Safety and Efficacy of Stand-Alone Bioactive Glass Injectable Putty or Granules in Posterior Vertebral Fusion for Adolescent Idiopathic and Non-Idiopathic Scoliosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
- -
- paediatric patient < 20 years old (AIS or NS);
- -
- scoliosis requiring posterior fusion posterior instrumentation;
- -
- use of bioactive glass (Glassbone Granules or Glassbone Injectable Putty, NORAKER, Lyon-France) as adjuvant fusion;
- -
- minimum of 2 years of follow-up.
- -
- The exclusion criteria were as follows:
- -
- surgical revision;
- -
- patient opposition to data collection.
2.2. Surgical Technique
2.3. Outcomes of Interest
2.4. Statistical Analysis
3. Results
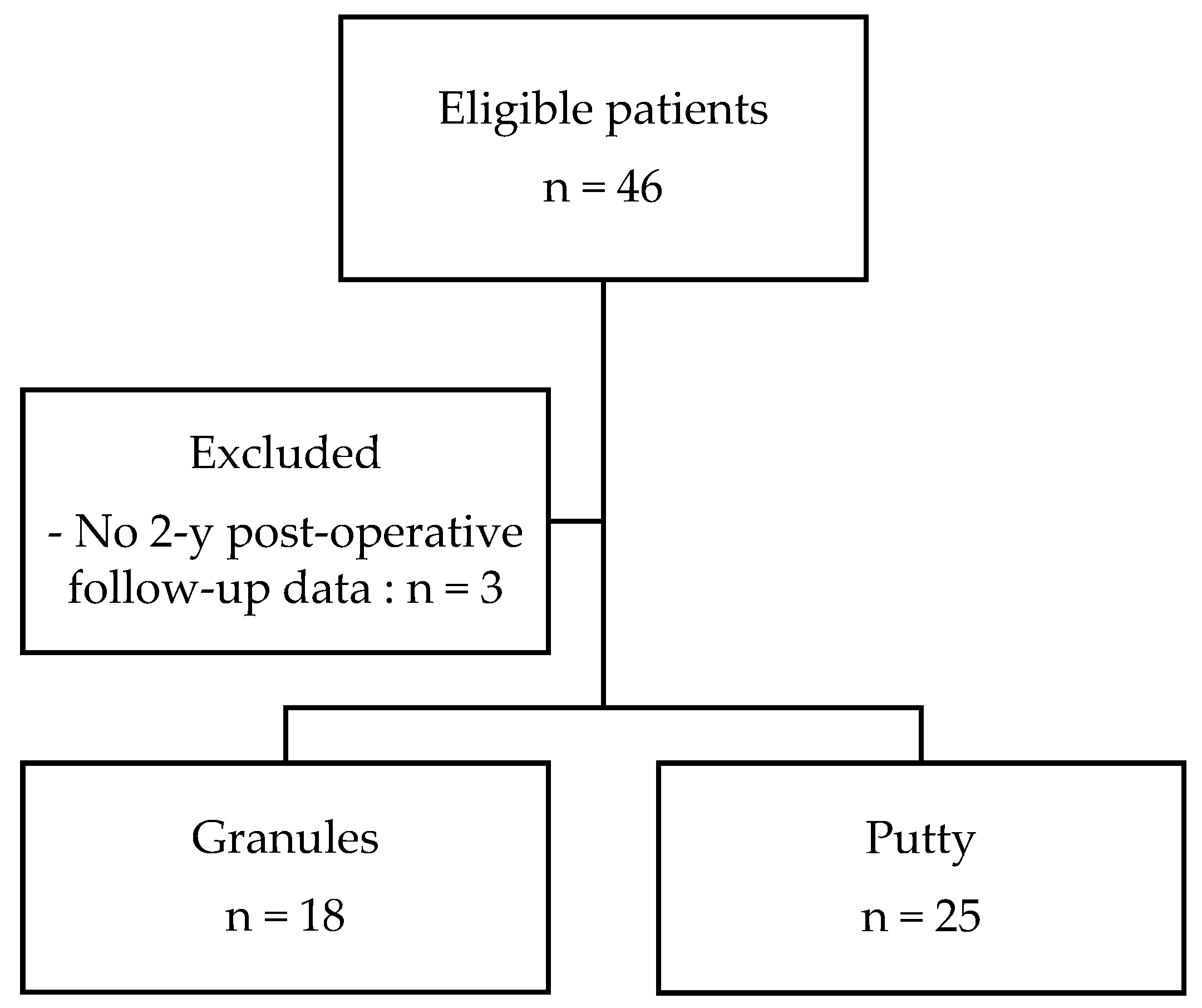
3.1. Patient Selection and Demographic Data
3.2. Peri-Operative Data
3.3. Safety
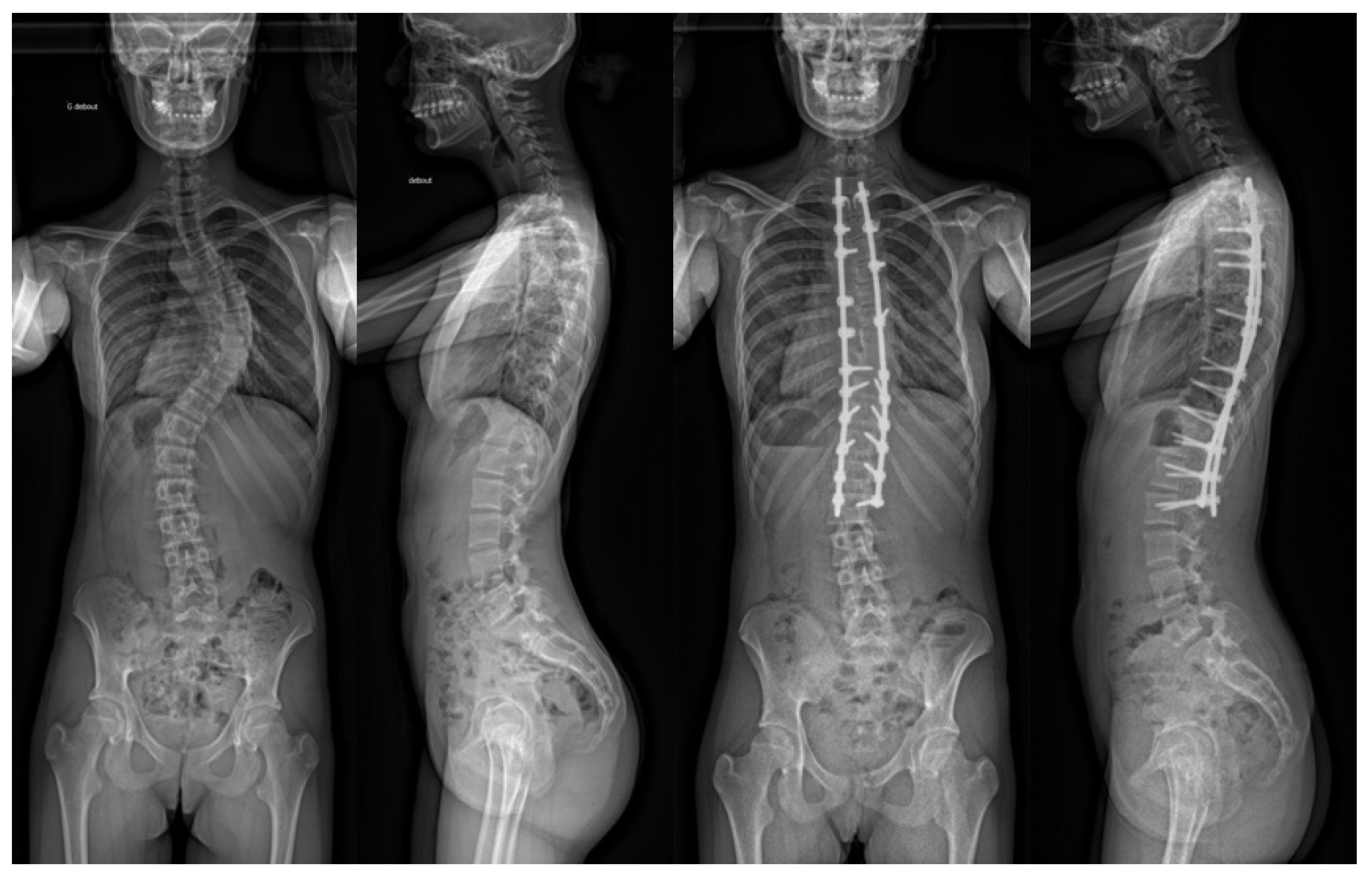
3.4. Radiographic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Hawary, H.; Shawky, M. Assessment Of The Sticky Bone Preparation Of BioActive bone Glass in Grafting Critical-Sized Surgical Bony Defects. Egypt. Dent. J. 2021, 67, 1899–1908. [Google Scholar] [CrossRef]
- Crawford, C.H., 3rd; Carreon, L.Y.; Lenke, L.G.; Sucato, D.J.; Richards, B.S., 3rd. Outcomes Following Posterior Fusion for Adolescent Idiopathic Scoliosis With and Without Autogenous Iliac Crest Bone Graft Harvesting. Spine Deform. 2013, 1, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Harshavardhana, N.S.; Noordeen, M.H. Surgical results with the use of Silicated Calcium Phosphate (SiCaP) as bone graft substitute in Posterior Spinal Fusion (PSF) for Adolescent Idiopathic Scoliosis (AIS). Scoliosis 2015, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Kirzner, N.; Hilliard, L.; Martin, C.; Quan, G.; Liew, S.; Humadi, A. Bone graft in posterior spine fusion for adolescent idiopathic scoliosis: A meta-analysis. ANZ J. Surg. 2018, 88, 1247–1252. [Google Scholar] [CrossRef]
