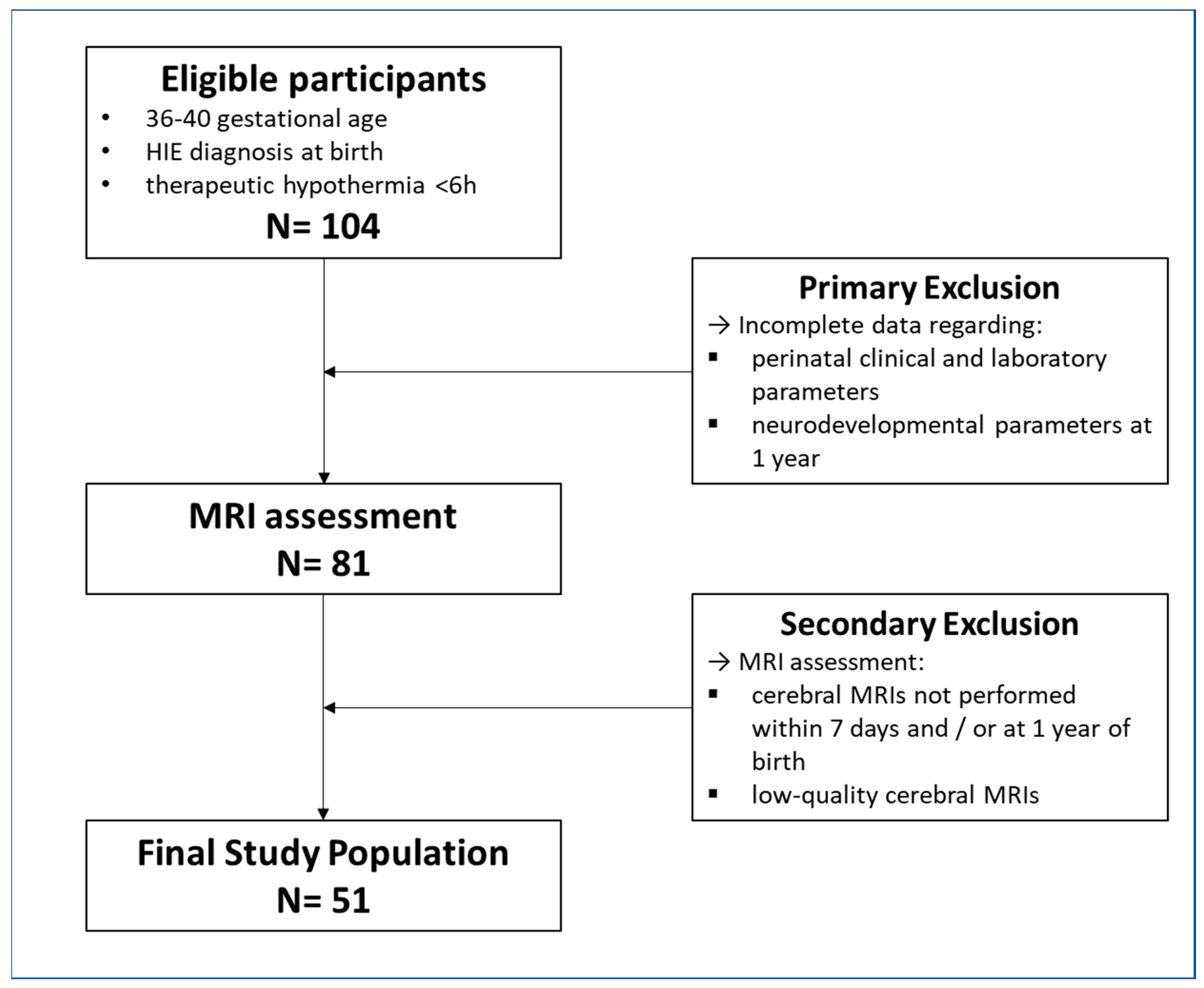
2.1. Participants
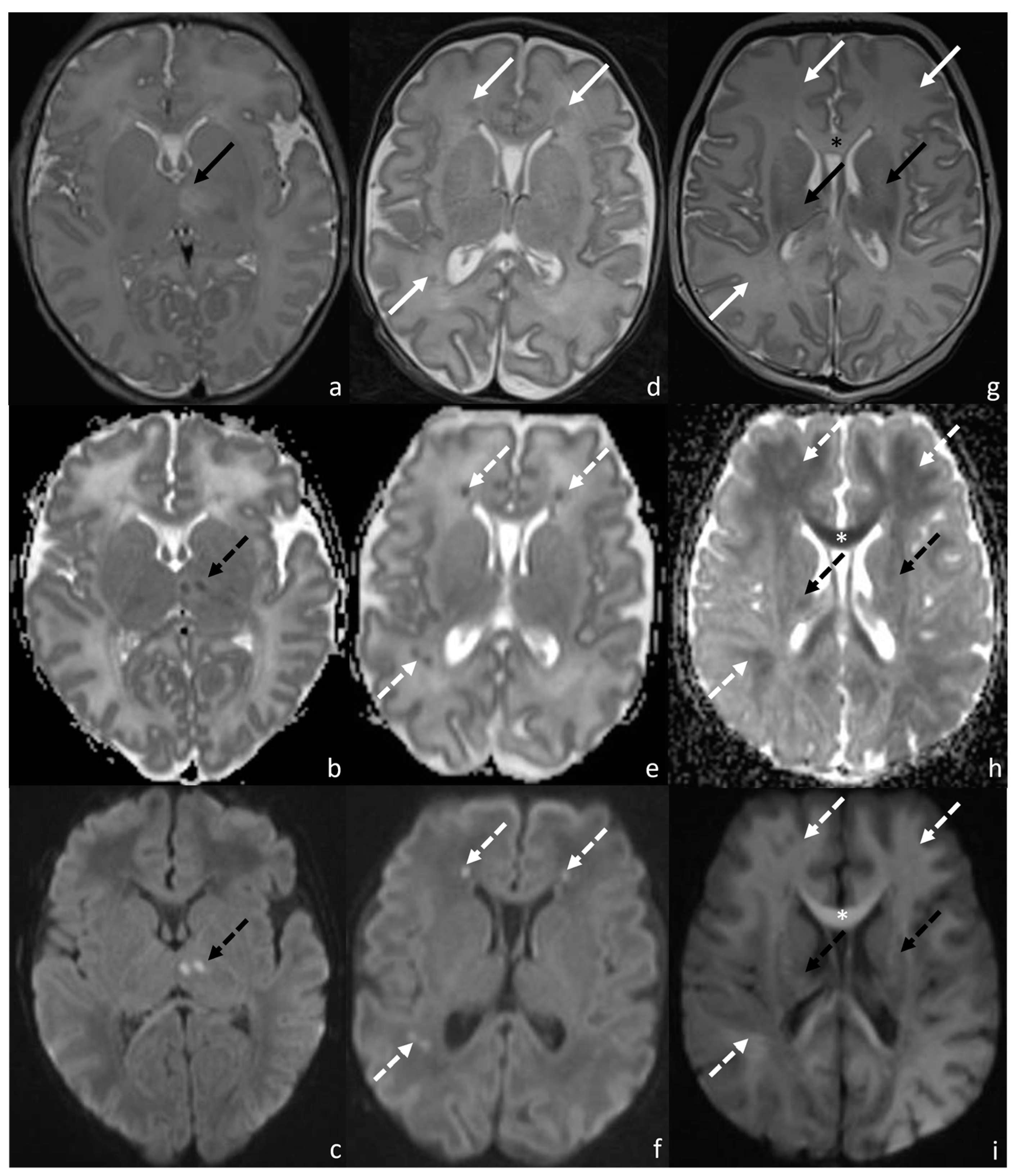
2.2. MRI Acquisition
2.3. Radiological Evaluation
2.3.1. Van Rooij Score
2.3.2. Measurement of ADC Values
2.4. Statistical Analysis
3. Results
3.1. Correlations between Perinatal and Outcome Parameters with Van Rooij Score at MRI0
3.2. Correlations between Perinatal and Outcome Parameters with ADC Values at MRI0 and ROC Analysis
3.3. Fluctuations in ADC Values between MRI0 and MRI1 and Their Correlation with Perinatal and Outcome Parameters
4. Discussion
4.1. Correlations between Perinatal and Outcome Parameters with Van Rooij Score and with ADC Values at MRI0, and ROC Analysis
4.2. Fluctuations in ADC Values between MRI0 and MRI1 and Their Correlation with Perinatal and Outcome Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- García-Alix, A.; Martínez-Biarge, M.; Diez, J.; Gayá, F.; Quero, J. [Neonatal hypoxic-ischemic encephalopathy: Incidence and prevalence in the first decade of the 21st century]. An. Pediatr. 2009, 71, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Levene, M.I.; Grindulis, H.; Sands, C.; Moore, J.R. Comparison of Two Methods of Predicting Outcome in Perinatal Asphyxia. Surv. Anesthesiol. 1986, 30, 286. [Google Scholar] [CrossRef]
- Vannucci, R.C. Current and Potentially New Management Strategies for Perinatal Hypoxic-Ischemic Encephalopathy. Pediatrics 1990, 85, 961–968. [Google Scholar] [CrossRef]
- Alderliesten, T.; de Vries, L.S.; Benders, M.J.N.L.; Koopman, C.; Groenendaal, F. MR imaging and Outcome of Term Neonates with Perinatal Asphyxia: Value of Diffusion-Weighted MR imaging and 1H MR Spectroscopy. Radiology 2011, 261, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B.; Sarnat, M.S. Neonatal Encephalopathy Following Fetal Distress. A Clinical and Electroencephalographic Study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef]
- Sakr, M.; Balasundaram, P. Neonatal Therapeutic Hypothermia; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Umamaheswari, B.; Amboiram, P.; Adhisivam, B.; Bhat, B.V. Therapeutic Hypothermia for Perinatal Asphyxia in India-Experience and Evidence. Indian J. Pediatr. 2022, 89, 804–811. [Google Scholar]
- Boichot, C.; Walker, P.M.; Durand, C.; Grimaldi, M.; Chapuis, S.; Gouyon, J.B.; Brunotte, F. Term Neonate Prognoses after Perinatal Asphyxia: Contributions of MR imaging, MR Spectroscopy, Relaxation Times, and Apparent Diffusion Coefficients. Radiology 2006, 239, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Liauw, L.; van Wezel-Meijler, G.; Veen, S.; van Buchem, M.A.; van der Grond, J. Do Apparent Diffusion Coefficient Measurements Predict Outcome in Children with Neonatal Hypoxic-Ischemic Encephalopathy? AJNR Am. J. Neuroradiol. 2009, 30, 264–270. [Google Scholar] [CrossRef]
- Rutherford, M.; Counsell, S.; Allsop, J.; Boardman, J.; Kapellou, O.; Larkman, D.; Hajnal, J.; Edwards, D.; Cowan, F. Diffusion-Weighted Magnetic Resonance imaging in Term Perinatal Brain Injury: A Comparison with Site of Lesion and Time from Birth. Pediatrics 2004, 114, 1004–1014. [Google Scholar] [CrossRef]
- Thayyil, S.; Chandrasekaran, M.; Taylor, A.; Bainbridge, A.; Cady, E.B.; Chong, W.K.K.; Murad, S.; Omar, R.Z.; Robertson, N.J. Cerebral Magnetic Resonance Biomarkers in Neonatal Encephalopathy: A Meta-Analysis. Pediatrics 2010, 125, e382–e395. [Google Scholar] [CrossRef]
- Hunt, R.W.; Neil, J.J.; Coleman, L.T.; Kean, M.J.; Inder, T.E. Apparent Diffusion Coefficient in the Posterior Limb of the Internal Capsule Predicts Outcome after Perinatal Asphyxia. Pediatrics 2004, 114, 999–1003. [Google Scholar] [CrossRef]
- Al Amrani, F.; Kwan, S.; Gilbert, G.; Saint-Martin, C.; Shevell, M.; Wintermark, P. Early imaging and Adverse Neurodevelopmental Outcome in Asphyxiated Newborns Treated With Hypothermia. Pediatr. Neurol. 2017, 73, 20–27. [Google Scholar] [CrossRef]
- Ouwehand, S.; Smidt, L.C.A.; Dudink, J.; Benders, M.J.N.L.; de Vries, L.S.; Groenendaal, F.; van der Aa, N.E. Predictors of Outcomes in Hypoxic-Ischemic Encephalopathy Following Hypothermia: A Meta-Analysis. Neonatology 2020, 117, 411–427. [Google Scholar] [CrossRef]