- Courvoisier, A.; Ahmad, E.; Griffet, J. Minimally Invasive System for Dynamic Correction of a Spinal Deformity. U.S. Patent No. 10,426,519, 1 October 2019. [Google Scholar]
- Yataganbaba, A.; Gahukamble, A.; Antoniou, G.; Freeman, B.J.C.; Cundy, P.J. Local Bone Grafting Is Sufficient for Instrumented Adolescent Idiopathic Scoliosis Surgery: A Preliminary Study. J. Pediatr. Orthop. 2021, 41, e641–e645. [Google Scholar] [CrossRef]
- Price, C.T.; Connolly, J.F.; Carantzas, A.C.; Ilyas, I. Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine 2003, 28, 793–798. [Google Scholar] [CrossRef]
- Crostelli, M.; Mazza, O.; Mariani, M.; Mascello, D.; Iorio, C. Adolescent idiopathic scoliosis correction by instrumented vertebral arthrodesis with autologous bone graft from local harvesting without bone substitute use: Results with mean 3 year follow-up. Eur. Spine J. 2018, 27, 175–181. [Google Scholar] [CrossRef]
- Ilharreborde, B.; Morel, E.; Fitoussi, F.; Presedo, A.; Souchet, P.; Penneçot, G.F.; Mazda, K. Bioactive glass as a bone substitute for spinal fusion in adolescent idiopathic scoliosis: A comparative study with iliac crest autograft. J. Pediatr. Orthop. 2008, 28, 347–351. [Google Scholar] [CrossRef]
- Ameri, E.; Behtash, H.; Mobini, B.; Omidi-Kashani, F.; Nojomi, M. Bioactive Glass versus Autogenous Iliac Crest Bone Graft in Adolescent Idiopathic Scoliosis Surgery. Acta Med. Iran. 2009, 47, 41–45. [Google Scholar]