- Troha Gergeli, A.; Škofljanec, A.; Neubauer, D.; Paro Panjan, D.; Kodrič, J.; Osredkar, D. Prognostic Value of Various Diagnostic Methods for Long-Term Outcome of Newborns After Hypoxic-Ischemic Encephalopathy Treated With Hypothermia. Front. Pediatr. 2022, 10, 856615. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, L.G.M.; Toet, M.C.; van Huffelen, A.C.; Groenendaal, F.; Laan, W.; Zecic, A.; de Haan, T.; van Straaten, I.L.M.; Vrancken, S.; van Wezel, G.; et al. Effect of Treatment of Subclinical Neonatal Seizures Detected with aEEG: Randomized, Controlled Trial. Pediatrics 2010, 125, e358–e366. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development, Third Edition. PsycTESTS Dataset 2012. [Google Scholar] [CrossRef]
- van Kooij, B.J.M.; van Handel, M.; Nievelstein, R.A.J.; Groenendaal, F.; Jongmans, M.J.; de Vries, L.S. Serial MRI and Neurodevelopmental Outcome in 9- to 10-Year-Old Children with Neonatal Encephalopathy. J. Pediatr. 2010, 157, 221–227.e2. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.S.; de Vries, L.S.; Jongmans, M.J. Long-Term Outcome after Neonatal Hypoxic-Ischaemic Encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F220–F224. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Ritter, S.; Brotschi, B.; Werner, H.; Caflisch, J.; Martin, E.; Latal, B. Long-Term Neurodevelopmental Outcome with Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2013, 163, 454–459. [Google Scholar] [CrossRef] [PubMed]
- van Schie, P.E.M.; Schijns, J.; Becher, J.G.; Barkhof, F.; van Weissenbruch, M.M.; Jeroen Vermeulen, R. Long-Term Motor and Behavioral Outcome after Perinatal Hypoxic-Ischemic Encephalopathy. Eur. J. Paediatr. Neurol. 2015, 19, 354–359. [Google Scholar] [CrossRef]
- McQuillen, P.S.; Ferriero, D.M. Selective Vulnerability in the Developing Central Nervous System. Pediatr. Neurol. 2004, 30, 227–235. [Google Scholar] [CrossRef]
- Imai, K.; de Vries, L.S.; Alderliesten, T.; Wagenaar, N.; van der Aa, N.E.; Lequin, M.H.; Benders, M.J.N.L.; van Haastert, I.C.; Groenendaal, F. MRI Changes in the Thalamus and Basal Ganglia of Full-Term Neonates with Perinatal Asphyxia. Neonatology 2018, 114, 253–260. [Google Scholar] [CrossRef]
- Herrero, M.-T.; Barcia, C.; Navarro, J. Functional Anatomy of Thalamus and Basal Ganglia. Child’s Nerv. Syst. 2002, 18, 386–404. [Google Scholar] [CrossRef]
- McKinstry, R.C.; Miller, J.H.; Snyder, A.Z.; Mathur, A.; Schefft, G.L.; Almli, C.R.; Shimony, J.S.; Shiran, S.I.; Neil, J.J. A Prospective, Longitudinal Diffusion Tensor imaging Study of Brain Injury in Newborns. Neurology 2002, 59, 824–833. [Google Scholar] [CrossRef]
- Cao, Z.; Harvey, S.S.; Bliss, T.M.; Cheng, M.Y.; Steinberg, G.K. Inflammatory Responses in the Secondary Thalamic Injury After Cortical Ischemic Stroke. Front. Neurol. 2020, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Necula, D.; Cho, F.S.; He, A.; Paz, J.T. Secondary Thalamic Neuroinflammation after Focal Cortical Stroke and Traumatic Injury Mirrors Corticothalamic Functional Connectivity. J. Comp. Neurol. 2022, 530, 998–1019. [Google Scholar] [CrossRef] [PubMed]
- Hooks, B.M.; Mao, T.; Gutnisky, D.A.; Yamawaki, N.; Svoboda, K.; Shepherd, G.M.G. Organization of Cortical and Thalamic Input to Pyramidal Neurons in Mouse Motor Cortex. J. Neurosci. 2013, 33, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Okoro, S.U.; Goz, R.U.; Njeri, B.W.; Harish, M.; Ruff, C.F.; Ross, S.E.; Gerfen, C.; Hooks, B.M. Organization of Cortical and Thalamic Input to Inhibitory Neurons in Mouse Motor Cortex. J. Neurosci. 2022, 42, 8095–8112. [Google Scholar] [CrossRef]
- de Leon, A.S.; Das, J. Neuroanatomy, Dentate Nucleus; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bond, K.M.; Brinjikji, W.; Eckel, L.J.; Kallmes, D.F.; McDonald, R.J.; Carr, C.M. Dentate Update: Imaging Features of Entities That Affect the Dentate Nucleus. AJNR Am. J. Neuroradiol. 2017, 38, 1467–1474. [Google Scholar] [CrossRef]
- Bostan, A.C.; Strick, P.L. The Basal Ganglia and the Cerebellum: Nodes in an Integrated Network. Nat. Rev. Neurosci. 2018, 19, 338–350. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. An Unfolded Map of the Cerebellar Dentate Nucleus and Its Projections to the Cerebral Cortex. J. Neurophysiol. 2003, 89, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Pehere, N.K.; Narasaiah, A.; Dutton, G.N. Cerebral Visual Impairment Is a Major Cause of Profound Visual Impairment in Children Aged Less than 3 Years: A Study from Tertiary Eye Care Center in South India. Indian J. Ophthalmol. 2019, 67, 1544–1547. [Google Scholar] [CrossRef]