- Pesenti, S.; Ghailane, S.; Varghese, J.J.; Ollivier, M.; Peltier, E.; Choufani, E.; Bollini, G.; Blondel, B.; Jouve, J.L. Bone substitutes in adolescent idiopathic scoliosis surgery using sublaminar bands: Is it useful? A case-control study. Int. Orthop. 2017, 41, 2083–2090. [Google Scholar] [CrossRef]
- Van Dijk, L.A.; Barrère-de Groot, F.; Rosenberg, A.; Pelletier, M.; Christou, C.; de Bruijn, J.D.; Walsh, W.R. MagnetOs, Vitoss, and Novabone in a Multi-endpoint Study of Posterolateral Fusion: A True Fusion or Not? Clin. Spine Surg. 2020, 33, E276–E287. [Google Scholar] [CrossRef] [PubMed]
- Delécrin, J.; Takahashi, S.; Gouin, F.; Passuti, N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: A prospective, randomized study. Spine 2000, 25, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.E.; Mesregah, M.K.; Fresquez, Z.; Stanton, E.W.; Buser, Z.; Wang, J.C. Use of graft materials and biologics in spine deformity surgery: A state-of-the-art review. Spine Deform. 2022, 10, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Isik, M.; Ozdemir, H.M.; Sakaogullari, A.; Cengiz, B.; Aydogan, N.H. The efficacy of in situ local autograft in adolescent idiopathic scoliosis surgery: A comparison of three different grafting methods. Turk. J. Med. Sci. 2017, 47, 1728–1735. [Google Scholar] [CrossRef]
- Remes, V.; Helenius, I.; Schlenzka, D.; Yrjonen, T.; Ylikoski, M.; Poussa, M. Cotrel-Dubousset (CD) or Universal Spine System (USS) instrumentation in adolescent idiopathic scoliosis (AIS): Comparison of midterm clinical, functional, and radiologic outcomes. Spine 2004, 29, 2024–2030. [Google Scholar] [CrossRef]
- Kalifa, G.; Charpak, Y.; Maccia, C.; Fery-Lemonnier, E.; Bloch, J.; Boussard, J.M.; Attal, M.; Dubousset, J.; Adamsbaum, C. Evaluation of a new low-dose digital x-ray device: First dosimetric and clinical results in children. Pediatr. Radiol. 1998, 28, 557–561. [Google Scholar] [CrossRef]