- Tian, Q.; Pan, Y.; Zhang, Z.; Li, M.; Xu, L.-X.; Gong, M.; Miao, P.; Jiang, X.; Yang, X.; Feng, C.-X.; et al. Predictive Value of Early Amplitude Integrated Electroencephalogram (aEEG) in Sleep Related Problems in Children with Perinatal Hypoxic-Ischemia (HIE). BMC Pediatr. 2021, 21, 410. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Rubens, E.O.; Yap, V.L.; Ross, G.; Engel, M.; Perlman, J.M. Delayed Onset of Sleep-Wake Cycling with Favorable Outcome in Hypothermic-Treated Neonates with Encephalopathy. J. Pediatr. 2011, 159, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cheng, Z.; Sun, B.; Huang, J.; Wang, L.; Han, X.; Yang, Y.; Xu, W.; Cao, X.; Miao, P.; et al. Distinctive Sleep Problems in Children with Perinatal Moderate or Mild Hypoxic-Ischemia. Neurosci. Lett. 2016, 614, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, E.; Mercuri, E.; Rutherford, M.; Cowan, F.; Azzopardi, D.; Frisone, M.F.; Cioni, G.; Dubowitz, L. Combined Use of Electroencephalogram and Magnetic Resonance imaging in Full-Term Neonates with Acute Encephalopathy. Pediatrics 2001, 107, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Ginet, V.; Pittet, M.P.; Rummel, C.; Osterheld, M.C.; Meuli, R.; Clarke, P.G.H.; Puyal, J.; Truttmann, A.C. Dying Neurons in Thalamus of Asphyxiated Term Newborns and Rats Are Autophagic. Ann. Neurol. 2014, 76, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, N.; Mathur, A.; Inder, T.; Wilkinson, J.; Neil, J.; Shimony, J. Impact of Therapeutic Hypothermia on MRI Diffusion Changes in Neonatal Encephalopathy. Neurology 2012, 78, 1420–1427. [Google Scholar] [CrossRef]
- Shibasaki, J.; Niwa, T.; Piedvache, A.; Tomiyasu, M.; Morisaki, N.; Fujii, Y.; Toyoshima, K.; Aida, N. Comparison of Predictive Values of Magnetic Resonance Biomarkers Based on Scan Timing in Neonatal Encephalopathy Following Therapeutic Hypothermia. J. Pediatr. 2021, 239, 101–109.e4. [Google Scholar] [CrossRef] [PubMed]
- Twomey, E.; Twomey, A.; Ryan, S.; Murphy, J.; Donoghue, V.B. MR imaging of Term Infants with Hypoxic-Ischaemic Encephalopathy as a Predictor of Neurodevelopmental Outcome and Late MRI Appearances. Pediatr. Radiol. 2010, 40, 1526–1535. [Google Scholar] [CrossRef]
- Sarioglu, F.C.; Sarioglu, O.; Guleryuz, H.; Deliloglu, B.; Tuzun, F.; Duman, N.; Ozkan, H. The Role of MRI-Based Texture Analysis to Predict the Severity of Brain Injury in Neonates with Perinatal Asphyxia. Br. J. Radiol. 2022, 95, 20210128. [Google Scholar] [CrossRef] [PubMed]
- Heursen, E.-M.; Zuazo Ojeda, A.; Benavente Fernández, I.; Jimenez Gómez, G.; Campuzano Fernández-Colima, R.; Paz-Expósito, J.; Lubián López, S.P. Prognostic Value of the Apparent Diffusion Coefficient in Newborns with Hypoxic-Ischaemic Encephalopathy Treated with Therapeutic Hypothermia. Neonatology 2017, 112, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Fernández, I.; Morales-Quezada, J.L.; Law, S.; Kim, P. Prognostic Value of Brain Magnetic Resonance Imaging in Neonatal Hypoxic-Ischemic Encephalopathy: A Meta-Analysis. J. Child Neurol. 2017, 32, 1065–1073. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Patients |
|---|---|
| N° of subjects | 51 |
| N° F/M | 18/33 |
| Eutocic/Dystocic delivery | 23/28 |
| Apgar 10 min < 5/≥5 | 23/28 |
| Blood lactate < 3.9/≥3.9 | 31/20 |
| Base Excess < −12/≥−12 | 35/16 |
| Umbilical cord pH < 7/≥7 | 18/33 |
| Cardiac Massage Y/N | 18/33 |
| Mechanical Ventilation Y/N | 18/33 |
| RDS | 17/34 |
| Bayley III—motion impairment < 85 at 1 year Y/N | 6/45 |
| Sleep Disorders at 1 year Y/N | 6/45 |
| Physiokinesitherapy at 1 year Y/N | 7/44 |
| Results | ||
| Correlations between the van Rooij score at MRI0 and perinatal/outcome parameters | ||
| Perinatal Parameters | Outcome Parameters | |
| - | sleep disorders (p < 0.0014) | |
| Correlations between perinatal and outcome parameters with ADC values at MRI0 and perinatal/outcome parameters | ||
| Brain region | Perinatal Parameters | Outcome Parameters |
| left thalamus | pH <7 (p < 0.026) BE < −12 mmol/L (p < 0.025) lactate <3.9 (p < 0.029) RDS (p < 0.041) cardiac massage (p < 0.032) | sleep disorders (p < 0.026) motor impairment (p < 0.015) physiokinesitherapy (p < 0.044) |
| right thalamus | lactate <3.9 (p < 0.004) | sleep disorders (p < 0.041) motor impairment (p < 0.021) |
| right visual cortex | BE < −12 mmol/L (p < 0.011) cardiac massage (p < 0.047) | - |
| left frontal white matter | BE < −12 mmol/L (p < 0.041) RDS (p < 0.038) cardiac massage (p < 0.043) | sleep disorders (p < 0.022) motor impairment (p < 0.014) |
| right frontal white matter | RDS (p < 0.026) cardiac massage (p < 0.039) | motor impairment (p < 0.046) |