- Dubousset, J.; Charpak, G.; Skalli, W.; Kalifa, G.; Lazennec, J.Y. EOS stereo-radiography system: Whole-body simultaneous anteroposterior and lateral radiographs with very low radiation dose. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2007, 93, 141–143. [Google Scholar] [CrossRef]
- Humbert, L.; De Guise, J.A.; Aubert, B.; Godbout, B.; Skalli, W. 3D reconstruction of the spine from biplanar X-rays using parametric models based on transversal and longitudinal inferences. Med. Eng. Phys. 2009, 31, 681–687. [Google Scholar] [CrossRef]
- Pomero, V.; Mitton, D.; Laporte, S.; de Guise, J.A.; Skalli, W. Fast accurate stereoradiographic 3D-reconstruction of the spine using a combined geometric and statistic model. Clin. Biomech. 2004, 19, 240–247. [Google Scholar] [CrossRef]
- Gille, O.; Champain, N.; Benchikh-El-Fegoun, A.; Vital, J.M.; Skalli, W. Reliability of 3D reconstruction of the spine of mild scoliotic patients. Spine 2007, 32, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Ilharreborde, B.; Dubousset, J.; Le Huec, J.C. Use of EOS imaging for the assessment of scoliosis deformities: Application to postoperative 3D quantitative analysis of the trunk. Eur. Spine J. 2014, 23 (Suppl. S4), 397–405. [Google Scholar] [CrossRef]
- Letchuman, V.; Ampie, L.; Choy, W.; DiDomenico, J.D.; Syed, H.R.; Buchholz, A.L. Bone grafting and biologics for spinal fusion in the pediatric population: Current understanding and future perspective. Neurosurg. Focus 2021, 50, E8. [Google Scholar] [CrossRef]
- Fiani, B.; Jarrah, R.; Shields, J.; Sekhon, M. Enhanced biomaterials: Systematic review of alternatives to supplement spine fusion including silicon nitride, bioactive glass, amino peptide bone graft, and tantalum. Neurosurg. Focus 2021, 50, E10. [Google Scholar] [CrossRef]