| left dentate nucleus | - | motor impairment (p < 0.028) |
| Fluctuations in ADC values between MRI0 and MRI1, and correlation with perinatal and outcome parameters | ||
| Brain region | Perinatal Parameters | Outcome Parameters |
| right visual cortex | Dystocic birth (p < 0.001) Apgar10 <5 (p < 0.001) pHuc <7 (p < 0.001) BE < −12 mmol/L (p < 0.001) RDS (p < 0.001) mechanical ventilation (p < 0.001) cardiac massage (p < 0,0) | sleep disorders (p < 0.001) motor impairment (p < 0.007) physiokinesitherapy (p < 0.001) |
| left dentate nuclei | Dystocic birth (p < 0.001) Apgar10 (p < 0.001) pHuc < 7 (p < 0.001) BE < −12 mmol/L (p < 0.001) RDS (p < 0.001) mechanical ventilation (p < 0.001) cardiac massage (p < 0.001) | sleep disorders (p < 0.009) motor impairment (p < 0.007) physiokinesitherapy (p < 0.002) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnera, A.; Lucignani, G.; Parrillo, C.; Rossi-Espagnet, M.C.; Carducci, C.; Moltoni, G.; Savarese, I.; Campi, F.; Dotta, A.; Milo, F.; et al. Predictive Value of MRI in Hypoxic-Ischemic Encephalopathy Treated with Therapeutic Hypothermia. Children 2023, 10, 446. https://doi.org/10.3390/children10030446
Guarnera A, Lucignani G, Parrillo C, Rossi-Espagnet MC, Carducci C, Moltoni G, Savarese I, Campi F, Dotta A, Milo F, et al. Predictive Value of MRI in Hypoxic-Ischemic Encephalopathy Treated with Therapeutic Hypothermia. Children. 2023; 10(3):446. https://doi.org/10.3390/children10030446
Chicago/Turabian StyleGuarnera, Alessia, Giulia Lucignani, Chiara Parrillo, Maria Camilla Rossi-Espagnet, Chiara Carducci, Giulia Moltoni, Immacolata Savarese, Francesca Campi, Andrea Dotta, Francesco Milo, and et al. 2023. "Predictive Value of MRI in Hypoxic-Ischemic Encephalopathy Treated with Therapeutic Hypothermia" Children 10, no. 3: 446. https://doi.org/10.3390/children10030446
APA StyleGuarnera, A., Lucignani, G., Parrillo, C., Rossi-Espagnet, M. C., Carducci, C., Moltoni, G., Savarese, I., Campi, F., Dotta, A., Milo, F., Cappelletti, S., Capitello Grimaldi, T., Gandolfo, C., Napolitano, A., & Longo, D. (2023). Predictive Value of MRI in Hypoxic-Ischemic Encephalopathy Treated with Therapeutic Hypothermia. Children, 10(3), 446. https://doi.org/10.3390/children10030446