- Larson, A.N.; Schueler, B.A.; Dubousset, J. Radiation in Spine Deformity: State-of-the-Art Reviews. Spine Deform. 2019, 7, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Courvoisier, A.; Sailhan, F.; Laffenetre, O.; Obert, L.; French Study Group of BMP in Orthopedic Surgery. Bone morphogenetic protein and orthopaedic surgery: Can we legitimate its off-label use? Int. Orthop. 2014, 38, 2601–2605. [Google Scholar] [CrossRef]
- Allan, I.; Newman, H.; Wilson, M. Particulate Bioglass reduces the viability of bacterial biofilms formed on its surface in an in vitro model. Clin. Oral Implants Res. 2002, 13, 53–58. [Google Scholar] [CrossRef]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef]

| Characteristic | N = 43 | Granules (n = 18) | Putty (n = 25) | p Value between Groups | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 15.4 ± 1.9 [11–19] | 15.7 ± 1.7 [13–19] | 15.2 ± 2.0 [11–19] | p = 0.466—NS | ||||
| Female | 30 (69.8%) | 10 (55.6%) | 20 (80%) | / | ||||
| Male | 13 (30.2%) | 8 (44.4%) | 5 (20%) | / | ||||
| Weight (kg) | 49.4 ± 9.9 [31–77] | 47.9 ± 11.5 [31–71] | 50.4 ± 8.7 [37–77] | p = 0.413—NS | ||||
| Size | 1.60 ± 0.06 [1.50–1.75] | 1.58 ± 0.07 [1.50–1.70] | 1.61 ± 0.06 [1.50–1.75] | p = 0.402—NS | ||||
| BMI (kg/m2) | 19.9 ± 3.3 [15.8–29.7] | 20.8 ± 3.6 [15.8–26.1] | 19.5 ± 3.2 [16.0–29.7] | p = 0.290—NS | ||||
| Smoking | None | / | / | / | ||||
| Indication | ||||||||
| Adolescent idiopathic scoliosis | 34 (79.1%) | 9 (50%) | 25 (100%) | / | ||||
| Neurologic scoliosis | 7 (16.3%) | 7 (38.9%) | / | / | ||||
| Neuromuscular scoliosis | 2 (4.7%) | 2 (11.1%) | / | / | ||||
| Lenke classification | 1A 2A 1B 3C 5C 1C | 20 (46.5%) 2 (4.7%) 3 (7.0%) 1 (2.3%) 16 (37.2%) 1 (2.3%) | 1A 2A 1B 3C 5C 1C | 6 (33.3%) 0 (0%) 2 (11.1%) 0 (0%) 9 (50%) 1 (5.6%) | 1A 2A 1B 3C 5C 1C | 14 (56%) 2 (8.0%) 1 (4.0%) 1 (4.0%) 7 (28%) 0 (0%) | / | |
| Risser classification | 1 2 3 4 5 | 2 (4.7%) 3 (7.0%) 3 (7.0%) 30 (69.8%) 5 (11.6%) | 1 2 3 4 5 | 2 (11.1%) 2 (11.1%) 2 (11.1%) 11 (61.1%) 1 (5.6%) | 1 2 3 4 5 | 0 (0.0%) 1 (4%) 1 (4%) 19 (76%) 4 (16%) | / | |
| N (%) | Granules (%) | Putty (%) | |
|---|---|---|---|
| Mean number of levels | 10 ± 3 [4–15] | 12 ± 3 [5–15] | 8 ± 3 [4–12] |
| Number of instrumented levels | |||
| ≥10 | 27 (62.8%) | 16 (88.9%) | 11 (44%) |
| 8–9 | 7 (16.3%) | 1 (5.6%) | 6 (24%) |
| 6–7 | 0 (0%) | 0 (0%) | 0 (0%) |
| ≤5 | 9 (20.9%) | 1 (5.6%) | 8 (32%) |
| N = 43 | p Value | Granules (n = 18) | p Value | Putty (n = 25) | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (n) | Range | Mean | Range | Mean | Range | ||||
| Cobb angle | |||||||||
| Pre-op | 62.7 ± 22.7 (43) | [30–130] | / | 70.4 ± 24.9 (18) | [42–130] | / | 57.2 ± 19.6 (25) | [30–120] | / |
| 1st erect | 26.5 ± 16.4 (43) | [0–68] | p < 0.05 from pre-op (7.10−13) | 30.1 ± 17.9 (18) | [2–68] | p < 0.05 from pre-op (3.10−6) | 23.9 ± 15.1 (25) | [0–50] | p < 0.05 from pre-op (2.10−8) |
| 3–6 months | 24.0 ± 13.9 (25) | [0–50] | p < 0.05 from pre-op (8.10−11) | 23.0 ± 7.1 (2) | [18–28] | p < 0.05 from pre-op (0.02) | 24.1 ± 14.5 (23) | [0–50] | p < 0.05 from pre-op (3.10−8) |
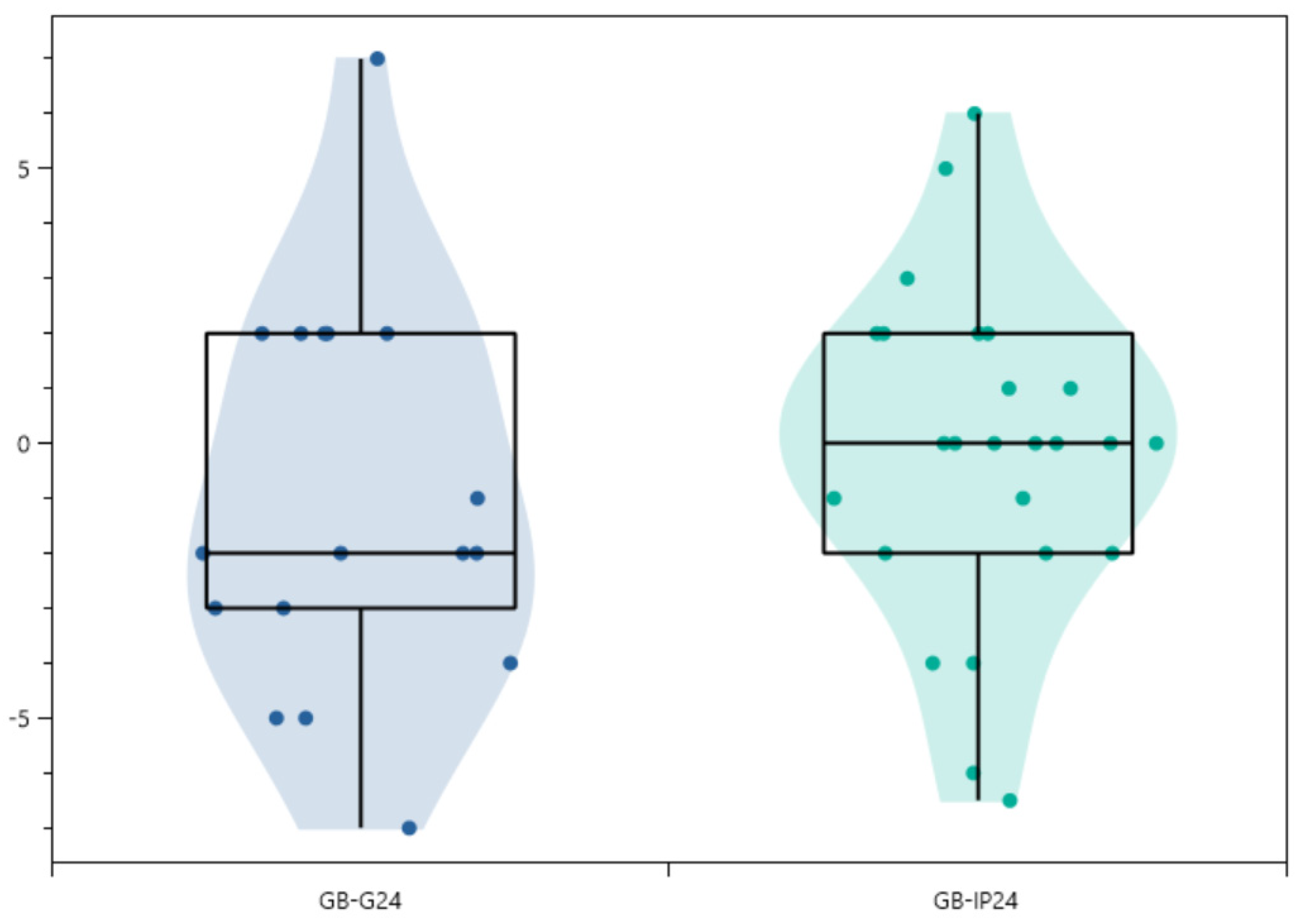
| 24 months | 27.1 ± 16.1 (42) | [0–70] | p < 0.05 from pre-op (10−12) | 31.2 ± 18.6 (17) | [7–70] | p < 0.05 from pre-op (9.10−6) | 24.1 ± 14.1 (25) | [0–52] | p < 0.05 from pre-op (10−8) |
| Correction rate | |||||||||
| Pre-op vs. 1st erect (°) | 36.2 ± 12.0 (43) | [15–70] | / | 40.3 ± 11.4 (18) | [24–70] | / | 33.2 ± 11.7 (25) | [15–70] | / |
| Loss of correction | |||||||||
| 1st erect vs. 3/6 months (°) | −0.55 ± 3.32 (25) | [−8.0–5.0] | p = 0.874—NS | 0.00 ± 5.7 (2) | [−4.0–4.0] | p = 0.950—NS | −0.60 ± 3.25 (23) | [−8.0–5.0] | p = 0.773—NS |
| 1st erect vs. 24 months (°) | −0.65 ± 3.24 (42) | [−7.0–7.0] | p = 0.671—NS | −1.12 ± 3.52 (17) | [−7.0–7.0] | p = 0.685—NS | −0.18 ± 2.97 (25) | [−6.5–6.0] | p = 0.868—NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courvoisier, A.; Maximin, M.-C.; Baroncini, A. Safety and Efficacy of Stand-Alone Bioactive Glass Injectable Putty or Granules in Posterior Vertebral Fusion for Adolescent Idiopathic and Non-Idiopathic Scoliosis. Children 2023, 10, 398. https://doi.org/10.3390/children10020398
Courvoisier A, Maximin M-C, Baroncini A. Safety and Efficacy of Stand-Alone Bioactive Glass Injectable Putty or Granules in Posterior Vertebral Fusion for Adolescent Idiopathic and Non-Idiopathic Scoliosis. Children. 2023; 10(2):398. https://doi.org/10.3390/children10020398
Chicago/Turabian StyleCourvoisier, Aurélien, Marie-Christine Maximin, and Alice Baroncini. 2023. "Safety and Efficacy of Stand-Alone Bioactive Glass Injectable Putty or Granules in Posterior Vertebral Fusion for Adolescent Idiopathic and Non-Idiopathic Scoliosis" Children 10, no. 2: 398. https://doi.org/10.3390/children10020398
APA StyleCourvoisier, A., Maximin, M.-C., & Baroncini, A. (2023). Safety and Efficacy of Stand-Alone Bioactive Glass Injectable Putty or Granules in Posterior Vertebral Fusion for Adolescent Idiopathic and Non-Idiopathic Scoliosis. Children, 10(2), 398. https://doi.org/10.3390/children10020398






